Abstract
OBJECTIVE
ob/ob and db/db mice manifest myocardial hypertrophy, insulin resistance, altered substrate utilization, mitochondrial dysfunction, and lipid accumulation. This study was designed to determine the contribution of central and peripheral leptin signaling to myocardial metabolism and function in ob/ob and db/db mice in the absence of diabetes and morbid obesity.
RESEARCH DESIGN AND METHODS
Male ob/ob mice (aged 4 weeks) were caloric restricted by pairfeeding to a leptin-treated ob/ob group. In addition to determining glucose tolerance and circulating lipid concentrations, myocardial substrate metabolism and mitochondrial function were determined in saponin-permeabilized cardiac fibers. Second, experiments were performed to determine whether leptin treatment by intraperitoneal injection or intracerebroventricular infusion could normalize myocardial palmitate oxidation in caloric-restricted ob/ob mouse hearts.
RESULTS
Despite normalizing body weight and glucose tolerance, fat mass and circulating lipid levels remained increased in caloric-restricted ob/ob animals. Palmitate oxidation remained elevated in caloric-restricted ob/ob hearts and was normalized by intraperitoneal or intracerebroventricular leptin. Intraperitoneal and intracerebroventricular treatment also normalized circulating free fatty acid levels, myocardial fatty acid oxidation gene expression, and myocardial insulin sensitivity.
CONCLUSIONS
These data suggest that impaired hypothalamic leptin signaling is sufficient to increase myocardial fatty acid oxidation by increasing delivery of free fatty acid substrates and peroxisome proliferator–activated receptor-α ligands to the heart.
Leptin is an adipocyte-secreted hormone that acts at the level of the hypothalamus to decrease appetite and increase energy expenditure (1). Leptin also increases fatty acid oxidation (FAO) rates in peripheral tissues by central and peripheral signaling via leptin receptors (Ob-Rs) (2). Previous studies have suggested that leptin may directly influence myocardial metabolism and function (2–7). Incubation of cultured neonatal cardiomyocytes with leptin leads to cellular hypertrophy, suggesting that leptin is prohypertrophic in vitro, but one study has suggested an antihypertrophic effect of leptin in vivo (3,6,7). Leptin is cardioprotective in ischemia/reperfusion injury and activates the cardioprotective Akt and mitogen-activated protein kinase (MAPK) pathways (8). Chronic leptin administration increases heart rate in mouse and rat models, and serum leptin levels are positively correlated with heart rate in modestly obese and hypertensive subjects (9,10). These observations support a putative role for leptin signaling in the heart in vivo and suggest that hyperleptinemia and leptin resistance might independently impact heart function. However, the consequences of impaired leptin action, both centrally and peripherally, on myocardial function and metabolism in vivo remain incompletely understood.
A widely studied genetic model of leptin deficiency is the ob/ob mouse, which develops morbid obesity and the characteristic hyperinsulinemia and hyperglycemia of type 2 diabetes by 8 weeks of age (4,11). ob/ob hearts exhibit functional and metabolic defects, including cardiac hypertrophy, insulin resistance, increased palmitate oxidation, and reduced cardiac power and efficiency, which precede the development of diabetes (11). Similar changes have been described in db/db mice (11). In db/db mice, peripheral leptin signaling via the short forms of the leptin receptor is presumably intact, but they lack the long form of the leptin receptor in the hypothalamus as well as in the periphery (12–14). Circulating leptin levels are elevated in db/db mice, reflecting severe leptin resistance (15). In both of these models, it is unclear which of the well-described cardiac defects are caused by leptin deficiency, versus effects that are secondary to obesity and type 2 diabetes.
Previous reports have shown that a significant reduction in caloric intake below that of wild-type mice is sufficient to normalize body weight and glucose tolerance in ob/ob mice (16–19). Therefore, we used a pairfeeding model of caloric restriction to evaluate the consequences of leptin deficiency on myocardial metabolism and function in vivo by minimizing the confounding effects of obesity and hyperglycemia. We hypothesized that leptin deficiency contributed to the myocardial metabolism in ob/ob mice via mechanisms that are independent of obesity, insulin resistance, and hyperglycemia. If true, hearts from caloric-restricted ob/ob mice should reveal persistent metabolic and functional abnormalities despite normalization of body weight. Because our goal was to elucidate the impact of leptin deficiency in the absence of obesity we elected to pairfeed young (aged 4 weeks) ob/ob mice before the onset of morbid obesity. Palmitate oxidation remained elevated in caloric-restricted ob/ob hearts under basal and insulin-perfused conditions and central leptin infusion was sufficient to normalize myocardial FAO, circulating free fatty acid (FFA) levels, and myocardial FAO gene expression in caloric-restricted ob/ob mice. Together, these results suggest that central leptin signaling regulates myocardial FAO in part by regulating the delivery of fatty acids (FA) and PPAR-α ligands to the heart.
RESEARCH DESIGN AND METHODS
Animals.
The Institutional Animal Care and Use Committee at the University of Utah approved these studies. Male (aged 4 to 5 weeks), ob/ob, db/db, and wild-type mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were singly housed in temperature-controlled facilities operating on a 12-h:12-h light/dark cycle and were random fed standard chow (24.53% protein, 4.38% fat; Teklad 8656, Madison, WI) or were caloric restricted according to the protocol discussed below. All mice had access to water ad libitum.
Caloric restriction: pairfeeding studies.
ob/ob mice were treated with leptin (National Hormone and Peptide Program, Dr. A.F. Parlow, Torrance, CA) twice daily (1 mg/kg at 9:00 a.m. and 2 mg/kg at 6:00 p.m.). A set of 4-week-old male ob/ob mice was fed the amount of food that the leptin-treated ob/ob group consumed the previous day. These pairfed mice were given half of their daily food at the 9:00 a.m. and 6:00 p.m. treatment times and are designated ob/ob CR (caloric restriction). Other control groups included: random-fed ob/ob mice, leptin-treated/random-fed wild-type mice, and random-fed wild-type mice. All nonleptin-treated mice received vehicle injections of PBS (pH 7.8). Caloric-restricted db/db mice were also fed an isocaloric diet matching that of the leptin-treated ob/ob mice. Mouse weights and food intake were measured daily. Treatments continued for 3 weeks. This duration of caloric restriction was based on body weight and glucose tolerance responses of caloric-restricted ob/ob mice. Our preliminary data suggested that 2 weeks of caloric restriction was sufficient to normalize glucose tolerance and body weight, although serum insulin levels remain elevated. Body weight and glucose tolerance were not changed further during the 3rd week of treatment, and there was no further reduction in serum insulin levels. Thus a 3-week time point was used for all studies given that body weights and glucose tolerance had been normalized and remained stable for an additional 7 days. For intraperitoneal and intracerebroventricular studies, 5-week-old ob/ob mice were used for these studies since 4-week-old mice subsequently became unavailable from The Jackson Laboratory. Mice were caloric restricted to 2.5 g/day or random fed for 3 weeks as described above. Mice were then treated with intraperitoneal leptin (3 mg/kg/day, as described above) or intracerebroventricular leptin infusion (15 ng/h) by osmotic minipump as described under intracerebroventricular protocol while continuing caloric restriction. Controls received vehicle, i.e., PBS (pH 7.8). Treatment continued for 1 week, after which the mice were killed.
