Abstract
OBJECTIVE
Increased plasma concentrations of apolipoprotein B100 often present in patients with insulin resistance and confer increased risk for the development of atherosclerosis. Naturally occurring polyphenolic compounds including flavonoids have antiatherogenic properties. The aim of the current study was to evaluate the effect of the polymethoxylated flavonoid nobiletin on lipoprotein secretion in cultured human hepatoma cells (HepG2) and in a mouse model of insulin resistance and atherosclerosis.
RESEARCH DESIGN AND METHODS
Lipoprotein secretion was determined in HepG2 cells incubated with nobiletin or insulin. mRNA abundance was evaluated by quantitative real-time PCR, and Western blotting was used to demonstrate activation of cell signaling pathways. In LDL receptor–deficient mice (Ldlr−/−) fed a Western diet supplemented with nobiletin, metabolic parameters, gene expression, fatty acid oxidation, glucose homeostasis, and energy expenditure were documented. Atherosclerosis was quantitated by histological analysis.
RESULTS
In HepG2 cells, activation of mitogen-activated protein kinase-extracellular signal–related kinase signaling by nobiletin or insulin increased LDLR and decreased MTP and DGAT1/2 mRNA, resulting in marked inhibition of apoB100 secretion. Nobiletin, unlike insulin, did not induce phosphorylation of the insulin receptor or insulin receptor substrate-1 and did not stimulate lipogenesis. In fat-fed Ldlr−/− mice, nobiletin attenuated dyslipidemia through a reduction in VLDL-triglyceride (TG) secretion. Nobiletin prevented hepatic TG accumulation, increased expression of Pgc1α and Cpt1α, and enhanced fatty acid β-oxidation. Nobiletin did not activate any peroxisome proliferator–activated receptor (PPAR), indicating that the metabolic effects were PPAR independent. Nobiletin increased hepatic and peripheral insulin sensitivity and glucose tolerance and dramatically attenuated atherosclerosis in the aortic sinus.
CONCLUSIONS
Nobiletin provides insight into treatments for dyslipidemia and atherosclerosis associated with insulin-resistant states.
Insulin resistance and type 2 diabetes are conditions classically defined by glucose intolerance but are also characterized by lipid abnormalities including an overproduction of hepatic VLDL (1). Increased VLDL secretion contributes to increased plasma concentrations of apolipoprotein B100 (apoB100)-containing lipoproteins, which represents an important risk factor for cardiovascular disease, especially because current pharmacological interventions provide limited benefit for correcting VLDL overproduction (2). Insulin resistance reduces the ability of the hepatic LDL receptor (LDLR) to mediate lipoprotein reuptake and degradation (3,4), increases microsomal triglyceride transfer protein (MTP) expression (5), and increases the hepatic synthesis of cholesterol and triglyceride (TG) (6). Hepatic accumulation of lipids and enhanced MTP-mediated transfer of these lipids onto the apoB100 backbone are key determinants of VLDL secretion (7,8). Concurrent with impaired lipid homeostasis, abnormal glucose metabolism is observed in insulin-resistant states as a result of impaired glucose uptake by peripheral tissues and enhanced hepatic gluconeogenesis (9).
Hyperinsulinemia can initiate the transcription of sterol regulatory element–binding protein 1c (SREBP1c), resulting in upregulation of genes involved in fatty acid (FA) synthesis (10), contributing to both hepatic steatosis and dyslipidemia (11). This is clearly observed in mice deficient in the hepatic insulin receptor (IR), where despite hyperinsulinemia, hepatic SREBP1c-mediated lipogenesis, hepatic TG, and VLDL-TG secretion are significantly reduced (12,13). Although not a target of SREBP1c, inhibition of insulin-induced mitogen-activated protein kinase-extracellular signal–related kinase (MAPK)erk signaling increases DGAT1/2 expression leading to microsomal TG accumulation and VLDL secretion (14). In concert with increased lipogenesis, the reduced FA oxidation observed in insulin resistance states further contributes to increased hepatic lipid availability (15). Pgc1α and Pparα are key regulators of hepatic FA oxidation, since in both Pgc1α−/− and Pparα−/− mice β-oxidation is impaired, leading to increased hepatic lipid accumulation (16,17).
The hypocholesterolemic and metabolically beneficial properties of citrus-derived flavonoids have been demonstrated (18–22). Tangeretin, a polymethoxylated flavonoid, reduced apoB100 secretion from human hepatoma (HepG2) cells through inhibition of both DGAT activity and microsomal TG accumulation (23). In a mouse model of dyslipidemia and hyperinsulinemia, naringenin prevented hyperinsulinemia-stimulated de novo lipogenesis, inhibited VLDL-apoB secretion, and improved plasma lipids (24). In insulin-resistant hamsters, a combination of tangeretin and another polymethoxylated flavonoid nobiletin ameliorated dyslipidemia and improved glucose tolerance (18). Nobiletin increased Glut4 expression in muscle and attenuated the hyperglycemia normally observed in ob/ob mice (25). In Ldlr−/− or apoE−/− mice, naringenin, resveratrol, and other polyphenolic compounds reduce atherosclerosis through a variety of mechanisms, suggesting they have powerful antiatherogenic properties (26–28).
The objective of the current study was to determine the effectiveness of the citrus-derived flavonoid nobiletin, to inhibit apoB100 secretion from HepG2 cells, and to attenuate dyslipidemia, insulin resistance, and atherosclerosis. We demonstrate that nobiletin, like insulin, inhibits apoB100 secretion from HepG2 cells through activation of MAPKerk signaling. In contrast with insulin, the effect of nobiletin was independent of either IR or IR substrate-1 (IRS-1) tyrosine phosphorylation. Activation of MAPKerk by both nobiletin and insulin decreased the expression of MTP and DGAT1/2 and increased LDLR expression. In Ldlr−/− mice fed a Western diet, nobiletin prevented dyslipidemia and hepatic lipid accumulation, without any change in caloric intake, intestinal TG absorption, or lean body mass. Hepatic TG accumulation was prevented by nobiletin through an increase in Pgc1α and Cpt1α expression leading to activation of hepatic FA oxidation. VLDL-TG secretion was significantly decreased by nobiletin. Nobiletin reduced peripheral lipid accumulation, improved glucose tolerance, and restored insulin sensitivity in liver and peripheral tissues, compared with Western-fed mice. Nobiletin prevented atherosclerotic lesion development, suggesting that amelioration of the dyslipidemia, hepatic steatosis, and insulin resistance in Western-fed Ldlr−/− mice attenuates plaque formation.
