Despite decades of acknowledging that a loss of insulin-producing pancreatic β-cells is central to the disorder now referred to as type 1 diabetes, the specific roles for genetic susceptibility, environmental factors, the immune system, and β-cells themselves in the pathogenic processes underlying the disorder remain unclear (1,2). Looking back over this period, one can identify a handful of conceptualizations that were seminal in their attempt to address this issue, including that posited by Dr. Gian Franco Bottazzo in his 1986 article, “Death of a Beta Cell: Homicide or Suicide?” (3). Bottazzo questioned whether the disorder’s pathogenesis weighed more heavily (or exclusively) on processes related to immune responsiveness (i.e., homicide) or the fragility of β-cells leading to self-destruction (i.e., suicide).
Many reasons exist with respect to why we are in this knowledge void, including the exceedingly complex nature of type 1 diabetes, the likelihood that this disorder may represent a disease with more than one etiology, as well as the complex interplay of genetics, the immune system, and the environment. One limitation in solving important pathogenic questions in type 1 diabetes has likely been suboptimal cross-talk among geneticists, epidemiologists, endocrinologists, and others. Our own approach to overcoming this limitation has been to try to increase collaboration between cell biologists and immunologists as a critical step in closing knowledge gaps regarding the disorder’s pathogenesis. The opinion put forward within this Perspectives article by this group of authors is one where multiple and clearly unique properties of the β-cell appear fundamental to the loss of immune tolerance, accompanied by immune-mediated destruction.
WHAT DID BOTTAZZO PORTEND?
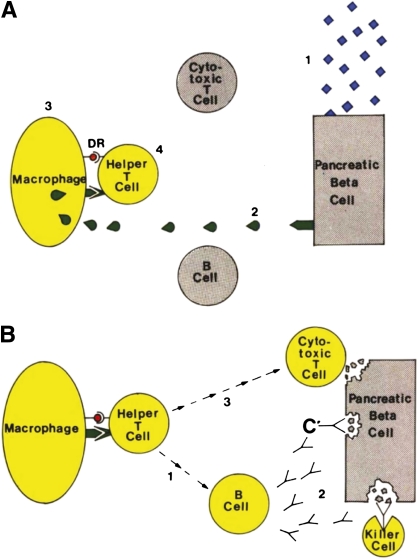
The Bottazzo article (3) was unique in its form of presentation, in that the prose represented the equivalent workings of a legal stenographer recording the debate between two counsels: one for the prosecution (i.e., β-cell homicide) the other representing the defense (i.e., β-cell suicide). More important than the means for its presentation was the evidence noted for each case. Arguments for homicide included, but were not limited to, a theoretic scenario wherein type 1 diabetes was posed to be initiated by an ill-defined environmental attack resulting in the release of β-cell autoantigens (Fig. 1). Subsequently, those self-antigens were thought to be scavenged by macrophages, presented by major histocompatibility complex (MHC) class II molecules (i.e., HLA-DR), leading to the activation of helper T-cells, which would in turn activate B-cells to produce antibodies (e.g., islet cell cytoplasmic autoantibodies and complement-fixing autoantibodies) as well as activate killer cells and cytotoxic T-cells. Interestingly, Bottazzo did note the potential role for “suppressor T-lymphocytes” (i.e., a forerunner of today’s regulatory T-cell), but left them out of the equation because of their ill-defined nature at the time of his writing. Other prosecutorial arguments included seasonality for the disease onset, typical age of onset, and questions related to genetic susceptibility to this disease.
FIG. 1.
Bottazzo’s “Exhibit 5.” Diagram shows the hypothetical steps leading to activation of the immune system against the β-cell. A: The triggering events: 1) Environmental attack. 2) Release of autoantigens from the β-cell. 3) The macrophage processes them on its surface membrane. D/DR molecules present islet autoantigens to the helper T-cell. 4) Activation of the helper T-cell. B: Closing the circle: 1) Activation of the B-cell to the helper T-cell. 2) Production of islet cell antibodies, followed by antibody-dependent complement (C’) and killer cell–mediated cytotoxicity. 3) Activation of the cytotoxic T-cell. Adapted (direct prose) from Bottazzo (3), reprinted with permission from John Wiley & Sons, Inc.
This prosecution was countered by a model of β-cell suicide, wherein the long latency period for type 1 diabetes formation was placed alongside a series of (then) timely genetic associations: chromosome 6 (HLA), 11 (insulin), 14 (Gm allotypes), and 2 (κ light chains), allowing for regimented β-cell death. Most controversial to this case was the added consideration involving an expression of HLA class II molecules on β-cells. This suicide model suggested the death of a β-cell was a matter of destiny, imprinted at the time of conception.
With a quarter century of research investigations since the initial presentation of these arguments, taken together with the benefit of hindsight, where do we currently stand in support of this polarizing issue of homicide versus suicide? Is it an either/or proposition? Do current genetic and physiologic insights enlighten the controversy? And finally, how will current technologies and basic biologic insights help to resolve this debate? Addressing these questions forms a major part of this Perspectives article.
TYPE 1 DIABETES FROM THE PERSPECTIVE OF THE IMMUNE RESPONSE: THE CURRENT CASE FOR HOMICIDE
Many of the concepts in the basic homicide model presented in the Bottazzo article remain intact. Indeed, a basic tenet that remains is the notion that type 1 diabetes is a disease of T-cells, but one highly influenced by the action of other components of the immune system, including B-cells, natural killer cells, and macrophages (2).
The role of the immune response in β-cell destruction.
With the caveat that no animal model (e.g., NOD mice) captures the complexity of human type 1 diabetes, but that some concepts can only be tested in such systems, studies of NOD mice since the time of Bottazzo have aided similar investigations of the immune response in human disease. Studies in animal models of type 1 diabetes have shown that a variety of pathogenic pathways can lead to the immune-mediated destruction of β-cells. For example, CD8+ cytolytic T-cells can kill β-cells directly in a perforin-dependent manner; but, at the same time, exposure of β-cells to cytokines can lead to their demise. In addition, apoptosis mediated by fatty acid synthase/fatty acid synthase ligand can play an important role (4), thus illustrating the potentially multifaceted face of β-cell demise.