Intracerebroventricular protocol.
Leptin or saline was administered using the Alzet Brain Infusion Kit 3 and micro-osmotic pump (1007D; DURECT, Cupertino, CA). Mice were anesthetized with chloral hydrate (400 mg/kg) and secured to a stereotactic apparatus adapted for mouse use (David Kopf Instruments, Tujunga, CA). The cannula was placed according to the stereotactic coordinates (A/P: −0.8 mm; M/L: −0.7 mm; D/V: −2.5 mm) and secured with Loctite 454 superglue (Loctite, Düsseldorf, Germany). Osmotic minipumps were implanted subcutaneously in the scapular region and connected to the cannula via Tygon S-54-HL tubing (Saint-Gobain Performance Plastics, Paris, France). The incision was sutured, and the mouse was placed on a 37° plate until conscious. The animals were treated with buprenorphrine hydrochloride (0.05 mg/kg) for 2 days after surgery.
Glucose tolerance tests.
Glucose tolerance tests (GTTs) were performed as previously described (20). Briefly, mice were injected with an intraperitoneal injection of glucose (1 mg/g) after 6 h of fasting. Blood glucose levels were measured using a standard glucometer (Bayer, Elkhart, IN). Blood was obtained from the tail vein for insulin measurements before and 30 min after intraperitoneal glucose injection in an Eppendorf tube containing a small ball of Teflon. The serum was removed and used to measure insulin levels using a sensitive rat insulin RIA kit (Linco, St. Charles, MO).
Serum fatty acids, triglyceride, adiponectin, and leptin levels.
Mice were fasted for 6 h, and blood was collected from the submandibular vein. The serum was collected as described above, and circulating triglycerides and FFA concentrations were determined using kits from Wako Diagnostics (Richmond, VA). Serum adiponectin and leptin were measured using specific radioimmunoassay kits (Linco).
Working hearts.
Hearts were excised and perfused in the working mode with Krebs-Henseleit buffer (KHB) containing 0.4 mM palmitate bound to 3% BSA and 5 mmol/L glucose at 37°C with or without 1 nmol/L insulin as previously described (4,11). The buffer was gassed with 95% O2 and 5% CO2. Palmitate oxidation rates were determined by measuring the amount of 3H2O released from [9–10–3H] palmitate during 60 min of perfusion.
Mitochondrial respirations.
Hearts were perfused via retrograde perfusion for 20 min with KHB as previously described (21). The buffer contained 1 mM palmitate preabsorbed to 3% BSA and 11 mmol/L glucose to mimic the diabetic milieu of ob/ob mice. Fibers were separated from the inner left ventricle and permeabilized as previously described (22). Oxygen consumption was measured at 25°C in KCl buffer supplemented with 20 μmol/L palmitoyl-carnitine and then subsequently with ADP, cytochrome C, or oligomycin. Respiration rates were normalized to dry fiber weight.
Electron microscopy.
Samples were removed from the inner left ventricle after Langendorff perfusion. They were fixed in 2.5% glutaraldehyde with 1% paraformaldehyde. They were postfixed in 2% osmium and embedded in resin as previously described (22).
Myocardial triglycerides.
Cardiac tissue was homogenized in 1 mL of 8:1 vol/vol chloroform/methanol. The tubes were rocked at room temperature for 3 h. H2SO4 (800 μL) was added to each tube and centrifuged at 218 g for 10 min. A sample (50 μL) was removed from the bottom (organic) layer and dried in a speedvac. Triglyceride assay buffer (300 μL; Sigma-Aldrich, St. Louis, MO) was added to each sample and incubated at 37° for 15 min with occasional mixing. Samples were loaded onto a 96-well plate and read at 562 nm. Glycerol was used to generate the standard curve (Sigma-Aldrich).
RNA extraction and quantitative PCR.
Heart tissue was obtained from hearts perfused in the working heart mode without insulin stimulation. The tissue was powdered and homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was extracted using manufacturer’s protocols. cDNA was synthesized from equivalent amounts of RNA using Superscript III Reverse Transcriptase (Invitrogen) and oligo dT primers. Quantitative (q)PCR products were normalized to the 16S ribosomal subunit gene and expressed as fold change from controls.
Statistics.
All statistics were performed by one-way ANOVA using the Statview 5.0.1 software (SAS Institute, Cary, NC). Statistical significance was accepted when the P value was less than 0.05.
RESULTS
Food intake, body composition, and systemic metabolism in caloric-restricted ob/ob and db/db mice.
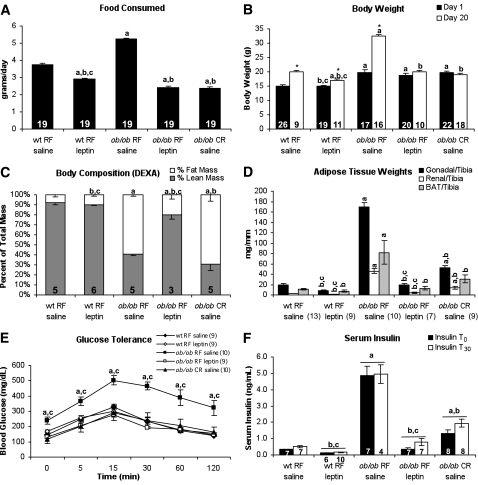
Random-fed ob/ob mice consumed significantly more food than wild-type controls, whereas leptin treatment significantly lowered food consumption below wild-type levels. Caloric-restricted ob/ob mice were fed an isocaloric diet to that of the leptin-treated ob/ob mice (Fig. 1A). Weight gain was accelerated in random-fed ob/ob mice compared with wild-type controls. Leptin treatment reduced body weight in wild-type mice and normalized body weight in ob/ob mice. Caloric restriction normalized body weight in ob/ob mice (Fig. 1B). Body composition was not normalized in caloric-restricted ob/ob mice despite normalization of body weight. Fat pad mass was significantly increased in random-fed and caloric-restricted ob/ob mice in comparison with wild-type mice, whereas leptin treatment restored fat mass to wild-type levels (Fig. 1C and D). Altered body composition in caloric-restricted ob/ob mice correlated with a decrease in oxygen consumption, heat production, and uncoupling protein-1 (UCP-1) content in brown adipose tissue (BAT) depots, despite an increase in movement (Supplementary Fig. 1).