RESEARCH DESIGN AND METHODS
Cell culture and chemicals.
Nobiletin (R&S PharmChem, Hangzhou City, China) was solubilized in DMSO. HepG2 cells from the American Type Culture Collection (Rockville, MD) were grown as described previously (29). All inhibitors and inactive isoforms (MEK1/2: U0126 and U0124; p38MAPK: SB203580 and SB248080 [Calbiochem, San Diego, CA]; MTP: BMS197636 [Bristol-Myers Squibb, Princeton, NJ]) were solubilized in DMSO. AICAR (Sigma, St. Louis, MO) was solubilized in H2O.
Cellular and tissue assays.
For gene expression, total RNA was isolated from HepG2 cells or mouse liver (TRIzol; Invitrogen, Mississauga, Ontario, Canada), and quantitative real-time PCR (qRT-PCR) was performed as previously described (24). Immunoprecipitation and immunoblotting of IR and IRS-1, apoB100 secretion into media, and pERK-to-ERK ratios in cell lysates was performed as described previously (20,21). A peroxisome proliferator–activated receptor (PPAR)-responsive element luciferase assay was carried out in HepG2 cells transfected with 0.01 μg/mL PPAR-α, -γ, or -δ.SG5 expression vectors; 0.5 μg/mL PPRE.tk.Luc; and 0.05 μg/mL tk.pRL vectors (provided by Dr. John Capone, McMaster University, Hamilton, Ontario, Canada) (30). FA oxidation assays were performed as previously described (31). DiI-LDL,1'-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (Biomedical Technologies, Stoughton, MA) was added at 10 ng/mL media for 5 h. Cells were trypsinized, suspended in PBS, and filtered. Mean fluorescent signals were determined by flow cytometry (104 cells/sample) using FACS Calibur (BD Biosciences). TG mass was measured as described previously (32). Synthesis of FA or TG was measured following incubation of cells in 5% human lipoprotein-deficient serum (LPDS) with insulin or nobiletin by incorporation of [1-14C] acetic acid (Amersham Biosciences, Piscataway, NJ) into FA or incorporation of [1-14C] oleic acid (Amersham Biosciences) into TG (33). MTP activity was determined using a modified isotopic transfer assay (32). See Supplementary Data for details.
Animals.
Male Ldlr−/− mice on the C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME). Studies were performed in accordance with the Canadian Guide for the Care and Use of Laboratory Animals and were approved by the University of Western Ontario Animal Care Committee. Twelve-week-old mice were fed ad libitum a chow diet (14% of calories from fat; Harlan Teklad T8604, Madison, WI), a high-fat Western diet (TD96125, 42% of calories from fat, no added cholesterol [0.05%] or cholate), or the Western diet supplemented with 0.1 or 0.3% (weight/weight) nobiletin for 8 or 26 weeks. Food intake was measured daily, and body weight was measured biweekly. Animals were fasted for 6 h before intervention. Intestinal fat absorption was determined using a modified fecal isotope ratio method (29).
Blood samples, tissue collection, and VLDL-TG secretion.
Blood and tissue collection from mice was as described previously (24). In vivo secretion rates of VLDL-TG into plasma were determined in conscious, unrestrained mice (18/group), following intraperitoneal injection of 1 g/kg tyloxapol (Ruger Chemical Company, Irvington, NJ) (34). See Supplementary Data for details.
Energy expenditure, adiposity index, and FA oxidation.
Energy expenditure was determined by an indirect open-circuit calorimeter (Oxylet; Panlab, Cornella, Spain), and adiposity index was determined as the wet weight of the epididymal fat pads/total body weight (24). Hepatic FA oxidation was determined as the conversion of [3H]palmitate to 3H20 (24). See Supplementary Data for details.
Hyperinsulinemic-euglycemic clamp and pyruvate tolerance test.
Clamps were performed as previously described (35,36). See Supplementary Methods for details. Pyruvate tolerance tests were performed in fasted mice (16 h) injected (intraperitoneally) with 2 g/kg sodium pyruvate (Sigma). Insulin and glucose tolerance tests were as described previously (24). Blood glucose was monitored by a glucometer (Ascensia Elite; Bayer Healthcare, Toronto, Canada).
Statistical analysis.
All data are presented as the mean ± SEM. Analysis was performed using Sigmaplot Version 14.0. Significant differences (P < 0.05) between groups were determined by a one-way ANOVA and post hoc Tukey test to determine statistical significance.
RESULTS
Nobiletin decreases apoB100 secretion through activation of MAPKerk.
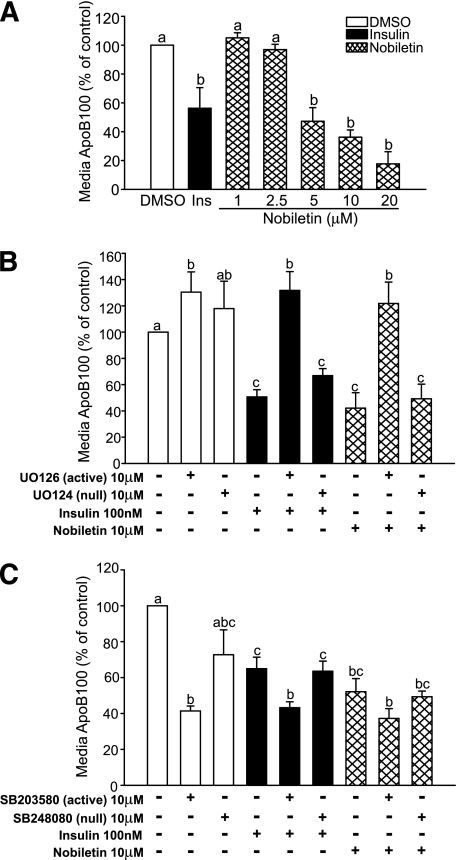
In HepG2 cells, nobiletin dose-dependently reduced the secretion of apoB100-containing lipoproteins into the media (Fig. 1A). Secretion of apoE was unaffected (data not shown). The concentration of nobiletin that inhibited apoB100 secretion by 50% (10 μM) was used for further experiments. Insulin has been shown to decrease apoB100 secretion through activation of MAPKerk signaling (20). Incubation of cells with a specific inhibitor of MEK1/2, U0126, demonstrated that nobiletin, like insulin, reduces apoB100 accumulation through activation of MAPKerk (Fig. 1B). The activity of MAPKp38, which normally attenuates MEK1/2 signaling, was inhibited by SB203580, which enhanced the inhibition of apoB100 secretion by nobiletin, confirming that nobiletin signals through MAPKerk (Fig. 1C). In contrast with insulin, incubation of cells with the PI3-kinase inhibitor wortmannin had no effect on the inhibition of apoB100 secretion by nobiletin (data not shown).
