It is hard to imagine, given the wealth of new data reported over the recent past, that adipose tissue at one time was primarily considered as a passive reservoir for energy deposition and storage. However, research beginning in the early 1990s on the role of tumor necrosis factor (TNF)-α ushered in a new era of investigation, and since that time, there has been an incredibly rapid and substantive increase in our understanding of underlying physiologic systems and molecular pathways linking obesity, inflammation, and insulin action (1,2). Specifically, our understanding of the link between obesity and carbohydrate metabolism has been significantly enhanced with the elucidation of key regulators of energy balance and cellular insulin signaling that are complex and highly integrated (3–6). We now readily accept adipose tissue as a key endocrine organ regulating processes throughout the body with its significant number of adipocyte secretions. What now appears to be emerging is the elucidation of cellular pathways that are operative at not only the level of the adipocyte, but appear in common with those reported in vascular tissue. Such a cellular mechanism that may link pathophysiologic processes between adipose and vascular tissue is the fractalkine chemokine system as reported in this issue of Diabetes by Shah et al. (7). Specifically, Shah et al. provide interesting data that suggest that fractalkine (CX3CL1), a chemokine whose source is the endothelium and is postulated to play a role in atherogenesis, is also expressed in obese adipose tissue and plays a role in monocyte adhesion processes. These data provide an intriguing molecular link between obesity-related metabolic dysfunction and cardiovascular disease (CVD) in humans.
Fractalkine, a chemokine that signals through a single known receptor (CX3CR1), has received considerable interest in relation to the contribution to atheroscleroisis. It has been described as a multidomain protein of exceptionally large size, i.e., 95 kDa, and is expressed on numerous cells, but more importantly, on activated endothelial, smooth muscle cells, and macrophages (8,9). In addition to its large size, another feature that is different from other members of the cytokine family is the presence of a transmembrane anchor, and as such, fractalkine is capable of mediating adhesion of cells expressing the G protein–coupled receptor CX3CR1. The expression of fractalkine has reportedly been enhanced by inflammatory stimuli, i.e., TNF-α, interferon (IFN)-γ and lipopolysaccharide (9). It is also reported that a soluble form can be released from its membrane form by extracellular cleavage and then act as a classical chemoattractant for leukocytes and also smooth muscle cells expressing the receptor, i.e., CX3CR1 (8,9). Recently, elegant studies in primary smooth muscle cell lines demonstrated that CX3CL1 may have novel functions in regard to antiapotosis and proliferation, and as such, have important implications for vascular pathologies where the balance of smooth muscle cell proliferation and apotosis plays a critical role in determining plaque stability and vessel stenosis (9).
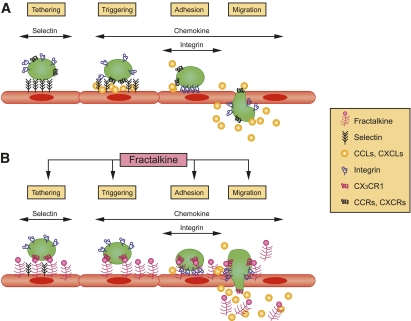
The chemokine family in general and fractalkine in particular are postulated to contribute significantly to the pathogenesis of CVD as leukocyte recruitment plays a role at all stages of CVD progression from early plaque formation to plaque rupture (10). The pathways by which fractalkine may participate in the modulation of adhesion processes at the cellular level are outlined schematically in Fig. 1 (11). As demonstrated in the figure, fractalkine may enhance leukocyte migration from the circulation into the tissue by enhancing the tethering (transient, selectin-mediated binding), triggering (activation of integrins by chemokines), adhesion, and finally migration through the endothelial layer. As such, enhancing or inhibiting any of these steps can either promote or attenuate atherogenic processes, respectively. In this regard, a preclinical study in mice evaluated anti-inflammatory therapy in the form of aspirin and plaque severity. Interestingly, anti-inflammatory treatment improved the atherosclerotic lesion as assessed by pathology image analysis. Such therapy also reportedly suppressed the fractalkine expression as assessed by both RT-PCR and immunohistochemical analysis, suggesting a cause and effect (12).
FIG. 1.
Schematic model of classical and fractalkine-mediated pathways in the adhesion cascade. Adapted from Umehara et al. (11). CCLs, CXCLs, chemokines; CCRs, CXCRs, chemokine receptors.
However, the clinical relevance in humans has been suggested by recent studies evaluating lesion extent and characteristics and the association to specific markers of the fractalkine-CX3CR1 chemokine system. Specifically, Ikejima et al. (10) demonstrated that plasma levels of fractalkine were significantly increased in patients with unstable angina pectoris with plaque rupture compared with patients with unstable angina pectoris without plaque rupture and patients with stable angina pectoris. In addition, they demonstrated that CX3CR1-expressing mononuclear cells were independent predictors of the presence of the plaque rupture (10). Other studies have suggested that fractalkine is increased in patients with coronary heart disease, but the beneficial effect of optimized anti-inflammatory therapy, i.e., statins, ACE inhibitors and ARBs, as part of coronary artery rehabilitation, may reduce the levels to those of control subjects (8). Additional studies in humans have evaluated polymorphisms of fractalkine that are postulated to alter ligand receptor affinity, and as such, potentially influence a patient’s susceptibility to CVD. One such study (13) demonstrated that a single nucleotide polymorphism (SNP) of the CX3CR1 gene (T280M) was associated with a lower risk of carotid atheromatous disease, independent from modifiable cerebrovascular risk factors. Interestingly, through a meta-analysis involving over 2,000 patients with coronary artery disease and 2,800 control subjects, the 280M allele was associated with a reduced risk of coronary artery disease in the heterozygous state (14). However, Debette et al. (15) evaluated 18 polymorphisms in CX3CL1 and CX3CR1 in over 2,700 German community individuals and over 6,000 French community individuals. They reported associations of common carotid artery intima-media thickness with three genetic variants of fractalkine and its receptor in the German cohort, but these were not replicated in the French cohort (15).