FIG. 1.
Altered body composition but normal glucose tolerance in caloric-restricted (CR) ob/ob mice. Average daily food intake (A), body weight (B), body composition (C), and adipose tissue weight (D) of ob/ob mice are shown. E: GTT performed on mice during 3rd week of treatment after 6 h of fasting. F: Serum insulin levels collected before and 30 min after glucose injection. Numbers of animals are shown in each figure. RF, random fed. aP < 0.05 vs. wt RF saline; bP < 0.05 vs. ob/ob RF saline; cP < 0.05 vs. ob/ob CR saline; *P < 0.05 vs. basal conditions of same treatment group. DEXA, dual-energy X-ray absorptiometry; RF, random fed; wt, wild type.
Glucose tolerance was significantly impaired in random-fed ob/ob mice when compared with wild-type controls. Leptin treatment normalized glucose tolerance in ob/ob mice, as did caloric restriction of ob/ob mice. Despite normalization of glucose tolerance, caloric restriction reduced fasting insulin concentrations in ob/ob mice by ∼50%, so that they remained two- to threefold higher than random-fed wild-type controls. Leptin treatment normalized insulin concentrations in ob/ob mice, by returning them to the levels observed in random-fed wild-type mice (Fig. 1E and F).
Circulating FFA concentrations were elevated in random-fed ob/ob mice, when compared with wild-type controls (Table 1). Triglyceride concentrations were not significantly elevated in random-fed ob/ob mice. FFA and triglyceride concentrations were further increased in caloric-restricted ob/ob mice (Tables 1 and 3) but were reduced by leptin treatment of ob/ob mice fed an isocaloric diet. Circulating adiponectin concentrations in random-fed ob/ob mice were comparable with random-fed wild-type mice, as were adiponectin levels in leptin-treated ob/ob mice. Adiponectin concentrations in caloric-restricted ob/ob mice were increased threefold to that of random-fed wild-type levels. Leptin was not detectable in untreated ob/ob mice, and leptin concentrations in leptin-treated ob/ob and wild-type mice were not significantly different than random-fed wild-type levels after 6 h of fasting.
TABLE 1.
Circulating lipid and hormone concentrations
| FFAs (mM) | Triglycerides (mg/dL) | Adiponectin (μg/mL) | Leptin (ng/mL) | |
|---|---|---|---|---|
| Pairfeeding studies | ||||
| Wt RF saline | 0.6 ± 0.1 (14) | 21.3 ± 3.0 (21) | 6.4 ± 1.0 (9) | 0.8 ± 0.2 (8) |
| Wt RF leptin | 0.6 ± 0.1c (11) | 15.9 ± 2.8b,c (21) | 6.4 ± 0.6c (10) | 1.2 ± 0.2 (6) |
| ob/ob RF saline | 0.9 ± 0.2a (11) | 30.8 ± 4.7 (11) | 8.7 ± 0.9 (11) | ND (6) |
| ob/ob RF leptin | 0.7 ± 0.1c (11) | 20.9 ± 2.2c (16) | 6.9 ± 0.8c (11) | 1.4 ± 0.3 (5) |
| ob/ob CR saline | 1.1 ± 0.2a (8) | 41.4 ± 5.3a (10) | 17.4 ± 1.3a,b (15) | ND (5) |
| db/db CR studies | ||||
| Wt RF | 0.7 ± 0.1 (8) | 21.3 ± 2.7 (13) | 5.6 ± 0.8 (6) | 0.8 ± 0.4 (7) |
| db/db RF | 1.5 ± 0.1a (4) | 42.6 ± 10.7a (7) | 8.9 ± 0.6 (7) | 63.8 ± 1.1a (4) |
| db/db CR | 1.4 ± 0.1a (6) | 34.6 ± 2. (11) | 14.6 ± 1.8a,b (10) | 17.6 ± 1.2a,b (4) |
Circulating concentrations of FFAs, triglycerides, adiponectin, and leptin were measured after 6 h of fasting (number of animals is indicated in parentheses).
aP < 0.05 vs. wt RF saline;
bP < 0.05 vs. ob/ob RF saline or db/db RF;
cP < 0.05 vs. ob/ob CR saline. ND, not detected; RF, random fed; wt, wild type.
TABLE 3.
Circulating lipids and hormones in caloric-restricted ob/ob mice with leptin treatment
| Treatment group | FFAs (mM) | Triglycerides (mg/dL) | Adiponectin (μg/mL) | Leptin (ng/mL) |
|---|---|---|---|---|
| Wt RF | 0.7 ± 0.1 (4) | 45.5 ± 5.2 (3) | 10.6 ± 1.2 (5) | 2.6 ± 0.5 (4) |
| Wt CR | 1.0 ± 0.1c (5) | 43.3 ± 4.8c (7) | 18.0 ± 1.4a,b,c (5) | 3.0 ± 0.2 (4) |
| Wt CR + L 1 week | 0.8 ± 0.2c (4) | 32.0 ± 1.9c (4) | 13.8 ± 1.3c (5) | 6.4 ± 1.9a (3) |
| ob/ob RF | 0.7 ± 0.1 (3) | 40.5 ± 2.0 (3) | 10.7 ± 1.4 (4) | ND (3) |
| ob/ob CR | 1.4 ± 0.02a,b (5) | 57.6 ± 3.6b (5) | 21.9 ± 0.6a,b (6) | ND (3) |
| ob/ob CR + L 1 week | 0.6 ± 0.1c (4) | 50.5 ± 2.6 (4) | 17.9 ± 2.0a,b,c (4) | 1.0 ± 0.8 (3) |
| Wt CR intracerebroventricular saline | 1.1 ± 0.20 (5) | 28.6 ± 1.5 (3) | 14.3 ± 1.3 (4) | 3.3 ± 0.9 (3) |
| Wt CR intracerebroventricular leptin | 0.1 ± 0.04a,c (6) | 17.6 ± 2.5c (6) | 8.6 ± 1.6a,c (5) | 3.5 ± 0.8 (3) |
| ob/ob CR intracerebroventricular saline | 1.6 ± 0.30a (5) | 43.8 ± 3.3a (4) | 23.6 ± 1.5a (6) | ND (3) |
| ob/ob CR intracerebroventricular leptin | 0.6 ± 0.05a,c (5) | 32.6 ± 3.5c (4) | 37.9 ± 2.0a,c (7) | ND (3) |
Circulating concentrations of FFAs, triglycerides, adiponectin, and leptin were measured in intraperitoneal (L 1 week)- and intracerebroventricular-treated mice after 6 h of fasting (number of animals is indicated in parentheses).