FIG. 1.
Nobiletin dose-dependently reduces apoB100 secretion through activation of MAPKerk signaling. A: HepG2 cells were incubated in 5% LPDS-minimum essential medium with insulin or increasing concentrations of nobiletin for 24 h. B: HepG2 cells were preincubated for 30 min in the absence or presence of DMSO U0126 (10 μM; active MEK1/2 inhibitor) or U0124 (10 μM; null inactive isoform). C: HepG2 cells were preincubated for 30 min in the absence or presence of DMSO, SB203580 (10 μM; active p38 MAPK inhibitor), or SB248080 (10 μM; null inactive isoform), followed by an additional 19.5-h incubation in the absence or presence of DMSO, insulin (100 nM), or nobiletin (10 μM). Media were collected, and apoB100 was measured by immunoblotting. Results are reported as a percentage of the DMSO control. Values are means ± SEM, and different letters are statistically different (P < 0.05).
Nobiletin activates signaling through MAPKerk to regulate LDLR, MTP, and DGAT1/2 mRNA expression, independent of the IR or IRS-1.
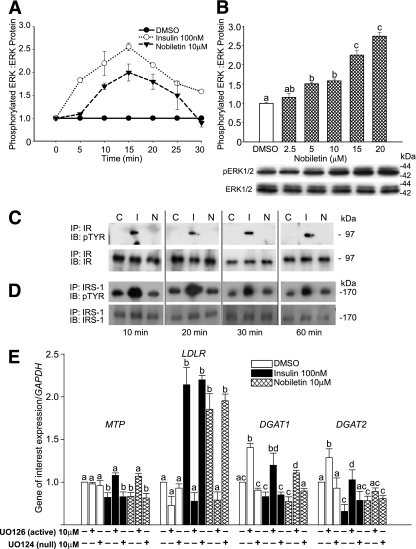
Activation of ERK, a protein downstream of MEK1/2, has been implicated in the regulation of apoB100 secretion (14,20). In HepG2 cells, the time course of nobiletin-induced activation of ERK1/2 was rapid and concentration dependent, with peak phosphorylation occurring at 15 min. (Fig. 2A and B). To determine whether MAPKerk activation by nobiletin was mediated through activation of the IR, we examined acute phosphorylation of both the IR and IRS-1. In contrast with insulin, nobiletin did not induce tyrosine phosphorylation of the IR or IRS-1 (Fig. 2C and D), demonstrating that nobiletin rapidly activates MAPKerk through a distinct mechanism. Decreased MTP and increased LDLR expression have been linked to inhibition of apoB100 secretion (20,22). Like insulin, MAPKerk activation by nobiletin decreased MTP mRNA (−30%) and increased LDLR mRNA 2.5-fold (Fig. 2E). Nobiletin significantly decreased DGAT1 and DGAT2 mRNA expression through activation of MAPKerk (Fig. 2E).
FIG. 2.
Nobiletin, unlike insulin, does not activate the IR and IRS-1 to stimulate ERK phosphorylation. A: HepG2 cells were incubated for 16 h in serum-free minimum essential medium (containing 0.5% insulin-free, FA-free BSA) and treated with DMSO, insulin (100 nM), or nobiletin (10 μM) for up to 30 min. B: HepG2 cells incubated in serum-free minimum essential medium were treated with DMSO (control) and increasing concentrations of nobiletin for 15 min. Total and phosphorylated ERK1/2 were measured by immunoblot analysis. Values are reported as a fold change compared with DMSO control and are means ± SEM. C and D: HepG2 cells were preincubated in serum-free minimum essential medium followed by addition of DMSO, insulin (100 nM), or nobiletin (10 μM) for up to 60 min, and cell lysates were immunoprecipitated with IR (C) or IRS-1 (D) antibodies. Proteins were separated by 6% SDS-PAGE and membranes probed with IR (C) or IRS-1 (D) and phosphotyrosine antibodies. E: HepG2 cells in 5% LPDS-minimum essential medium were preincubated for 30 min with DMSO, the MEK1/2 inhibitor UO126 (10 μM), or its null inactive isoform UO124 (10 μM) followed by a 5.5 h incubation with DMSO, insulin (100 nM), or nobiletin (10 μM). mRNA levels were quantitated by qRT-PCR. Results are normalized to GAPDH and reported relative to the DMSO control. Values are means ± SEM; n ≥5. Different letters are statistically different (P < 0.05).
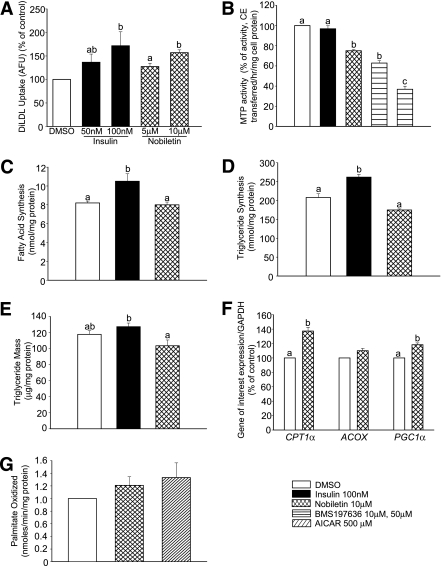
Nobiletin stimulates LDL uptake, inhibits MTP activity, but does not increase cellular TG synthesis or mass accumulation.