Turning to studies of humans, interferons, potentially produced in response to local or systemic infection events, can accelerate β-cell destruction by inducing the expression of islet antigen-presenting MHC class I molecules on β-cells (5). These observations blend well with those of other human studies where higher levels of MHC class I molecules are frequently detected on β-cells (6), and infiltration by CD8+ T-cells in inflamed pancreata from type 1 diabetic patients have been found (7).
Taken collectively, multiple pathways of β-cell elimination may be operational at the same time, suggesting a remarkable complementarity among the various pathways targeting β-cells for destruction. This multiparameter approach to β-cell destruction by the immune system suggests that individual immunotherapeutic treatments targeting any one pathway may not be as successful as those aimed at the initial target antigen recognition or active against multiple pathways (8,9), as will be discussed later.
As was the case in the era of the Bottazzo report (3), it remains unclear whether single or multiple primary specific antigenic targets initiate this disease. One leading hypothesis today is that insulin itself may be the crucial antigenic target, particularly in NOD mice, where mutating a single amino acid of insulin peptide 9-23 prevents all formation of diabetes (10). Further evidence of the key role of insulin derives from genetic studies in humans demonstrating genetic polymorphisms in the insulin gene that alter the expression of the protein, especially in the thymus, where central tolerance to autoantigens operates. This being said, there is also ample evidence from T-cell repertoire and autoantibody analyses to suggest that other pathogenic self-antigens (e.g., GAD, insulinoma-associated protein 2) with varying specificities may participate in the autoimmune response (11).
Beyond this, we have also learned much since the Bottazzo report about the processes of how the immune response develops a response against various antigens over time (i.e., intermolecular spreading of antigenicity), as well as that within a given antigen (i.e., intramolecular or epitope spreading), both of which may prove important to type 1 diabetes formation. Indeed, we now know that the initial antibody response in humans predominantly occurs against the insulin or GAD molecule, with spreading over time to other β-cell antigens. Interestingly, NOD mice produce high levels of insulin autoantibodies, and although NOD T-cells target multiple molecules (e.g., GAD, chromogranin A), it has been harder to demonstrate autoantibodies to many other T-cell targets using this animal model, including GAD.
Novel mechanisms of antigenic processing that lead to immune responsiveness.
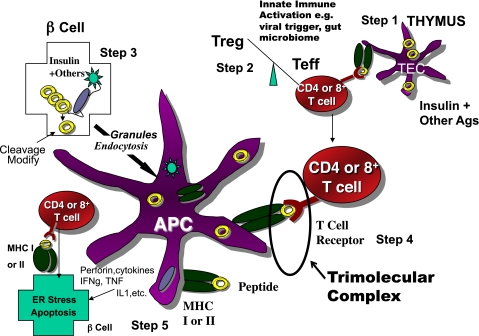
The initially trivial answer to the question of why β-cells are targeted and destroyed by the immune system was that the molecules targeted by the immune system were merely present in β-cells. Recent studies, however, indicate that T-cell targeting of specific autoantigens represents a far more complex process (Fig. 2) than once thought and may depend on tissue-specific localization and processing of a given target antigen(s). Indeed, many examples have been found of epitopes of islet antigens produced in β-cells that are then recognized by specific anti-islet T-cells, and with this, multiple new concepts (beyond those of the era of the Bottazzo article) need to be considered when type 1 diabetes is viewed from the perspective of homicide (Table 1).
FIG. 2.
Simplified model of the immune pathogenesis of type 1 diabetes. Major components include Step 1) The thymus where peptides of peripheral antigens are expressed and presented by HLA molecules on the surface of medullary thymic epithelial cells (mTECs cells) to T-cell receptors, leading to deletion of many but not all anti-islet autoreactive T-cells. Step 2) Regulatory T-cells (Treg) and effector T-cells are both produced, and their balance is crucial for maintaining tolerance. Innate immune activation can affect the balance in terms of activating autoimmunity. Step 3) The β-cell itself not only produces target antigens but also modifies molecules, such as chromogranin, by cleavage at critical sites, thus creating peptides recognized by pathogenic T-cells. There is evidence that processing of molecules such as insulin within the β-cell creates peptides that are then taken up by antigen-presenting cells either as whole, dead β-cells, or specifically, granules of β-cells, for eventual further processing and presentation of islet peptides to effector T-cells. Step 4) The trimolecular complex, involving the MHC-presenting molecule/peptide in the appropriate “register”/T-cell receptor recognizing both and, like a lock and key, is the essential recognition unit for adaptive organ-specific autoimmunity. Step 5) Finally, CD4 T-cells orchestrate multiple arms of the immune system (e.g., CD8 cytotoxic T-cells, pathogenic cytokine production), resulting in specific destruction of islet β-cells.
TABLE 1.
Five recent notations supporting the importance for homicide as a means for β-cell destruction
| 1. | Tissue-specific cleavage appears critical to produce target autoantigenic peptides. |
| 2. | Production of an antigenic peptide can occur directly in islet β-cells and not merely by antigen-presenting cells exposed to the target molecule. |
| 3. | T-cells targeting multiple islet antigens are required to cause type 1 diabetes. |
| 4. | Post-translational modification of peptide epitopes may be important to their antigenicity. |
| 5. | Recognition of specific islet peptides appears to occur by conserved sequences of T-cell receptors. |
First, tissue-specific cleavage may be critical to the production of a target peptide. For instance, the neuroendocrine molecule chromogranin A is cleaved to produce a specific peptide (WE14) within islets that is recognized by the pathogenic BDC2.5 T-cell receptor (TCR) of NOD mice in the context of pockets 5 through 9 of the I-Ag7 cMHC class II molecule, the only class II–presenting molecule in the NOD mouse (12). In contrast, other cells expressing or “fed” naked chromogranin A do not effectively produce this autoantigenic peptide. Of note, the tissue-specific cleavage to produce the WE14 peptide removes four N-terminal amino acids present in chromogranin A that, if present, would fill pockets 1 to 4 of I-Ag7. If these four amino acids are present, they block BDC 2.5 TCR stimulation. In addition, WE14 contains nine COOH-terminal amino acids projecting outside the I-Ag7 binding groove, which when removed, abrogate binding and, naturally, TCR stimulation. Thus, the specific cleavage of chromogranin A within the β-cell is essential for the diabetogenicity of BDC 2.5 T-cells.