aP < 0.05 vs. wt RF or wt CR intracerebroventricular saline;
bP < 0.05 vs. ob/ob RF;
cP < 0.05 vs. ob/ob CR or ob/ob CR intracerebroventricular saline. L 1 wk, 1 week of leptin treatment; ND, not detected; RF, random fed; wt, wild type.
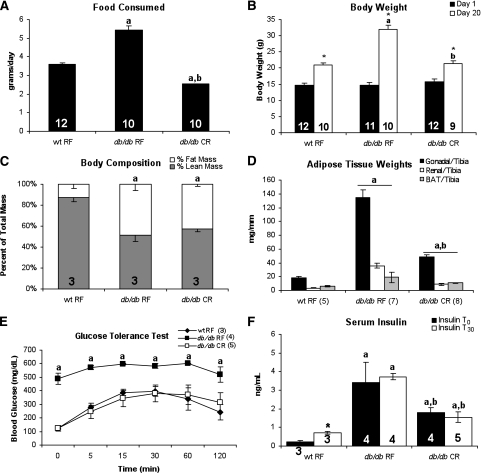
To determine whether similar changes in body composition and metabolism would occur in a model of leptin resistance, we applied the same degree and duration of caloric restriction to 4-week-old db/db mice. Adipose tissue weight and circulating insulin concentrations remained elevated in caloric-restricted db/db mice, despite normalization of body weight and glucose tolerance (Fig. 2).
FIG. 2.
Altered body composition but normal glucose tolerance in caloric-restricted db/db mice. Average daily food intake (A), body weight (B), body composition (C), and adipose tissue weight (D) of db/db mice are shown. E: GTT performed on mice during 3rd week of treatment after 6 h of fasting. F: Serum insulin levels collected before and 30 min after glucose injection. Numbers of animals are shown in each figure. aP < 0.05 vs. wt RF; bP < 0.05 vs. db/db RF; *P < 0.05 vs. basal conditions of same treatment group. RF, random fed; wt, wild type.
Myocardial function and FAO in ob/ob and db/db mice.
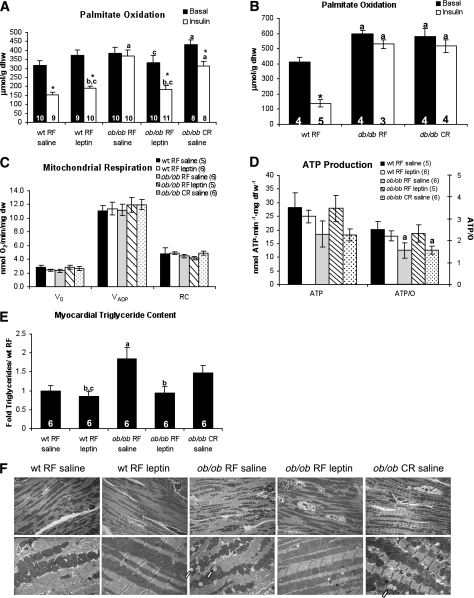
We performed isolated working heart perfusions to determine whether myocardial metabolism was altered in caloric-restricted ob/ob mice (Fig. 3A). Palmitate oxidation was elevated in random-fed ob/ob hearts when perfused with insulin. Leptin treatment restored insulin responsiveness in the hearts of ob/ob mice. Palmitate oxidation rates were increased in caloric-restricted ob/ob hearts when compared with random-fed wild-type hearts. Although insulin reduced palmitate oxidation rates in caloric-restricted ob/ob hearts, palmitate oxidation remained higher than rates observed in insulin-perfused, random-fed wild-type hearts. Similar findings were observed in random-fed and caloric-restricted db/db hearts, which showed higher palmitate oxidation and lower glucose oxidation when compared with random-fed wild-type controls (Fig. 3B and Supplementary Fig. 2). We measured dry heart weights postperfusion to determine the impact of caloric restriction on cardiac hypertrophy in ob/ob and db/db hearts. Heart weights from random-fed ob/ob mice but not db/db mice were increased when compared with random-fed wild-type hearts. Leptin treatment and caloric restriction normalized heart weights to levels similar to that of random-fed wild-type mice (Table 2).
FIG. 3.
Increased triglyceride content and mitochondrial uncoupling accompany elevated myocardial palmitate oxidation in caloric-restricted mouse hearts. Myocardial palmitate oxidation as measured by isolated working heart perfusion in ob/ob (A) and db/db mice (B). C: Mitochondrial respirations from saponin-permeabilized fibers isolated from ob/ob hearts. V0, basal respiration; VADP, maximal ADP-stimulated respiration; Voligo, oxygen consumption in the presence of oligomycin; RC, respiratory control ratio (VADP/Voligo). D: ATP production rate and ATP/state 3 respirations (ATP/O ratio) for saponin-permeabilized fibers. E: Myocardial triglyceride content. F: Transmission electron microscopy of myocardial tissue from inner left ventricle. Magnification ×2,000 (top row) and ×8,000 (bottom row). Arrows indicate lipid droplets. aP < 0.05 vs. wt RF saline; bP < 0.05 vs. ob/ob RF or db/db RF; cP < 0.05 vs. ob/ob CR saline; *P < 0.05 vs. basal conditions of same treatment group. dfw, dry fiber weight; dhw, dry heart weight; dw, dry weight; RF, random fed; wt, wild type.