The increased LDLR expression resulted in enhanced LDLR activity as diI-LDL uptake increased in HepG2 cells incubated with either nobiletin or insulin (Fig. 3A). Nobiletin reduced MTP activity (−25%) (Fig. 3B), whereas insulin had no effect, suggesting that inhibition of MTP by nobiletin was direct and not related to decreased mRNA expression. Insulin is known to stimulate FA and TG synthesis (22); however, nobiletin had no effect on either parameter (Fig. 3C and D). Consistent with decreased DGAT1/2 mRNA, TG synthesis and TG mass were significantly lower in nobiletin-treated cells, when compared with insulin-treated cells (Fig. 3D and E). Nobiletin increased CPT1-α and PGC1-α mRNA, which was associated with a modest, but not significant, increase in cellular FA oxidation (Fig. 3F and G).
FIG. 3.
Nobiletin modestly decreases FA and TG synthesis and TG mass in HepG2 cells despite inhibition of MTP activity in HepG2 cells. A: HepG2 cells in 5% LPDS-minimum essential medium were preincubated for 19 h with insulin (50 nM, 100 nM) or nobiletin (5 μM, 10 μM) followed by a 5-h incubation with 10 ng/mL diI-LDL. diI-LDL fluorescence was measured by flow cytometry. B: MTP activity was measured in homogenates from HepG2 cells incubated for 24 h with DMSO, insulin (100 nM), nobiletin (10 μM), or a specific MTP inhibitor, BMS-197636. Values are reported as percentage of controls and are means ± SEM. C: HepG2 cells were incubated in 5% LPDS-minimum essential medium with DMSO, insulin (100 nM), or nobiletin (10 μM) for 24 h in the presence of [14C]acetate. Lipids were extracted and saponified, and rates of FA synthesis are presented as nanomoles FA formed per milligrams cell protein. TG synthesis (D) and TG mass (E) were determined in HepG2 cells incubated with DMSO, insulin (100 nM), or nobiletin (10 μM) for 19 h followed by a 5-h incubation with [1-14C]oleic acid plus 0.1 mM cold oleic acid (D) or 0.1 mM cold oleic acid alone (E). Lipids were extracted with hexane-isopropanol and quantified. F: The mRNA abundance of CPT1α, ACOX, and PGC1α was quantified by qRT-PCR in HepG2 cells treated for 6 h with DMSO or nobiletin (10 μM). G: FA oxidation was determined in HepG2 cells by conversion of [3H]palmitate to [3H20] in cells treated with nobiletin (10 μM) or AICAR (500 μM). All values are means ± SEM; n ≥5. Different letters are statistically different (P < 0.05).
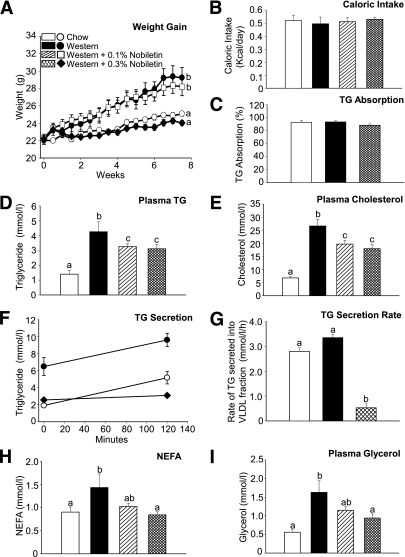
Nobiletin prevents diet-induced weight gain and reduces dyslipidemia in high-fat fed Ldlr−/− mice.
In Ldlr−/− mice, a Western diet induces many aspects of the metabolic syndrome including obesity, hyperinsulinemia, hyperlipidemia, and glucose intolerance (37). Using this model, we examined the effect of nobiletin on metabolic parameters including VLDL-TG production, hepatic steatosis, insulin resistance, and atherosclerosis. Nobiletin-treated mice resisted the Western diet-induced weight gain (Fig. 4A) despite no difference in caloric intake or intestinal TG absorption (Fig. 4B and C). The Western diet increased fasting plasma TG (threefold) and cholesterol (2.8-fold) compared with chow-fed animals. Nobiletin significantly decreased both plasma lipids by 35% (Fig. 4D and E). Metabolic studies with tyloxapol, which inhibits lipolysis of lipoproteins derived from both the intestine and the liver, demonstrated an overproduction of TG-rich lipoproteins induced by the Western diet (Fig. 4F). When compared with Western-fed mice, nobiletin supplementation significantly reduced the VLDL-TG secretion rate (Fig. 4G). Elevated plasma nonesterified fatty acid (NEFA) and glycerol, characteristic of insulin resistance, were observed in Western-fed animals, both of which were normalized by nobiletin (Fig. 4H and I).
FIG. 4.
Nobiletin dose-dependently inhibits weight gain in Western-fed Ldlr−/− mice. Mice were fed diets for 8 weeks and fasted for 6 h (n = 6/group). Body weight was measured biweekly (A), and caloric intake was measured daily over 8 weeks (B). C: TG absorption using a fecal dual isotope method. Percent TG absorption was calculated from the radiolabel ratio in feces collected over 48 h. D and E: Plasma TG and cholesterol concentrations. F: Mice were injected with tyloxapol, and TG was measured in the plasma VLDL/IDL fraction (d <1.019 g/mL) at 0 and 120 min (n = 5–6 mice/group/time point). G: The production rate was determined as micromoles per liter per hour. H and I: Plasma NEFA (H) and glycerol concentrations (I) are shown. Data represent the mean ± SEM, and different letters are statistically different (P < 0.05).
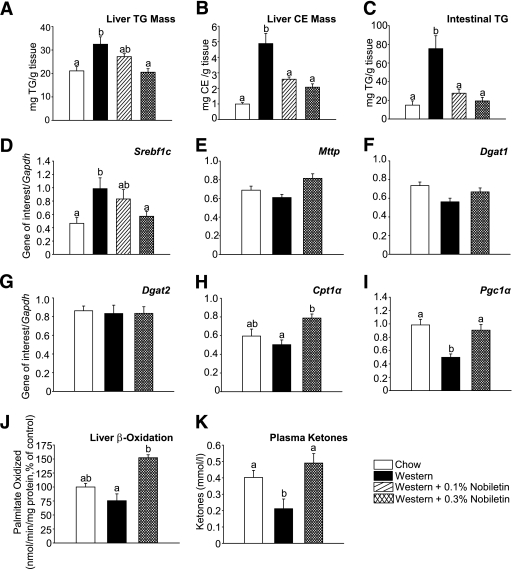
Nobiletin reduces TG in both the liver and intestine in high-fat fed Ldlr−/− mice.