Second, production of the antigenic peptide can occur in islet β-cells, that is, not necessarily in antigen-presenting cells (APCs) exposed to the target molecule. Unanue et al. (13) recently found that insulin peptide B:9-23 is produced within NOD islet β-cells, and that APCs process the peptide and the whole insulin secretory granules. Only APCs from islets are able to directly stimulate anti-B:9-23 T-cells (without exogenous antigen), and for many anti-B:9-23 T-cell clones, provision of insulin to APCs does not lead to stimulation, but the processed peptide needs to be presented. The B:9-23 peptide presentation is further unusual in that it binds in a very unusual low-affinity register to I-Ag7, and binding in this register is key for presentation to NOD anti-B:9-23 TCR (14).
Next, for type 1 diabetes to develop, it may be necessary for T-cells to target multiple islet antigens. For example, the CD8 T-cell clone targeting the islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) is very diabetogenic, but only when T-cells targeting insulin peptide B:9-23 or perhaps other class II–restricted peptides are also present. In contrast, removing IGRP autoreactivity does not decrease progression to diabetes in NOD mice (15). In other animal models of diabetes, however, CD8+ T-cells specific for a single antigen can cause loss of β-cells and diabetes (16). Taken together, it is likely that the functional synergy between CD4+ and CD8+ T-cells and their cytokine production, perhaps due to selective tissue targets, is essential for a potent pathogenic response (in the initial stages of) and disease development. However, whether targeting of multiple antigens and antigenic spreading is required for diabetes development and if so, at which stage, is not known.
There are several examples where autoreactive TCRs targeting a given epitope share sequence elements. Tollefsen et al. (17) have defined TCR sequences recognizing a peptide of IGRP with dominant use of Vα17-Jα42 by CD8 T-cells, both in spontaneous disease of NOD mice and in recurrent disease after islet transplantation. In addition, the B:9-23 peptide is targeted predominantly by NOD T-cells using the TRAV5D-4*04 germline–encoded TCR α-chain sequence, with highly variable α-chain N regions and variable TCR β-chains (18). Precise analysis of the repertoire and clonality of T-cells infiltrating human islets will be needed to finally answer the important question of whether a few specific culprits are destroying β-cells or whether multiple specificities are of comparable importance.
Lastly, post-translational modification of peptide epitopes could be important for fueling the process of immune destruction. A clear example of post-translational modification of the β-cell peptide essential for T-cell autoreactivity does not yet exist, but other disorders where transglutaminase modification of gliadin is essential (19), such as celiac disease, have illustrated the importance of this concept.
TYPE 1 DIABETES FROM THE PERSPECTIVE OF A β-CELL: THE CURRENT CASE FOR SUICIDE
The case for homicide presented, the converse notion would turn to that of suicide, a notion where evidence also continues to grow since the publication of Bottazzo’s work. There are at least three ways in which the β-cell might participate in its own demise:
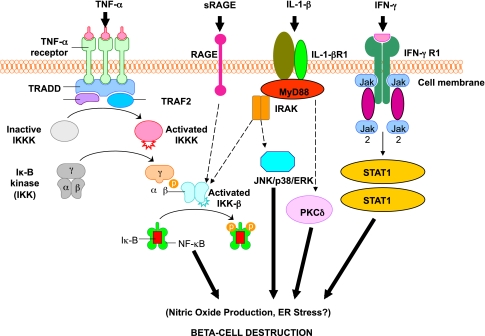
First, the β-cell may be a very friendly target for immune destruction. As suggested, β-cells may be vulnerable to stress-induced changes that may occur during local infections, causing production of specific autoantigenic peptides recognized by pathogenic T-cells. Second, there is documented evidence of increased β-cell sensitivity to cytokine-mediated killing. Indeed, β-cells appear to be particularly sensitive to the cytokine interleukin (IL)-1β (Fig. 3). Third, persistent changes in β-cell physiology (e.g., hyperexpression of class I molecules) once autoimmunity has been initiated likely enhance their sensitivity to autoimmune destruction. Thus, it might not be surprising that β-cells within islets are predominantly destroyed even though other cell types (e.g., islet cells producing glucagon) express many similar antigens and survive. Finally, the islet β-cells are extremely prone to self-directed cellular destruction because they are very sensitive to multiple forms of endoplasmic reticulum (ER) stress, as evidenced by mutations that affect insulin protein folding.
FIG. 3.