TABLE 2.
Heart weights in caloric-restricted ob/ob and db/db mice with leptin treatment
| Treatment group | DHW (mg) | Tibia length (mm) | DHW/Tibia length (mg/mm) |
|---|---|---|---|
| Wt RF saline (25) | 20.4 ± 0.7 | 15.6 ± 0.1 | 1.3 ± 4.3 E-05 |
| Wt RF leptin (22) | 18.1 ± 0.9a,b | 15.6 ± 0.1b | 1.2 ± 5.4 E-05a,b |
| ob/ob RF saline (19) | 25.1 ± 0.7a | 14.7 ± 0.1a | 1.7 ± 4.2 E-05a |
| ob/ob RF leptin (21) | 21.4 ± 0.6b | 15.6 ± 0.1b | 1.4 ± 3.5 E-05b |
| ob/ob CR saline (28) | 19.4 ± 0.6b | 14.5 ± 0.2a | 1.3 ± 3.7 E-05b |
| Wt RF (6) | 22.4 ± 0.6 | 16.7 ± 0.2 | 1.4 ± 0.05 |
| db/db RF (8) | 23.3 ± 0.9 | 15.2 ± 0.2a | 1.5 ± 0.08 |
| db/db CR (8) | 19.0 ± 0.4a,b | 14.7 ± 0.2a,b | 1.3 ± 0.02b |
Dry heart weights (DHW) obtained after working heart perfusions in 9-week-old male mice with 1 week of intraperitoneal or intracerebroventricular leptin are shown.
aP < 0.05 vs. wt RF saline;
bP < 0.05 vs. ob/ob RF saline or db/db RF. RF, random fed; wt, wild type.
Mitochondrial function in caloric-restricted ob/ob mouse hearts.
We previously reported that mitochondria from ob/ob mice were uncoupled after perfusion with FA (21). Thus we sought to determine whether mitochondria of caloric-restricted ob/ob mice remained uncoupled when hearts were perfused with palmitate before mitochondrial studies. We only performed these studies in ob/ob mice, since similar changes in systemic and myocardial metabolism were observed in both ob/ob and db/db mice. Oxygen consumption rates in saponin-permeabilized fibers exposed to palmitoyl carnitine were similar in all groups (Fig. 3C). However ATP production rates trended lower in fibers isolated from both random-fed and caloric-restricted ob/ob mice (Fig. 3D) resulting in significantly lower ATP/O ratios. Thus mitochondria from caloric-restricted ob/ob mice remained uncoupled despite normalization of body weight. This contrasts with normalization of mitochondrial coupling in leptin-treated ob/ob mouse hearts.
Transmission electron microscopy was performed to determine whether leptin treatment or caloric restriction modified mitochondrial morphology (Fig. 3F). Although major changes in mitochondrial morphology between groups were not observed, striking differences in lipid droplet accumulation were noted. Leptin treatment reversed myocardial lipid droplet accumulation. In contrast, the number of lipid droplets in hearts from caloric-restricted ob/ob mice was similar to those observed in random-fed ob/ob animals. To quantify these microscopic features, we measured myocardial triglyceride content. There was a significant elevation in myocardial triglyceride levels in random-fed ob/ob mice relative to wild-types (Fig. 3E) and normal levels in leptin-treated ob/ob hearts. Triglyceride levels were not significantly reduced in caloric-restricted ob/ob hearts compared with random-fed ob/ob mice, nor were they significantly elevated when compared with wild-type mice, indicating an intermediate degree of myocardial triglyceride accumulation.
Systemic metabolic milieu of caloric-restricted mice after intracerebroventricular or peripheral leptin treatment.
The experiments described thus far do not distinguish whether the altered myocardial FAO observed in caloric-restricted ob/ob mice was a result of caloric restriction or leptin deficiency per se. Moreover, studies in caloric-restricted db/db mice suggested that hypothalamic leptin signaling via the Ob-Rb isoform of the leptin receptor is important in maintaining normal myocardial metabolism in caloric-restricted mice. Therefore, we performed two studies in which caloric-restricted wild-type and ob/ob mice were treated with intraperitoneal or intracerebroventricular leptin to determine whether systemic or central leptin can normalize myocardial metabolism.
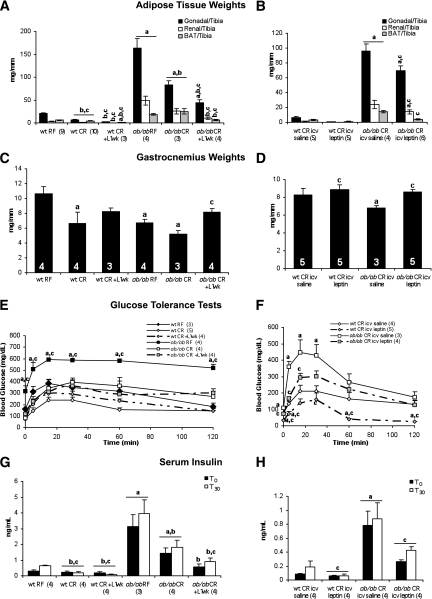
Male mice (aged 5 weeks) were obtained from The Jackson Laboratory and random fed or caloric restricted (2.5 g/day) for 3 weeks. Mice were then treated with systemic (intraperitoneal study; 3 mg/kg/day) or intracerebroventricular study (15 ng/h) leptin for 1 week. Mice were caloric restricted throughout the 4-week protocol. At the conclusion of the study, body weights of caloric-restricted mice were mildly elevated relative to wild-type controls (Supplementary Fig. 3A–D). Leptin treatment by intraperitoneal injection reduced body weight and adipose mass in caloric-restricted ob/ob mice (Fig. 4A). Caloric-restricted wild-type mice weighed significantly less than controls, and white adipose tissue mass was significantly reduced by leptin treatment. Intracerebroventricular infusion of leptin reduced body weight and white adipose tissue mass in caloric-restricted ob/ob mice (Fig. 4B). However, this reduction was not equivalent to the reduction observed in the intraperitoneal-treated mice. In contrast, intraperitoneal and intracerebroventricular leptin treatment were sufficient to normalize BAT weight in caloric-restricted ob/ob mice. Gastrocnemius weight was also increased by intraperitoneal and intracerebroventricular leptin treatment, suggesting that fat mass was spared at the expense of muscle mass in caloric-restricted ob/ob mice (Fig. 4C and D).
FIG. 4.