The Western diet significantly increased liver TG 1.5-fold and cholesteryl ester (CE) mass 4.8-fold compared with chow (Fig. 5A and B). Addition of 0.1 and 0.3% nobiletin dose-dependently decreased hepatic TG by 44 and 87%, and hepatic CE by 61 and 71%, respectively. The intestine contributes to VLDL-TG secretion under conditions described for tyloxapol experiments. Significant levels of TG remain within intestinal tissue in Western-fed mice following a 6-h fast (Fig. 5C). Nobiletin supplementation completely normalized intestinal TG (Fig. 5C), suggesting that nobiletin limits lipid availability in both liver and intestine for VLDL-TG secretion. Intestinal CE concentrations were unchanged (data not shown). The Western diet significantly increased liver Srebp1c expression (2.3-fold) compared with chow-fed mice. Srebp1c was dose-dependently reduced by 0.1% (−35%) and 0.3% nobiletin (−80%) (Fig. 5D), suggesting that reduced de novo lipogenesis contributes to the prevention of hepatic TG accumulation. In contrast with HepG2 cells, nobiletin did not affect hepatic expression of Mttp or Dgat1/2 (Fig. 5E–G). The marked reduction in hepatic TG concentrations and VLDL-TG secretion suggested that nobiletin stimulates FA oxidation. Expression of Cpt1α, Pgc1α, and rates of FA oxidation were depressed in Western-fed animals compared with chow. All three parameters were significantly increased in nobiletin-treated mice (Fig. 5H–J). Furthermore, nobiletin increased plasma ketones twofold, compared with Western-fed mice (Fig. 5K). Increased FA oxidation suggested that nobiletin functions as a PPAR activator. However, using a luciferase reporter assay in HepG2 cells, nobiletin did not significantly activate PPARα, PPARγ, or PPARδ (Supplementary Fig. 1). Furthermore, nobiletin had no effect on hepatic Pparα mRNA, Acox mRNA, or liver weight (data not shown).
FIG. 5.
Nobiletin improves hepatic steatosis in Ldlr−/− mice fed a Western diet for 8 weeks. Mice were fed diets for 8 weeks and fasted for 6 h (n = 6/group). Liver TG (A) and CE mass determined by lipid extraction (B) are shown. C: Intestinal TG content at sacrifice. D–I: Expression of Srebf1c (Srebp1c), Mttp, Dgat1, Dgat2, Cpt1α, and Pgc1α mRNA relative to Gapdh mRNA, quantitated by qRT-PCR. J: Fatty acid oxidation in liver (n = 4–6/group) determined by [3H]palmitate conversion to 3H2O. K: Plasma ketone bodies were quantitated by a kinetic rate method. Data represent the mean ± SEM, and different letters are statistically different (P < 0.05).
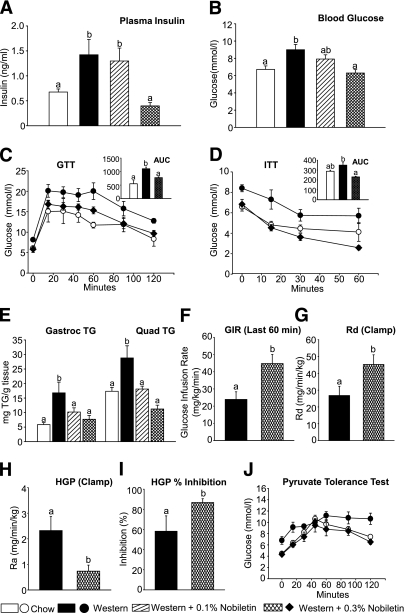
Nobiletin prevents hyperinsulinemia and improves glucose tolerance and insulin sensitivity.
Western-fed mice were hyperinsulinemic (1.4 ng/mL) and moderately hyperglycemic (9.0 mmol/L) compared with chow (0.7 ng/mL and 6.8 mmol/L), whereas fasting plasma insulin and glucose were significantly decreased by 0.3% nobiletin (0.4 ng/mL and 6.2 mmol/L), respectively (Fig. 6A and B). Glucose tolerance tests revealed that nobiletin normalized the impaired glucose tolerance observed in Western-fed mice (Fig. 6C). Nobiletin significantly improved the impaired insulin tolerance in Western-fed mice such that the area under the glucose curve was similar to chow-fed mice (Fig. 6D). The Western diet induced TG accumulation in gastrocnemius and quadriceps, whereas muscle lipid deposition was completely prevented by nobiletin supplementation (Fig. 6E).
FIG. 6.
Nobiletin improves glucose utilization and insulin sensitivity in Ldlr−/− mice fed a Western diet for 8 weeks. Mice were fed diets for 8 weeks and fasted for 6 h (n = 6/group). A: Plasma insulin was measured by enzyme-linked immunosorbent assay. B: Blood glucose was measured by glucometer. Glucose tolerance tests (C) or insulin tolerance tests (D) were performed in 6-h fasted mice by intraperitoneal injection of 1 g/kg glucose (C) or 0.6 IU/kg insulin (D) and blood glucose measured from 0 to 60 or 120 min. Glucose utilization or insulin sensitivity was determined as the area under the curve (AUC; inset graphs). E: TG content of gastrocnemius and quadriceps muscle. Hyperinsulinemic-euglycemic clamps were conducted in Ldlr−/− mice fed the Western diet or the Western diet supplemented with 0.3% nobiletin for 8 weeks. F: glucose infusion rate during the last 60 min of the clamp. G: Whole-body glucose uptake (Rd) during the clamp. HGP (H) and percent suppression of HGP (I) during the clamp are shown. J: Pyruvate tolerance tests were performed in 16-h fasted mice by intraperitoneal injection of pyruvate (2 g/kg). Data represent the mean ± SEM, and different letters are statistically different (P < 0.05).