Activation of inflammatory mediators in pancreatic β-cells in type 1 diabetes. Tumor necrosis factor-α (TNF-α), IL-1β, and interferon-γ (IFN-γ) are the most likely cytokines acting in synergy during inflammation of pancreatic β-cells, leading to the activation of a final common pathway, such as nuclear factor-κB (NF-κB) and, ultimately, to β-cell destruction. NF-κB can be activated by a variety of stimuli, including TNF-α, IL-1, receptor for advanced glycation end products (RAGE), and Toll-like receptors (TLRs). IL-1β is an inflammatory cytokine that plays a major role in immune-mediated β-cell destruction. Interestingly, in patients with type 2 diabetes, the IL-1 pathway blockade with an IL-1 receptor antagonist (Anakinra) improved glycemic control and β-cell secretory function and resulted in a significant reduction marker of systemic inflammation, namely, C-reactive protein and IL-6 (56). A recent clinical study indicated that the blockade of the IL-1β pathway in type 1 diabetes resulted in the reduced ability of mononuclear cells to traffic to sites of inflammation (57). The latter observations provide evidence for a possible mechanistic link between type 1 and type 2 diabetes, and additional studies are necessary to unravel the common inflammatory pathways involved in the pathologic etiology of these two diseases. Compelling evidence indicates that cytokines influence the expression of inducible NO synthase (iNOS) leading to NO production. IL-1β and IFNγ, by NO synthesis, were reported to markedly decrease sarco(endo)plasmic reticulum Ca2+ ATPase 2b (SERCA2b) protein expression, deplete Ca2+ stores, and activate ER stress pathway, which is a potential contributing mechanism to β-cell death. Furthermore, cytokine-induced (IL-1β + IFN-γ) apoptosis of INS-1 cells appears to depend on NO production, as demonstrated by the use of the NO dioxygenase blocker NG-methyl-l-arginine. NO also contributes to cytokine-induced apoptosis through potentiation of Jun NH2-terminal kinase (JNK) activity and suppression of Akt/protein kinase B. Although whether oxidative stress plays a key role in the pathogenesis of type 1 diabetes is still being discussed, a reduced antioxidant capacity has been demonstrated in patients with type 1 diabetes compared with healthy control subjects. To summarize the cytokine signaling, TNF-α signals through trimerized p60 receptors that interact with the TNF receptor type 1–associated death domain protein (TRADD). Fas-associated protein with death domain (FADD) is then recruited by TRADD, thus allowing binding of receptor-interacting protein (RIP) and TNF receptor–associated factor 2 (TRAF2) to the receptor complex. TRAF2 activates NF-κB through NF-κB–inducing kinase (NIK)–inhibitor of κB kinase (IKK) and activates the JNK/p38 pathways. TNF-α is an inflammatory cytokine that appears to be associated with a number of autoimmune disorders, including type 1 diabetes. TNF-α may activate intraislet resident macrophages, resulting in the release of IL-1β, which generates iNOS expression and the overproduction of NO in β-cells. Alterations in the number and function of CD4+CD25+ T-cells may be an additional mechanism by which TNF-α may cause type 1 diabetes in NOD mice. The role of RAGE mediated by NF-κB has not been entirely elucidated, although RAGE may be an important intermediary in causing monocyte production of inflammatory mediators such as TNF-α. It is possible that increased expression of RAGE in response to hyperglycemia may lead to activation of innate and even adaptive immune responses and enhance β-cell destruction. After IL-1β binding to IL-1βR1, MyD88 is recruited to the receptor complex. MyD88 interacts with IL-1 receptor–associated kinase (IRAK), allowing the binding of TRAF6 to IRAK. TRAF6 causes activation of mitogen-activated protein kinase/stress-activated protein kinase and activation of the NF-κB pathway by transforming growth factor-β–activated kinase 1 (TAK1)–mediated activation of IKK. IL-1β also stimulates activation of protein kinase C-δ (PKC-δ), possibly through phospholipase C generation of diacylglycerol. ERK, extracellular signal–regulated kinase; Jak, Janus kinase; STAT1, signal transducer and activator of transcription-1.
Indeed, the β-cell is exquisitely unique. They are the sole source for providing insulin when needed, but also in that their metabolic machinery is distinctively set up for generating secondary signals to control β-cell function (20). This provides an exclusive link for metabolic homeostasis to control cellular function; however, it might also be the Achilles’ heel of the β-cell, as articulated in the next section.
The β-cell’s unique metabolic regulation of insulin production.
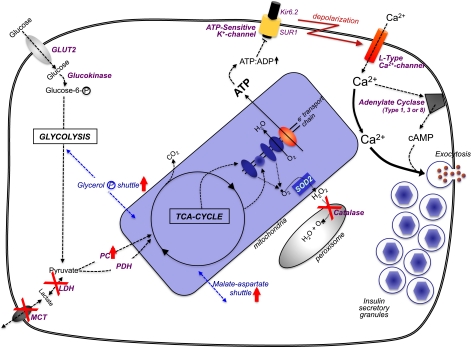
After a meal, under normal circumstances, the β-cell is stimulated not only to secrete insulin but also to replenish the intracellular stores by a parallel upregulation of proinsulin biosynthesis and processing (21). As much as 20% of the total protein synthesized by a β-cell is (pro)insulin and may occur under such conditions. The predominant physiologically relevant nutrient to regulate β-cell function is glucose, but certain amino acids, fatty acids, and incretin hormones can assist in this process (20,22). Nutrient control of β-cell function requires glucose metabolism to generate secondary signals, such as an elevation in cytosolic [Ca2+]i (23), and the carbon in glucose is uniquely channeled from glycolysis to mitochondrial trichloroacetic acid cycle metabolism for this purpose by anaplerosis (20,22). As a consequence, the conglomerate of β-cell metabolic enzymes is quite distinct from that of other cells (Fig. 4):
FIG. 4.
The pancreatic β-cell’s “metabolic wiring” is uniquely geared to generate secondary signals. Glucose enters the β-cell by the GLUT2 transporter, where it is phosphorylated by glucokinase and channeled to glycolysis. The combination of GLUT2 and glucokinase (with their Km values in the mmol/L range) sense glucose in the physiologic range that is unique to only a few nutrient-sensing cells in the body. Also relatively unique to β-cells is a negligible amount of lactate dehydrogenase (LDH) and the plasma membrane lactate/pyruvate transporter (MCT), so that the pyruvate resulting from glycolysis is channeled to the trichloroacetic acid cycle in the mitochondria. To counter for this, there is unusually high pyruvate carboxylase (PC) activity in β-cells to accompany pyruvate dehydrogenase (PDH). To rebalance β-cell redox in the absence of LDH/MCT, there is an increase in mitochondrial shuttle activities, particularly the glycerol-phosphate and malate-aspartate shuttles. Mitochondrial oxidative activity by the trichloroacetic acid cycle and electron transport chain generates [ATP] that is comparable to the glycolytic flux relative to increased circulating glucose. A byproduct of increased mitochondrial oxidative activity is a rise in oxygen radicals that can be converted to H2O2 in mitochondria by superoxide dismutase-2 (SOD2). But β-cells cannot dispose of H2O2 very well because they are uniquely deficient in catalase in their peroxisomes. An increase in ATP production in β-cells leads to a rise in the adenosine 5´-triphosphate (ATP)/adenosine 5´-diphosphate (ADP) ratio that shuts the ATP-sensitive potassium channel, which consists of the sulfonylurea receptor (SUR1) and Kir6.2 subunits. The consequential decreased efflux of K+ results in a depolarization of the β-cell’s plasma membrane, and voltage-sensitive Ca2+-channels open, increasing cytosolic [Ca2+]i. The rise in [Ca2+]i is the major trigger to induce insulin secretory granule exocytosis for release of insulin to the circulation. The rise in [Ca2+]i can also activate Ca2+/calmodulin–activated adenylate cyclases (type I, II, or VIII) to increase the cell’s [cAMP]i levels, which is an augmentation signal to potentiate the Ca2+-induced insulin secretion.