Intraperitoneal and intracerebroventricular leptin are sufficient to alter body composition and serum insulin levels. Adipose tissue and gastrocnemius muscle weights in mice treated with intraperitoneal (A, C) or intracerebroventricular (B, D) leptin for 1 week are shown. GTTs (E, F) and serum insulin levels (G, H) before and 30 min after glucose injection are shown. Numbers of animals are indicated in each figure. For intraperitoneal leptin study, aP < 0.05 vs. wt RF; bP < 0.05 vs. ob/ob RF; cP < 0.05 vs. ob/ob CR. For intracerebroventricular leptin study, aP < 0.05 vs. wt CR intracerebroventricular saline; cP < 0.05 vs. ob/ob CR intracerebroventricular saline. icv, intracerebroventricular; L 1 wk, 1 week of leptin treatment; RF, random fed; wt, wild type.
We measured circulating lipid and hormone levels to determine whether 1 week of leptin treatment via intraperitoneal or intracerebroventricular routes was sufficient to normalize systemic metabolism in caloric-restricted ob/ob mice (Table 3). Serum FFA levels were significantly increased by caloric restriction in ob/ob but not wild-type mice and were normalized by intraperitoneal and intracerebroventricular leptin treatment. Caloric restriction increased serum adiponectin levels in wild-type and ob/ob mice. Intracerebroventricular leptin treatment did not increase circulating leptin levels. Surprisingly, adiponectin levels were further increased in caloric-restricted ob/ob mice treated with intracerebroventricular leptin. Caloric restriction or leptin treatment by intraperitoneal injection or intracerebroventricular infusion improved glucose tolerance in ob/ob mice and significantly lowered glucose levels in caloric-restricted wild-type mice (Fig. 4E–H). Insulin levels were also reduced by intraperitoneal and intracerebroventricular leptin under basal and glucose-stimulated conditions.
Myocardial FAO in caloric-restricted mice after central or peripheral leptin repletion.
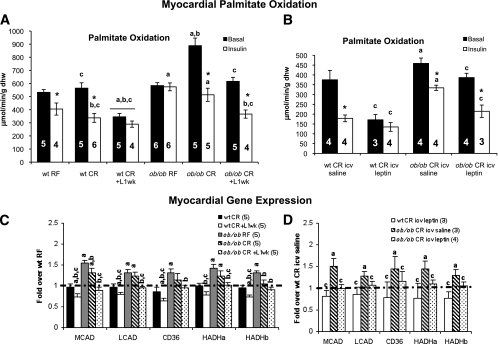
To determine whether myocardial FAO was normalized by leptin repletion, we performed isolated working heart perfusions on caloric-restricted ob/ob and wild-type mice treated with intraperitoneal and intracerebroventricular leptin (Fig. 5A and B). Myocardial FAO was increased in caloric-restricted ob/ob hearts as previously observed, but myocardial FAO was not changed in caloric-restricted wild-type hearts. Furthermore, FAO remained elevated in random-fed and caloric-restricted ob/ob mice under insulin-perfused conditions. Intraperitoneal and intracerebroventricular leptin treatment reduced basal and insulin-perfused FAO in caloric-restricted ob/ob hearts and further repressed myocardial FAO in caloric-restricted wild-type hearts. We measured Akt and S6 phosphorylation in these hearts to determine whether insulin sensitivity was altered by caloric restriction. Random-fed and caloric-restricted ob/ob mice were insulin resistant at the level of Akt and S6 phosphorylation. Intraperitoneal and intracerebroventricular leptin treatment normalized insulin-stimulated Akt and S6 phosphorylation in caloric-restricted ob/ob mice (Supplementary Fig. 4).
FIG. 5.
Intraperitoneal and intracerebroventricular leptin are sufficient to normalize basal and insulin-stimulated palmitate oxidation in caloric-restricted ob/ob hearts. Basal and insulin-stimulated palmitate oxidation after 1 week of intraperitoneal (A) or intracerebroventricular leptin (B). C and D: qPCR analysis from noninsulin-stimulated perfused hearts. WT RF values are normalized to 1 and shown as the dashed line. MCAD, medium chain acyl CoA dehydrogenase; LCAD, long chain acyl CoA dehydrogenase; CD36, CD36 antigen; HADHa, hydroxyacyl-CoA dehydrogenase α-subunit; HADHb, hydroxyacyl-CoA dehydrogenase β-subunit. For intraperitoneal leptin study, aP < 0.05 vs. wt RF; bP < 0.05 vs. ob/ob RF; cP < 0.05 vs. ob/ob CR; *P < 0.05 vs. noninsulin-stimulated hearts from the same treatment group. For intracerebroventricular leptin study, aP < 0.05 vs. wt CR intracerebroventricular saline; cP < 0.05 vs. ob/ob CR intracerebroventricular saline; *P < 0.05 vs. noninsulin-stimulated hearts from the same treatment group. CR, caloric restriction; dhw, dry heart weight; icv, intracerebroventricular; L 1 wk, 1 week of leptin treatment; RF, random fed; wt, wild type.
Transcript levels of FAO regulatory genes were measured to determine whether myocardial FAO in caloric-restricted ob/ob mice was elevated as a result of increased FAO capacity (Fig. 5C and D). FAO gene expression was increased in the hearts of random-fed and caloric-restricted ob/ob mice. These genes include the PPAR-α targets: medium and long chain acyl CoA dehydrogenases, the FA transporter CD36, and Hadha /Hadhb, the subunits of the mitochondrial trifunctional protein. These increases were not observed in caloric-restricted wild-type mice. Myocardial FAO gene expression was normalized by intraperitoneal and intracerebroventricular leptin in caloric-restricted ob/ob mouse hearts. Furthermore, intraperitoneal and intracerebroventricular leptin tended to reduce FAO gene expression in caloric-restricted wild-type mice. No significant changes were observed in PPAR-α, PPARγ coactivator-1α, malonyl CoA decarboxylase (MCD), UCP-2, or UCP-3 expression levels (data not shown).
DISCUSSION
Intraperitoneal and intracerebroventricular leptin normalize myocardial FAO potentially by normalizing FFA delivery to the heart and reducing myocardial FAO capacity.