To determine the locus of insulin resistance in Western-fed mice and its prevention by nobiletin, hyperinsulinemic euglycemic clamp studies were performed to assess whole-body insulin sensitivity as well as hepatic versus peripheral insulin action. As shown in Fig. 6F, the glucose infusion rate required to maintain euglycemia was significantly increased twofold in nobiletin-supplemented mice compared with Western-fed mice. This improved insulin sensitivity was explained by both an increase in peripheral glucose disposal (Fig. 6G) and enhanced suppression of hepatic glucose production (HGP) (Fig. 6H and I). During the clamp, HGP was significantly lower (−70%) in nobiletin-supplemented mice compared with Western-fed mice because of an increase in the ability of insulin to blunt HGP. Hepatic gluconeogenesis was assessed using a pyruvate tolerance test in which injection of the gluconeogenic substrate pyruvate increases blood glucose by promoting hepatic gluconeogenesis. In Western-fed mice, pyruvate increased blood glucose concentrations to a greater extent, compared with both nobiletin- and chow-fed animals (Fig. 6J).
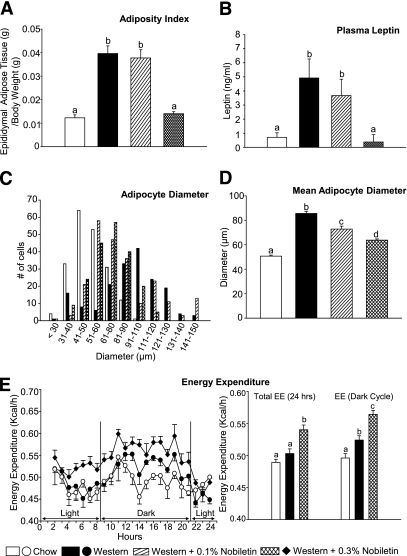
Nobiletin inhibits diet-induced obesity and adipocyte hypertrophy.
Treatment with 0.1% nobiletin had no effect on diet-induced adiposity, whereas 0.3% nobiletin completely prevented adipose tissue accumulation (Fig. 7A and Supplementary Fig. 2A and B). Western-fed mice exhibited elevated plasma leptin levels (Fig. 7B) and adipocyte hypertrophy in epididymal fat pads and brown adipose tissue (Fig. 7C and D and Supplementary Fig. 3A and B), which were completely normalized by 0.3% nobiletin. These changes were independent of caloric intake (Fig. 4B) and muscle mass (Supplementary Fig. 2C and D), suggesting effects on energy metabolism. Total energy expenditure increased significantly in mice fed 0.3% nobiletin, compared with both Western and chow-fed mice. This was primarily because of enhanced energy expenditure during the dark cycle (Fig. 7E). Respiratory quotients were not statistically different among the dietary groups: 0.95 ± 0.02, 0.87 ± 0.02, and 0.93 ± 0.02 for chow, Western, and Western plus 0.3% nobiletin, respectively.
FIG. 7.
Nobiletin prevents adipose tissue accumulation and adipocyte hypertrophy in Ldlr−/− mice fed a Western diet for 8 weeks. Mice were fed diets for 8 weeks and fasted for 6 h (n = 6/group). A: Adiposity index was determined as the weight of epididymal adipose stores per gram of body weight. B: Plasma leptin concentrations. C and D: Adipocyte size was determined by measuring the diameter (in μm) of adipocytes from hematoxylin and eosin-stained sections of epididymal adipose tissue (n = 200 adipocytes/group). E: Energy expenditure was determined via indirect calorimetry during both the light and dark cycle (6:00 a.m.–6:00 p.m.). Measurements were collected every 15 min, and each data point, expressed as kilocalories per hour per mouse, represents a rolling average of four time points. Values are the mean ± SEM, and different letters are statistically different (P < 0.05).
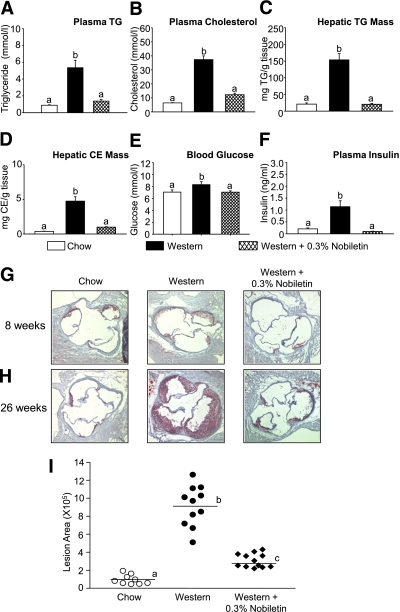
Nobiletin prevents dyslipidemia, hepatic steatosis, and atherosclerosis in Ldlr−/− mice.
To evaluate whether correction of dyslipidemia and insulin resistance by nobiletin prevents atherosclerosis, mice were fed chow or the Western diet ±0.3% nobiletin (weight/weight) for 8 or 26 weeks. By 26 weeks, Western-fed mice developed extensive dyslipidemia. Both plasma TG and cholesterol concentrations increased sixfold compared with chow-fed mice. Nobiletin supplementation significantly decreased plasma TG and cholesterol by 74 and 67%, respectively (Fig. 8A and B). The Western diet induced marked hepatic steatosis resulting in a 7.3-fold increase in liver TG and a 13-fold increase in liver CE compared with chow-fed mice. Nobiletin almost completely prevented hepatic lipid accumulation such that lipid levels were similar to chow-fed mice (Fig. 8C and D). At 26 weeks, Western-fed mice continued to be hyperglycemic and hyperinsulinemic. Nobiletin normalized fasting glucose and insulin concentrations to levels observed in chow-fed mice (Fig. 8E and F).
FIG. 8.
Nobiletin improves dyslipidemia, hepatic steatosis, and hyperinsulinemia and reduces atherosclerosis in Ldlr−/− mice fed a Western diet for 26 weeks. A–F: Mice were fed diets for 26 weeks and fasted for 6 h (n = 12/group). Plasma TG (A) and cholesterol concentrations (B) are shown. TG (C) and cholesteryl ester concentrations (D) in liver are shown. E: Blood glucose concentrations. F: Plasma insulin concentrations. G and H: Mice were fed diets for 8 weeks (n = 3/group) or 26 weeks (n = 12/group) and fasted for 6 h before being killed. Representative frozen serial sections (10 μm) of the aortic sinus, prepared using a cryostat, stained with Oil Red O and counterstained with hematoxylin. I: Lesion area for all mice at 26 weeks was quantitated and expressed as total plaque area. Values are the mean ± SEM, and different letters are statistically different (P < 0.05). (A high-quality digital representation of this figure is available in the online issue.)