First, there is a β-cell–specific isoform of glucokinase that, together with the glucose transporter GLUT2 and low hexokinase activity, enables the β-cell to sense circulating glucose concentrations in the physiologic range of 2 to 20 mmol/L (19,23).
Second, β-cells are deficient in lactate dehydrogenase and plasma membrane monocarboxylate transporters, but have a marked increase in pyruvate carboxylase activity, all to focus channeling pyruvate into mitochondrial metabolism/anaplerosis (22). Because of the lack of lactate dehydrogenase, β-cells reoxidize NADH and maintain cellular redox by highly active mitochondrial shuttles, particularly the glycerol phosphate and malate-aspartate shuttles (22).
Third, β-cells have a low capacity to oxidize fatty acids and relatively little pentose phosphate pathway activity (22,24), underlining the focus of β-cells on glycolytic metabolism.
Finally, β-cells appear to have a low capacity for disposing of reactive oxygen species (ROS) generated from mitochondrial metabolism, in particular H2O2 (25), which has led some to suggest that ROS could be additional metabolic secondary signals for control of β-cell function (26).
Although the inimitable metabolic wiring of the β-cell works extraordinarily well to control β-cell functions under normal conditions, this might well be its undoing under the pathologic circumstances of type 1 diabetes (Fig. 5).
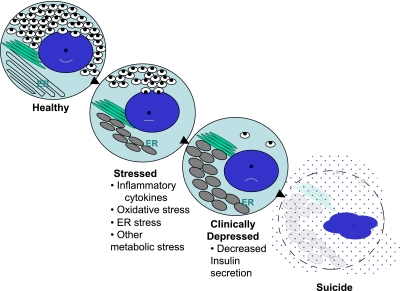
FIG. 5.
The progressive state of β-cell dysfunction in type 1 diabetes—a model for stages of depression? As noted in the text, increasing data support a model of progressive β-cell dysfunction in type 1 diabetes. Meaning, from the activities of a variety of “stressors,” (e.g., inflammation, glucolipotoxicity, ER stress, etc.), β-cells move from a state of normalcy to a “stressed” state by a process that depletes the insulin storage pool. Therefore, β-cells proceed to a state where dysfunctional insulin secretion occurs, along with expansion and distortion of the ER. The end stage is one of “assisted suicide,” where the immunologic parameters, when combined with unique susceptibilities inherent to β-cells, lead to their ultimate demise.
Setting the stage: metabolism and hyperglycemia as a key component for β-cells in type 1 diabetes progression.
Hyperglycemia may contribute to acceleration of type 1 diabetes through enhanced antigen exposure by β-cells or through other mechanisms such as enhancing their suicide. Indeed, the National Institutes of Health Diabetes Care and Complications Trial (DCCT) showed that the decline in β-cell function was reduced in participants in the group that received intensive insulin treatment versus the control group that received standard insulin treatment (27). At the same time, even mild persistent hyperglycemia results in significant depletion of insulin stores with decreased insulin release from the remaining β-cells, leading to a vicious cycle and ultimate deterioration of glycemic control (28,29). These observations provide a motivation underlying metabolic therapies that provide β-cells rest to allow for insulin repletion and future secretory responses (30). Early and subtle islet secretory defects are an established component in at least a subset of patients that develop type 1 diabetes (31).
Well before the onset of diabetic ketoacidosis but after onset of autoimmunity, mild hyperglycemia may be present. In turn, mild persistent hyperglycemia triggers ER stress and oxidative stress in pancreatic β-cells (32), even as inflammatory signaling, such as through IL-1, may also trigger ER stress and mitochondrial stress in β-cells—effecting a “two-hit” injury (33). Thus, metabolic contributions, with their obvious linkage to type 2 diabetes, also appear unavoidable in our approach to type 1 diabetes (34).
An interesting result supporting such an interface between metabolism and autoimmunity is how islet transplantation (combined with immunomodulation), with metabolic control recovered at least temporarily by insulin secretion from the transplant, may be linked to neogenesis and recovery of endogenous pancreatic β-cells (35,36). The concept of such interplay is clinically relevant, because metabolic therapies at the time of the initial overt type 1 diabetes often trigger a honeymoon period that reflects the recovery from the acute stress-related insults that led to the diagnosis (37). Such thinking has led many to aim for strategies for providing enhanced β-cell function with immunomodulation for reversal of type 1 diabetes (38).
A prototypic model for the β-cell’s unique demise in the pathogenesis of type 1 diabetes.
Proinsulin biosynthesis is dynamically regulated at the translational level (39,40). Also regulated in parallel are most of the protein components of the insulin secretory granule (ISG), many of which, in addition to insulin itself, are major islet cell antigens, such as IGRP, insulinoma-associated protein 2, chromogranin A, and ZnT8, among others (40).