Based on the above, it is apparent that fractalkine, given its function and source of expression, has an important role in CVD. However, the importance of the current study in this issue of Diabetes as reported by Shah et al. (7) is the fact that this chemokine appears to also be a novel chemokine induced in human adipose tissue. The studies of Shah et al. (7) were well conceived, well conducted, and represented a comprehensive evaluation of the novel adiopochemokine system from basic to translational studies. This was achieved using a variety of approaches that included in vitro studies on primary human adipocytes, in vivo endotoxemia studies in humans, subcutaneous adipose tissue biopsies in both lean and obese subjects, and visceral adipose tissue studies in obese subjects. To support the data obtained in the laboratory and from the human metabolic studies, a well designed case-control study was performed in individuals with and without type 2 diabetes in addition to association studies of the CX3CR1 gene variants in over 2,000 well characterized subjects. Thus, support for the hypothesis was confirmed at many different levels of basic and clinical research.
The results definitively demonstrated that CX3CL1 is significantly increased in obesity and can be evoked by induction of inflammation in vivo. They demonstrated that not only is the chemokine secreted by stromal vascular fraction of the adipose tissue, but it is also secreted by the specific adipocytes. In addition, they demonstrated that the fractalkine chemokine system mediates monocyte adhesion to human adipocytes. The secretion of fractalkine by the adipocyte and the observation that the system modulates adhesion processes are important observations of the study and clearly represent the novelty of the research. In the case-control study, patients with type 2 diabetes were observed to have higher levels of plasma fractalkine than nondiabetic subjects. Finally, it was also shown that individuals that had two common nonsynonymous coding SNPs in CX3CR1 (i.e., T280M and V249I) tended to have greater waist circumference, were more insulin resistant, and had higher leptin, but lower adiponectin levels. Further, these alleles tended to be associated with the presence of metabolic syndrome and type 2 diabetes.
The data clearly suggest that the fractalkine system represents a novel potential target for therapeutic intervention for obesity and type 2 diabetes. Future studies evaluating this system with genetic models in preclinical studies and with pharmacologic modulation in humans will be important in order to further define the clinical relevance of the system. But, to date, the data presented do provide for an intriguing unifying cellular mechanism contributing to the understanding of obesity-related dysfunction and vascular pathologies.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1512.
REFERENCES
- 1.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 2.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-α suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem 1993;268:26055–26058 [PubMed] [Google Scholar]
- 3.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med 2004;10:355–361 [DOI] [PubMed] [Google Scholar]
- 4.Vernochet C, Peres SB, Farmer SR. Mechanisms of obesity and related pathologies: transcriptional control of adipose tissue development. FEBS J 2009;276:5729–5737 [DOI] [PubMed] [Google Scholar]
- 5.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32(Suppl. 7):S109–S119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring) 2008;16(Suppl. 3):S5–S10 [DOI] [PubMed] [Google Scholar]
- 7.Shah R, Hinkle CC, Ferguson JF, et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 2011;60:1512–1518 [DOI] [PMC free article] [PubMed]
- 8.Maegdefessel L, Schlitt A, Pippig S, et al. Patients with insulin-dependent diabetes or coronary heart disease following rehabilitation express serum fractalkine levels similar to those in healthy control subjects. Vasc Health Risk Manag 2009;5:849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res 2010;85:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikejima H, Imanishi T, Tsujioka H, et al. Upregulation of fractalkine and its receptor, CX3CR1, is associated with coronary plaque rupture in patients with unstable angina pectoris. Circ J 2010;74:337–345 [DOI] [PubMed] [Google Scholar]
- 11.Umehara H, Tanaka M, Sawaki T, et al. Fractalkine in rheumatoid arthritis and allied conditions. Mod Rheumatol 2006;16:124–130 [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Jiang D, Zhang S, Ou B. Aspirin inhibits fractalkine expression in atherosclerotic plaques and reduces atherosclerosis in ApoE gene knockout mice. Cardiovasc Drugs Ther 2010;24:17–24 [DOI] [PubMed] [Google Scholar]
- 13.Kimouli M, Miyakis S, Georgakopoulos P, Neofytou E, Achimastos AD, Spandidos DA. Polymorphisms of fractalkine receptor CX3CR1 gene in patients with symptomatic and asymptomatic carotid artery stenosis. J Atheroscler Thromb 2009;16:604–610 [DOI] [PubMed] [Google Scholar]
- 14.Apostolakis S, Amanatidou V, Papadakis EG, Spandidos DA. Genetic diversity of CX3CR1 gene and coronary artery disease: new insights through a meta-analysis. Atherosclerosis 2009;207:8–15 [DOI] [PubMed] [Google Scholar]
- 15.Debette S, Bevan S, Dartigues JF, et al. Fractalkine receptor/ligand genetic variants and carotid intima-media thickness. Stroke 2009;40:2212–2214 [DOI] [PubMed] [Google Scholar]