After 8 weeks, Western-fed mice demonstrated modest lesion development as assessed by Oil Red O-staining, within the aortic sinus, when compared with chow-fed mice or nobiletin-treated mice (Fig. 8G). After 26 weeks on diet, lesion area within the aortic sinus of Western-fed animals was significantly increased by 12-fold, compared with chow-fed animals. Nobiletin supplementation significantly reduced plaque area by 83%, compared with Western-fed mice (Fig. 8H and I).
DISCUSSION
In the current study, we evaluated the role of nobiletin in the regulation of apoB100 secretion from hepatoma cells and the ability of nobiletin to prevent dyslipidemia, insulin resistance, and atherogenesis induced in mice by high-fat feeding. We show that nobiletin inhibits apoB100 secretion from HepG2 cells through activation of MAPKerk, in a manner similar to insulin, although nobiletin does not activate the IR and IRS-1. In Ldlr−/− mice, nobiletin attenuates diet-induced obesity, hepatic steatosis, VLDL-TG secretion, and dyslipidemia and increases hepatic FA oxidation. Furthermore, nobiletin restores glucose tolerance and insulin sensitivity in liver and peripheral tissues. Collectively, improvement in these metabolic parameters leads to the prevention of atherosclerosis.
Atherogenic lipoprotein profiles are characterized by increased concentrations of apoB100-containing lipoproteins. Previously, it has been shown that the citrus flavonoid naringenin inhibits apoB100 secretion from HepG2 cells through a mechanism similar to insulin (20,22). Nobiletin is a significantly more potent inhibitor; its half-maximal inhibitory concentration (IC50) for reduction of apoB100 secretion from HepG2 cells is ∼10-fold lower, compared with naringenin (29). Nobiletin, like insulin, decreases apoB100 secretion through rapid activation of signaling through MAPKerk, leading to the enhanced expression and activity of the LDLR, decreased expression, and activity of MTP and decreased expression of DGAT1/2, all of which are known to contribute to the inhibition of apoB100 secretion (14,20–22). Nobiletin activates MAPKerk through a mechanism distinct from insulin, since nobiletin did not induce tyrosine phosphorylation of the IR or IRS-1. This observation demonstrates that nobiletin has the potential to regulate hepatic lipid and lipoprotein metabolism, in vivo, in the context of insulin resistance.
Activation of MAPKerk signaling by polyphenols in HepG2 cells has been shown to increase LDLR mRNA (38–40). Naringenin increases LDLR expression in HepG2 cells and requires enhanced processing of SREBP1 (22). Berberine increases LDLR mRNA via ERK1/2, through a mechanism involving message stabilization, resulting in reduced plasma LDL-C concentrations in vivo (41). Activation of MAPKerk by nobiletin also decreased DGAT1/2 mRNA expression. In HepG2 cells, inhibition of MAPKerk enhances DGAT1/2 expression and microsomal TG availability for VLDL assembly (14). We demonstrate that nobiletin decreases expression of DGAT mRNA, which is associated with reduced TG synthesis, indicating one mechanism whereby nobiletin limits TG availability for apoB100 secretion. MTP mRNA and MTP activity were both decreased by nobiletin in HepG2 cells, similar to the effect of naringenin (21,32). These data suggest that reduced lipid transfer activity also contributes to reduced apoB100 secretion in nobiletin-treated HepG2 cells.
To extend our in vitro findings, we determined the effect of nobiletin in high-fat fed Ldlr−/− mice, a model of diet-induced insulin resistance and atherosclerosis (37). Nobiletin supplementation resulted in a dramatic reduction in both hepatic and intestinal TG accumulation, attenuation of VLDL-TG secretion, and normalization of glucose homeostasis and conferred an almost complete resistance to obesity, without effect on caloric consumption or fat absorption. Many variables can contribute to hepatic lipid accumulation including increased flux of dietary and liberated visceral FA, increased FA synthesis, and reduced FA oxidation (8). In contrast with the Western diet, nobiletin normalizes insulin concentrations, reduces plasma NEFA, and decreases hepatic Srebp1c mRNA expression demonstrating that nobiletin reduces both NEFA flux and endogenous lipogenesis. It has been shown that blocking the effect of hyperinsulinemia on Srebp1c-stimulated lipogenesis dramatically decreases hepatic TG content and VLDL-TG secretion (12,13). Nobiletin also decreases hepatic TG availability through enhanced expression of Pgc1α and Cpt1α, leading to a significant increase in hepatic β-oxidation. Hepatic-specific overexpression of malonyl-CoA decarboxylase, an enzyme that stimulates β-oxidation, decreases hepatic TG content, reduces plasma NEFA, prevents hyperinsulinemia, and improves whole body glucose tolerance and insulin sensitivity (42). These data indicate that prevention of the hepatic lipid load by nobiletin limits lipid availability for hepatic lipid storage, lipoprotein secretion, and lipid deposition in peripheral tissues.
The striking improvement in hepatic steatosis and prevention of adiposity in nobiletin-treated mice was associated with a significant reduction of TG in muscle. Reduced muscle TG is known to restore muscle function and insulin sensitivity (43–45). Studies in ob/ob mice treated with a PPARα agonist demonstrated improved insulin sensitivity in both liver and muscle, which was accompanied by a significant reduction of lipid accumulation in both tissues (46). This concept is supported in the current study by nobiletin-induced normalization of peripheral glucose disposal, which is primarily a reflection of increased skeletal muscle insulin sensitivity. Furthermore, nobiletin improved hepatic insulin sensitivity as evidenced by enhanced insulin-mediated suppression of HGP and gluconeogenesis; assessed in hyperinsulinemic-euglycemic clamps and pyruvate tolerance tests, respectively. Nobiletin-induced increases in hepatic FA oxidation and reduced lipogenic gene expression would be expected to improve hepatic insulin sensitivity and enhance insulin-mediated suppression of HGP. The restored insulin sensitivity and glucose disposal in peripheral tissues of nobiletin-treated mice could be a secondary consequence of reduced exposure of muscle to both VLDL-derived and plasma NEFA.