How could this exquisite machinery, which keeps the β-cell well occupied even under physiologic conditions, be derailed? Environmental factors, such as viral infections, have long been discussed as contributing factors to type 1 diabetes pathogenesis because of the discordance in disease onset in monozygotic twins (41). Indeed, viruses must use the host cell’s protein synthesis machinery to replicate, but internal defense mechanisms exist to prevent this, including the unfolded protein response and activation of interferon pathways. Both could be detrimental for the β-cell while attempting to combat an ongoing infection. A virally infected β-cell would synthesize viral protein in parallel to proinsulin and ISG proteins, and the β-cell’s unfolded protein response could be increased in an attempt to rid foreign viral proteins, while protecting the capacity for the host cell’s normal protein biosynthesis. However, despite the huge capacity for the β-cell secretory pathway in proinsulin biosynthesis, the extra viral protein synthesis may push ER stress to the point that limits the ability to replenish stored insulin pools, and making β-cells susceptible to apoptosis.
Also because of the high capacity of the β-cell for proinsulin/ISG-protein biosynthesis, ER stress is likely to cause collateral damage to β-cell proteins, with peptides generated that could be presented by MHC to attract an autoimmune response, thus adding to β-cell susceptibility for destruction. In parallel, viral peptide fragments, derived from the unfolded protein response, would then be presented by MHC class I, which is possibly upregulated due to the antiviral interferon response (see the discussion in the previous section) to engage the immune system and reduce the viral infection. This could lead to direct β-cell lysis by antiviral T-cells as well as damage to the β-cell by upregulating interferons, leading to apoptosis in conjunction with other inflammatory cytokines such as IL-1β.
The unique metabolic signaling in the β-cell (Fig. 4) could well contribute to the susceptibility for β-cell destruction, especially in a relative inability to dispose of ROS (25), such as nitric oxide (NO) and associated ROS production in the cytokine-mediated destruction of β-cells (Fig. 3). Then, once a significant (i.e., critical) number of functional β-cells are lost, this metabolic susceptibility accelerates the selective β-cell destructive process. Excursions in postprandial glucose levels further deplete the size of the residual pancreatic insulin pool, while compensatory proinsulin/ISG-protein biosynthesis, which in turn could further raise ER stress, may result in increased β-cell antigen presentation by the MHC, thus fueling a vicious cycle. In this condition, the “metabolic wiring” of the β-cell would not help.
Glycolytic flux and mitochondrial oxidation would generate increased ROS, which would contribute to oxidative stress that enhances the β-cell apoptosis (42). Cytokine attack from autoimmunity would increase NO production and add to the oxidative stress and β-cell damage (43). Formation of the disulfide-bonds of proinsulin also contributes to ROS, so increased proinsulin biosynthesis itself is likely to add to ER stress and also to oxidative stress.
Finally, because the metabolic enzyme makeup of β-cells is designed to generate secondary signals, chronically increased glycolytic flux could lead to such an elevation of intracellular signals such as [Ca2+]i so that the normal buffering capacity to handle fluxes of [Ca2+]i is overcome and it becomes cytotoxic (44). Try as it may to rectify the situation by producing more insulin, the unique metabolic regulation of the β-cell function tends to further its own demise (Fig. 5).
Nonetheless, hyperglycemia alone is not sufficient to induce β-cell death but can impart dysfunction (45). Rather modest hyperglycemia, together with cytokine-mediated oxidative and ER stress, will exacerbate β-cell death and dysfunction. The contribution from hyperglycemia in accelerating this β-cell destructive process could be important when considering the recovery of β-cell function that occurs in the honeymoon period for many new-onset type 1 diabetic patients, once the hyperglycemia is treated.
BRIDGING THE GAP: β-CELL HOMICIDE AND SUICIDE TAKEN TOGETHER
More recently, it has become apparent that the onset of type 1 diabetes may not be solely a consequence of irreversible β-cell death. Loss of insulin production more likely results from a combination of β-cell destruction alongside partially reversible loss of β-cell function caused by inflammatory cytokines. In support of this notion, studies of human pancreata suggest that small numbers of insulin-positive β-cells are present in many patients with established type 1 diabetes, suggesting that β-cells can survive but are not able to secrete sufficient insulin to overcome hyperglycemia. In addition, some recent immune intervention trials observed rapid recovery of β-cell function that was too rapid to be explained by β-cell replication or neogenesis. For example, Couri et al. (46) achieved at least temporary insulin independence and increased C-peptide in most type 1 diabetic patients who were treated with a nonmyeloablative bone marrow transplant after strong immunosuppression. Thus, aborting the inflammatory and autoreactive response in conjunction with relieving metabolic stress might be sufficient to restore function in surviving β-cells. Crucial aspects that need to be studied in the future include determining the frequency of metabolically inactive surviving β-cells and identifying the pathways within β-cells that are affected by inflammatory immune mediators in conjunction with metabolic stress.
This is a significant issue, because mature β-cells have very little ability to proliferate and make up for the loss caused by an autoimmune insult. Specifically, although β-cell proliferation is readily detectable in children, new evidence suggests the proliferative capacity is already markedly reduced during early adolescence (47). This reduction is partly due to the increased expression of the cyclin-dependent kinase inhibitor p16 in aging β-cells that is partly mediated by components of the polycomb group of histone methyltransferases (48,49). Thus, even when the immune insult is blocked, it may be critical to develop approaches to induce the proliferation of remaining β-cells in older patients with long-standing type 1 diabetes to restore normoglycemia.
That said, recent studies have suggested that new β-cells can be generated from progenitor tissue. Indeed, studies in mice that have undergone pancreatic injury have demonstrated that other cell types, including duct or duct-associated cells, can reactivate the endocrine program and generate insulin-producing cells (50). Recent efforts also suggest that the immune system itself may be important in this regeneration process. Thus, future studies will have to address whether the β-cells found in patients with long-standing type 1 diabetes are derived from the few surviving β-cells that have escaped the immune insult or whether they have formed by neogenesis from a non–β-cell population. Importantly, understanding the origin of the β-cells present in type 1 diabetic patients might influence the choice of immune regulatory drug therapies to maximize islet regeneration and increase β-cell mass, while at the same time halting the immune assault.