The main findings of this study are that metabolic abnormalities that characterize the heart in ob/ob and db/db mice, which are commonly used to model the cardiomyopathy of obesity and diabetes, are not reversed by caloric restriction, despite normalization of glucose tolerance and body weight. Specifically, in caloric-restricted ob/ob and db/db mice these changes occur in parallel with increasing circulating concentrations of FFA and triglycerides and modest hyperinsulinemia. Repletion of leptin both systemically and directly to the central nervous system (intracerebroventricular) normalized circulating FFA and triglyceride concentrations and expression levels of PPAR-α target genes that regulate FAO capacity in the heart, in concert with normalization of myocardial metabolism and mitochondrial function. Abnormalities in myocardial FAO in ob/ob and db/db mice are multifactorial, reflecting consequences of hyperglycemia, hyperinsulinemia, and increased myocardial lipid delivery. However, the major impact of leptin repletion in caloric-restricted ob/ob mice appears to be repression of FAO gene expression, which we propose is the result of reduced delivery of FA, which are PPAR-α ligands, to the heart. Moreover, hyperinsulinemia might promote increased FA utilization in the heart by increasing CD36 translocation (23), and normalization of circulating insulin concentrations would reverse this. Given the impact of leptin to lower FAO rates and FAO gene expression in caloric-restricted wild-type mice, the possibility remains that leptin might also directly regulate myocardial FAO capacity independently of circulating FA and insulin concentrations. Although we did not directly measure glucose utilization rates in this study, our prior studies have shown that in hearts perfused with glucose and palmitate, lowering of FA utilization by decreasing FA concentrations or by perfusing hearts with insulin is associated with an increase in glucose utilization (4). Thus we expect that the normalization of FA utilization in caloric-restricted ob/ob mice by leptin will increase myocardial glucose utilization.
The current study also raises the intriguing possibility that leptin may regulate mitochondrial coupling in the heart. We previously reported that mitochondrial uncoupling developed in hearts from ob/ob and db/db mice after exposure of these hearts to FA (21,22,24). We confirmed these findings in the current study, but now also show that mitochondrial uncoupling persists in caloric-restricted ob/ob mice in which glucose tolerance is normalized. In contrast, leptin treatment, which also normalized glucose tolerance and circulating FFA levels, restored coupled mitochondrial respiration. Although the mechanisms responsible for persistent mitochondrial dysfunction in hearts from caloric-restricted ob/ob mice remain to be elucidated, our observations are consistent with a role for persistent myocardial FA delivery in contributing to mitochondrial uncoupling in these hearts. Taken together, the current study underscores potential mechanisms by which impaired hypothalamic leptin signaling may contribute to altered myocardial substrate metabolism and mitochondrial function in obesity and diabetes. Given that leptin resistance is a uniform characteristic of individuals with acquired obesity (25), our studies raise the possibility that impaired central leptin action may contribute to altered cardiac metabolism and mitochondrial function in these individuals.
Caloric restriction further increased circulating concentrations of FFA and triglycerides in ob/ob mice, and fat pad weight was increased at the expense of lean mass, confirming previous reports that caloric-restricted ob/ob mice retain features of insulin resistance, characterized by increased adiposity (16–19). Thus leptin deficiency promotes myocardial FA utilization by increasing FA substrate availability. The lipolytic response to caloric restriction in ob/ob mice resembles an exaggerated fasting response, which under physiological circumstances is driven in part by reduced adipocyte insulin signaling coupled with stimulation of lipolysis by counterregulatory hormones. Thus although insulin levels in caloric-restricted ob/ob mice remain elevated, our observations suggest that adipose tissue remains insulin insensitive in caloric-restricted ob/ob mice and that this is reversed by central repletion of leptin. The mechanisms by which caloric restriction increased lipolysis in ob/ob mice despite an apparent modest increase in whole animal insulin sensitivity and normalization of glucose tolerance are incompletely understood. Future studies will be needed to elucidate these mechanisms by examining activities and expression levels of regulators of lipolysis such as adipose triglyceride lipase and lipoprotein lipase. Leptin has been suggested to increase whole animal FA utilization by inhibiting stearoyl-CoA desaturase 1 in the liver and increasing AMPK and ACC activation in the liver and skeletal muscle (26–29). We did not observe any changes in ACC or AMPK phosphorylation in liver, heart, or skeletal muscle (data not shown) in our experimental paradigm. These differences might reflect the chronic nature of leptin administration in these studies, which contrasts with other studies that suggest that the ability of leptin to activate AMPK in peripheral tissues is an acute response (30).
Caloric restriction reduced the respiratory exchange ratios in ob/ob mice, which suggests that in addition to the increase in cardiac FAO rates, whole body rates of FA utilization were also increased. Conversely, the increase in respiratory exchange ratios, insulin sensitivity, and reduction in myocardial palmitate oxidation in leptin-treated ob/ob mice suggests an overall increase in glucose utilization. We also hypothesized that leptin repletion reduced FFA delivery to the heart by increasing FA utilization in BAT. To test this hypothesis we measured UCP-1 content in BAT and observed that intraperitoneal and intracerebroventricular leptin increased UCP-1 content in BAT from caloric-restricted ob/ob mice (Supplementary Fig. 5). These data suggest that some of the FFAs released by lipolysis in caloric-restricted ob/ob mice were used by BAT thereby lowering FFA delivery to the heart upon leptin repletion. Thus, although leptin may acutely increase FA utilization in vivo, chronic leptin repletion predominantly decreases the relative contribution of FA oxidation to energy expenditure by increasing insulin sensitivity, glucose utilization, and heat production in caloric-restricted ob/ob mice.
In earlier studies, we observed that myocardial insulin resistance in ob/ob mice occurred at the level of phosphoinositide 3-kinase activation despite increased tyrosine phosphorylation of the insulin receptor (4). Leptin repletion to caloric-restricted ob/ob mice restored insulin-mediated activation of Akt in the heart. These changes occur independently of changes in AMPK activity or activation of mammalian target of rapamycin. Our present study design does not allow us to determine whether these changes are secondary to normalization of the metabolic milieu or to direct effects of central leptin signaling on myocardial insulin signaling. Myocardial hypertrophy was observed in random-fed ob/ob mice, but not in caloric-restricted ob/ob mice. An earlier study suggested that leptin is antihypertrophic and that caloric restriction was not able to reverse cardiac hypertrophy (3). However, these observations were obtained after significant hypertrophy had developed in ob/ob mice. In contrast, our studies suggest that the development of cardiac hypertrophy in ob/ob mice is not a direct consequence of leptin deficiency but is likely secondary to the hemodynamic consequences of obesity.