Finally, it is likely that physiologic changes of β-cells in response to autoimmunity or unknown environmental factors may be critical to perpetuating islet autoimmunity. Studies of pancreatic sections from patients with both new-onset and a subset of patients with long-term type 1 diabetes reveal β-cell destruction in a lobular pattern, with some regions of the pancreas where all islets contain β-cells, whereas in other regions, all β-cells have been destroyed. As the number of whole pancreata from deceased donors with type 1 diabetes become more readily available, such as those in the Juvenile Diabetes Research Foundation (JDRF) Network for Pancreatic Donors with Diabetes (nPOD) resource (histology available online for research viewing at www.jdrfnpod.org), it is becoming clear that the degree of insulitis of most patients with type 1 diabetes is not only lobular but is also much less than that present in the NOD mouse or the BB rat animal models. This may relate to the usual very slow progression of β-cell loss in humans, occurring over years.
The nPOD resource also highlights the existence of heterogeneity of pancreatic pathology for patients who were diagnosed with type 1 diabetes. A subset of the pancreata from patients lacking islet autoantibodies or high-risk HLA have no pseudoatrophic islets (islets lacking all β-cells, typically associated with type 1A diabetes) but rather have decreased numbers of β-cells per islet rather than increased islets lacking all β-cells (51). Both for humans (of pancreata with pseudoatrophic islets) and the NOD mouse, the surviving β-cells are not normal and hyperexpress MHC class I alleles and the survivin molecule. In addition, hyperexpression of MHC class I alleles is seen in a lobular fashion up to 8 years after diagnosis, independently of islet infiltration (51). Unfortunately, the specific initiators of increased expression of MHC class I molecules on β-cells are not yet clear (see our earlier discussion).
USING KNOWLEDGE OF HOMICIDE AND SUICIDE OF β-CELLS TO DEVELOP THERAPIES FOR TYPE 1 DIABETES
Taken collectively, these concepts suggest how, through targeting of mechanisms of homicide and suicide, therapies seeking to prevent or reverse type 1 diabetes could be optimized (Fig. 5). This would include combining therapies that have already shown some degree of promise in modulating the immunologic attack, such as antibodies against lymphoid cell subsets (e.g., anti-CD3 or anti-CD20 monoclonal antibodies) or antigen-specific therapies (e.g., alum/GAD65 immunization), with agents that would preserve β-cell function (i.e., prevent suicide) or induce their replication (Fig. 6) (52–55). Indeed, by blocking inflammatory pathways together with modulation of adaptive immunity, homicide and suicide might be prevented and the direct effects of the immune therapy might be improved.
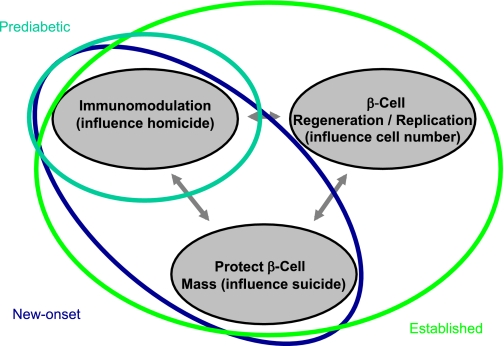
FIG. 6.
An idealized model illustrates intervention in type 1 diabetes by the degree of disease state and improved knowledge of understanding the role of β-cell homicide (i.e., immune-mediated death) and suicide (i.e., mechanisms that contribute to cellular death) in the processes underlying the formation of this disorder. Subjects would, theoretically, be placed into different means and methods of therapy by the status of their disease state, such as prediabetic, new-onset diabetes, or established diabetes. On the basis of our understanding of the natural history of diabetes, an increasingly intensive intervention may be required to provide therapeutic benefit to those with this disease. For example, in those with ongoing anti–β-cell autoimmunity but who lack overt type 1 diabetes (aqua line), therapy with a single agent capable of restoring immune tolerance and disrupting immune-mediated death may be beneficial. However, for patients with new-onset disease (blue line), combination therapy that provides an agent capable of restoring immune tolerance along with a drug that preserves and protects the remaining β-cells may prove ideal. Finally, for the patient with established (i.e., long-term) type 1 diabetes (green line), a three-way combination therapy that includes the aforementioned agent forms with a therapy capable of inducing β-cell replication/neogenesis may prove most beneficial.
Furthermore, these concepts suggest a potential role for metabolic control to enhance any effect of an immune modulator. The honeymoon that is experienced soon after clinical presentation in patients with type 1 diabetes may indeed reflect a reversal of the pathways of suicide that have been described. Unfortunately, this alone is not sufficient to prevent autoimmunity, because deterioration in insulin secretion invariably occurs. This is consistent with the modest effect of blockade of anti-inflammatory mediators such as IL-1β and tumor necrosis factor-α alone on progression of diabetes. Nonetheless, when combined with an immune therapy, the effects may be synergistic and long lasting.
In referring back to the question once posed by G. Franco Bottazzo some 25 years ago, is the death of a β-cell in the pathogenesis of diabetes homicide or suicide? given what we have learned about the immune response and the β-cell, our belief is that type 1 diabetes appears to be a case of (immune) self-assisted homicide.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
M.A.A., J.A.B., and G.S.E. wrote, reviewed, and edited the manuscript. M.H., K.C.H., and D.A. contributed to discussion and reviewed and edited the manuscript. M.P., P.R.A., M.V.H., D.S.M., and C.J.R. wrote, reviewed, and edited the manuscript.
The Brehm Coalition (www.brehmcoalition.org) was designed to bring together investigators (the authors of this article) with diverse backgrounds in these two disciplines (immunology and cell biology) in a forum that would promote close working relationships. The concepts put forward in this Perspectives article and presented by the coauthors were developed with the support of many governmental and nongovernmental agencies, and to them the authors extend their thanks. For this particular effort, the authors thank Dee and William Brehm for the creation and support of the Brehm Coalition, and Cyndi Souder of Moonlighting Studios for editorial assistance.
REFERENCES
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottazzo GF. Lawrence lecture. Death of a beta cell: homicide or suicide? Diabet Med 1986;3:119–130 [DOI] [PubMed] [Google Scholar]
- 4.Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. Beta cell apoptosis in diabetes. Apoptosis 2009;14:1389–1404 [DOI] [PubMed] [Google Scholar]
- 5.Seewaldt S, Thomas HE, Ejrnaes M, et al. Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes 2000;49:1801–1809 [DOI] [PubMed] [Google Scholar]
- 6.Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1987;30:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993;92:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schatz D, Gale EA, Atkinson MA. Why can’t we prevent type 1 diabetes?: maybe it’s time to try a different combination. Diabetes Care 2003;26:3326–3328 [DOI] [PubMed] [Google Scholar]
- 9.Roep BO, Peakman M. Surrogate end points in the design of immunotherapy trials: emerging lessons from type 1 diabetes. Nat Rev Immunol 2010;10:145–152 [DOI] [PubMed] [Google Scholar]
- 10.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev 2005;204:250–263 [DOI] [PubMed] [Google Scholar]
- 12.Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol 2010;11:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol 2010;11:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA 2010;107:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy B, Dudek NL, McKenzie MD, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 2006;116:3258–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1994;1:231–242 [DOI] [PubMed] [Google Scholar]
- 17.Tollefsen S, Arentz-Hansen H, Fleckenstein B, et al. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest 2006;116:2226–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity 2010;32:437–445 [DOI] [PubMed] [Google Scholar]
- 19.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 1995;64:689–719 [DOI] [PubMed] [Google Scholar]
- 20.Deeney JT, Prentki M, Corkey BE. Metabolic control of β-cell function. Semin Cell Dev Biol 2000;11:267–275 [DOI] [PubMed] [Google Scholar]
- 21.Rhodes CJ. Processing the insulin molecule. In Diabetes Mellitus: A Fundamental and Clinical Text 3rd ed. LeRoith D, Taylor SI, Olefsky JM, Eds. Philadelphia, Lippincott-Raven Publishers, 2004, p. 27–50 [Google Scholar]
- 22.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 2010;53:1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Mol Cell Endocrinol 2009;297:58–72 [DOI] [PubMed] [Google Scholar]
- 24.Hedeskov CJ, Capito K. The pentose cycle and insulin release in isolated mouse pancreatic islets during starvation. Biochem J 1975;152:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–466 [DOI] [PubMed] [Google Scholar]
- 26.Pi J, Zhang Q, Fu J, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 2010;244:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 28.Vague P, Moulin JP. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism 1982;31:139–142 [DOI] [PubMed] [Google Scholar]
- 29.Imamura T, Koffler M, Helderman JH, et al. Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes 1988;37:600–609 [DOI] [PubMed] [Google Scholar]
- 30.Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem 2007;282:287–293 [DOI] [PubMed] [Google Scholar]
- 31.Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes 2002;51:951–957 [DOI] [PubMed] [Google Scholar]
- 32.Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 2007;50:2486–2494 [DOI] [PubMed] [Google Scholar]
- 33.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol 2010;6:158–166 [DOI] [PubMed] [Google Scholar]
- 34.Wentworth JM, Fourlanos S, Harrison LC. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat Rev Endocrinol 2009;5:483–489 [DOI] [PubMed] [Google Scholar]
- 35.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant 2009;9:2716–2726 [DOI] [PubMed] [Google Scholar]
- 36.Bottino R, Criscimanna A, Casu A, et al. Recovery of endogenous beta-cell function in nonhuman primates after chemical diabetes induction and islet transplantation. Diabetes 2009;58:442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aly H, Gottlieb P. The honeymoon phase: intersection of metabolism and immunology. Curr Opin Endocrinol Diabetes Obes 2009;16:286–292 [DOI] [PubMed] [Google Scholar]
- 38.Waldron-Lynch F, Herold KC. Advances in type 1 diabetes therapeutics: immunomodulation and beta-cell salvage. Endocrinol Metab Clin North Am 2009;38:303–317, viii [DOI] [PubMed] [Google Scholar]
- 39.Itoh N, Okamoto H. Translational control of proinsulin synthesis by glucose. Nature 1980;283:100–102 [DOI] [PubMed] [Google Scholar]
- 40.Uchizono Y, Alarcón C, Wicksteed BL, Marsh BJ, Rhodes CJ. The balance between proinsulin biosynthesis and insulin secretion: where can imbalance lead? Diabetes Obes Metab 2007;9(Suppl. 2):56–66 [DOI] [PubMed] [Google Scholar]
- 41.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008;359:2849–2850 [DOI] [PubMed] [Google Scholar]
- 42.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDaniel ML, Corbett JA, Kwon G, Hill JR. A role for nitric oxide and other inflammatory mediators in cytokine-induced pancreatic beta-cell dysfunction and destruction. Adv Exp Med Biol 1997;426:313–319 [DOI] [PubMed] [Google Scholar]
- 44.Lee MS, Chang I, Kim S. Death effectors of beta-cell apoptosis in type 1 diabetes. Mol Genet Metab 2004;83:82–92 [DOI] [PubMed] [Google Scholar]
- 45.Fontés G, Zarrouki B, Hagman DK, et al. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia 2010;53:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 47.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 2009;23:975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev 2009;23:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 51.Gianani R, Campbell-Thompson M, Sarkar SA, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010;53:690–698 [DOI] [PubMed] [Google Scholar]
- 52.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 53.Keymeulen S, Candon S, Fafi-Kremer S, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes mellitus. N Engl J Med 2008;362:726–738 [Google Scholar]
- 54.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 55.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 57.Sanda S, Bollyky J, Standifer N, Nepom G, Hamerman JA, Greenbaum C. Short-term IL-1beta blockade reduces monocyte CD11b integrin expression in an IL-8 dependent fashion in patients with type 1 diabetes. Clin Immunol 2010;136:170–173 [DOI] [PubMed] [Google Scholar]