In conclusion, the current study demonstrates that changes in cardiac metabolism that characterize obesity and diabetes persist in leptin-deficient mice in which obesity and glucose intolerance is prevented by reducing caloric intake and are likely mediated by increased FFA delivery to the heart. These observations suggest that leptin resistance may indirectly contribute to altered cardiac metabolism in obesity and insulin-resistant states by regulating the delivery of FA to the heart. Not only are these FA metabolic substrates but they also act as ligands for PPAR-α. Our study also suggests that hypothalamic leptin signaling may normalize myocardial insulin resistance, potentially improving glucose oxidation in caloric-restricted ob/ob hearts and reducing the overall contribution of FA to ATP production in the heart. These studies are not without limitations, since they were performed before development of overt obesity and diabetes, and it remains to be shown that intracerebroventricular leptin is sufficient to normalize myocardial metabolism in older ob/ob mice, with diabetes of longer duration. Moreover, the timing of pairfeeding and intraperitoneal leptin injections do not mimic the nocturnal feeding patterns or diurnal rhythms of leptin release. Nevertheless, these observations raise the possibility that hypothalamic leptin resistance as develops in individuals with obesity and type 2 diabetes could contribute to altered myocardial substrate metabolism and myocardial insulin resistance. Enhancing hypothalamic leptin sensitivity not only represents an important approach for promoting weight loss and reversing obesity, but the current study provides evidence that reversal of maladaptive patterns of myocardial substrate utilization in obesity and insulin-resistant states is another important consequence of increasing hypothalamic leptin action. Thus future studies should seek to determine whether increasing hypothalamic leptin signaling will normalize myocardial metabolism in obese and insulin-resistant patients.
ACKNOWLEDGMENTS
This work was supported by grants R01-HL-73167, U01-HL-70525, and U01-HL-087947 from the National Institutes of Health to E.D.A., who is an established investigator of the American Heart Association. K.N. and J.F. were supported by summer undergraduate research fellowships from the American Heart Association Western Affiliates, and N.W., T.S., and L.A. were supported by the University of Utah undergraduate LEAP (Learning, Engagement, Achievement, Progress) program. H.B. was supported by a postdoctoral fellowship from the German Research Foundation.
No potential conflicts of interest relevant to this article were reported.
C.S. wrote the manuscript and researched data. J.T. performed the working heart experiments. K.N. and J.F. helped in researching data. J.S. performed saponin-permeabilized fiber experiments. N.W., T.S., and L.A. helped in researching data. H.B. aided in collection and analysis of transmission electron microscopy imaging. E.D.A. directed the study and edited the manuscript.
Parts of this study were presented in abstract form at the Scientific Sessions of the American Heart Association, Chicago, Illinois, 13–17 November 2010.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1106/-/DC1.
REFERENCES
- 1.Haynes WG. Role of leptin in obesity-related hypertension. Exp Physiol 2005;90:683–688 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem 2002;277:29424–29430 [DOI] [PubMed] [Google Scholar]
- 3.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 2003;108:754–759 [DOI] [PubMed] [Google Scholar]
- 4.Mazumder PK, O’Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004;53:2366–2374 [DOI] [PubMed] [Google Scholar]
- 5.Nickola MW, Wold LE, Colligan PB, Wang GJ, Samson WK, Ren J. Leptin attenuates cardiac contraction in rat ventricular myocytes. Role of NO. Hypertension 2000;36:501–505 [DOI] [PubMed] [Google Scholar]
- 6.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res 2003;93:277–279 [DOI] [PubMed] [Google Scholar]
- 7.Tajmir P, Ceddia RB, Li RK, Coe IR, Sweeney G. Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology 2004;145:1550–1555 [DOI] [PubMed] [Google Scholar]
- 8.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol 2006;149:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 2002;39:496–501 [DOI] [PubMed] [Google Scholar]
- 10.Ren J. Leptin and hyperleptinemia–from friend to foe for cardiovascular function. J Endocrinol 2004;181:1–10 [DOI] [PubMed] [Google Scholar]
- 11.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005;146:5341–5349 [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996;84:491–495 [DOI] [PubMed] [Google Scholar]
- 13.Chua SC, Jr, Koutras IK, Han L, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics 1997;45:264–270 [DOI] [PubMed] [Google Scholar]
- 14.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996;379:632–635 [DOI] [PubMed] [Google Scholar]
- 15.Palmer G, Aurrand-Lions M, Contassot E, et al. Indirect effects of leptin receptor deficiency on lymphocyte populations and immune response in db/db mice. J Immunol 2006;177:2899–2907 [DOI] [PubMed] [Google Scholar]
- 16.Alonso LG, Maren TH. Effect of food restriction on body composition of hereditary obese mice. Am J Physiol 1955;183:284–290 [DOI] [PubMed] [Google Scholar]
- 17.Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE. Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol 1999;276:E443–E449 [DOI] [PubMed] [Google Scholar]
- 18.Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci USA 1984;81:1835–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito M, Bray GA. Adrenalectomy and food restriction in the genetically obese (ob/ob) mouse. Am J Physiol 1984;246:R20–R25 [DOI] [PubMed] [Google Scholar]
- 20.Tabbi-Anneni I, Buchanan J, Cooksey RC, Abel ED. Captopril normalizes insulin signaling and insulin-regulated substrate metabolism in obese (ob/ob) mouse hearts. Endocrinology 2008;149:4043–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 2005;112:2686–2695 [DOI] [PubMed] [Google Scholar]
- 22.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 2007;56:2457–2466 [DOI] [PubMed] [Google Scholar]
- 23.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 2010;90:367–417 [DOI] [PubMed] [Google Scholar]
- 24.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258 [DOI] [PubMed] [Google Scholar]
- 25.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 2000;62:413–437 [DOI] [PubMed] [Google Scholar]
- 26.Cohen P, Miyazaki M, Socci ND, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002;297:240–243 [DOI] [PubMed] [Google Scholar]
- 27.Dobrzyn A, Dobrzyn P, Lee SH, et al. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol Endocrinol Metab 2005;288:E599–E607 [DOI] [PubMed] [Google Scholar]
- 28.Dobrzyn P, Dobrzyn A, Miyazaki M, et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 2004;101:6409–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ntambi JM, Miyazaki M, Stoehr JP, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 2002;99:11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002;415:339–343 [DOI] [PubMed] [Google Scholar]