The prevention of ectopic lipid accumulation by nobiletin may be partially mediated through increased energy metabolism. Addition of nobiletin to the high-fat diet moderately stimulates whole-body energy expenditure, particularly during the dark period, and thus makes a contribution to the dramatic reductions in hepatic and peripheral tissue fat content. However, nobiletin does not appear to affect metabolic fuel preference since the respiratory quotient did not differ among dietary groups. Although cold tolerance was not measured, mean body temperatures were similar among the dietary groups (data not shown) suggesting that brown adipose tissue adaptive thermogenesis did not contribute to enhanced energy expenditure. Interestingly, attenuation of diet-induced adiposity by nobiletin does not account entirely for the prevention of dyslipidemia and hepatic lipid accumulation. Nobiletin at 0.1% had no effect on adiposity but reduced plasma concentrations of cholesterol, TG, glycerol, and NEFA to the same extent as 0.3% nobiletin. Furthermore, 0.1% nobiletin reduced hepatic TG and CE and reduced muscle lipid accumulation. However, plasma concentrations of insulin, glucose, and leptin in 0.1% nobiletin-treated mice were not different from Western-fed animals. This suggests that prevention of dyslipidemia and hepatic lipid accumulation is more sensitive to nobiletin and is independent of decreased diet-induced adiposity. The prevention of insulin resistance, glucose intolerance, and adiposity requires 0.3% nobiletin, suggesting that over 8 weeks normalization of these metabolic parameters by nobiletin is mechanistically linked.
Nobiletin increased hepatic Pgc1α and Cpt1α mRNA, leading to increased FA oxidation. However, in a luciferase reporter assay, nobiletin did not activate any PPAR, including PPARα, nor did nobiletin increase hepatic Pparα expression or liver weight, suggesting activation of FA oxidation was not mediated through classic PPARα activation. In contrast with our in vitro studies, Dgat1/2 or Mttp mRNA abundance was unaffected by any dietary treatment. This is consistent with previous studies in which Mttp expression is unchanged in hepatic IR–deficient (L1B6Ldlr−/−), Lxr−/−, and Ldlr−/− mice fed a high-fat diet (13,24,47), suggesting that neither the high-fat diet nor flavonoid supplementation affect Mttp or Dgat1/2 expression in this model.
Hyperlipoproteinemia and type 2 diabetes are thought to contribute synergistically to inflammation and atherosclerosis (48). Nobiletin-treated mice had substantially reduced lesion area within the aortic sinus, compared with Western-fed mice, which was evident as early as 8 weeks of treatment. These data suggest that nobiletin supplementation reduces the atherosclerotic disease process, primarily through prevention of dyslipidemia, hepatic steatosis, and improved insulin sensitivity. In macrophages, nobiletin has been shown to suppress proinflammatory cytokine expression (49) and reduce the uptake of acetylated LDL (50). Although not evaluated in this study, a direct effect on inflammation and foam cell formation may also contribute to the attenuation of atherosclerosis.
In conclusion, our studies provide physiological and molecular evidence that nobiletin regulates hepatic lipid metabolism to prevent many of the abnormalities associated with insulin resistance. The correction of hepatic steatosis, dyslipidemia, and glucose homeostasis by nobiletin protects against the development of atherosclerosis through a range of mechanisms. The use of nobiletin provides insight into potential targets for the treatment of abnormal lipoprotein and glucose metabolism, characteristic of insulin-resistant states, and premature atherosclerosis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Pfizer Canada (NR2580078), the Heart and Stroke Foundation of Ontario (HSFO; T-7007 and PG-5967 [to M.W.H.]), an HSFO Masters Award (to E.E.M. and J.M.A.), and a Canadian Institute of Health Research-Canada Graduate Scholarship Doctoral Award (to E.E.M.).
No potential conflicts of interest relevant to this article were reported.
E.E.M. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. J.M.A. researched data, contributed to discussion, and reviewed and edited the manuscript. J.K.L. researched data. E.M.A. and B.G.S. researched data, contributed to discussion, and reviewed and edited the manuscript. J.B.K. researched data and reviewed and edited the manuscript. C.G.S., J.Y.E., and D.E.T. researched data, contributed to discussion, and reviewed and edited the manuscript. A.C., P.S., and A.M. researched data and contributed to discussion. M.W.H. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript.
The authors acknowledge Christine Dion (Laval University, Quebec City, Quebec, Canada) for expert technical and surgical assistance and Dr. Robert Hegele (Robarts Research Institute, The University of Western Ontario, London, Ontario, Canada) for his insight and helpful suggestions in the preparation of this manuscript.
Parts of this study were presented in abstract form at the Arteriosclerosis, Thrombosis and Vascular Biology Scientific Sessions, San Francisco, California, 8–10 April 2010.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0589/-/DC1.
REFERENCES
- 1.Steiner G, Lewis GF. Hyperinsulinemia and triglyceride-rich lipoproteins. Diabetes 1996;45(Suppl. 3):S24–S26 [DOI] [PubMed] [Google Scholar]
- 2.Watts GF, Ooi EM, Chan DC. Therapeutic regulation of apoB100 metabolism in insulin resistance in vivo. Pharmacol Ther 2009;123:281–291 [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia 2003;46:733–749 [DOI] [PubMed] [Google Scholar]
- 5.Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 2008;118:2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verges B. Abnormal hepatic apolipoprotein B metabolism in type 2 diabetes. Atherosclerosis 2010;211:353–360 [DOI] [PubMed]
- 7.Thompson GR, Naoumova RP, Watts GF. Role of cholesterol in regulating apolipoprotein B secretion by the liver. J Lipid Res 1996;37:439–447 [PubMed] [Google Scholar]
- 8.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res 2005;36:232–240 [DOI] [PubMed] [Google Scholar]
- 9.Fisher SJ, Kahn CR. Insulin signaling is required for insulin’s direct and indirect action on hepatic glucose production. J Clin Invest 2003;111:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002;109:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grefhorst A, Parks EJ. Reduced insulin-mediated inhibition of VLDL secretion upon pharmacological activation of the liver X receptor in mice. J Lipid Res 2009;50:1374–1383 [DOI] [PMC free article] [PubMed]
- 12.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 2008;7:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S, Liang CP, Westerterp M, et al. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest 2009;119:1029–1041 [DOI] [PMC free article] [PubMed]
- 14.Tsai J, Qiu W, Kohen-Avramoglu R, Adeli K. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler Thromb Vasc Biol 2007;27:211–218 [DOI] [PubMed] [Google Scholar]
- 15.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O’Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab 2008;294:E969–E977 [DOI] [PubMed] [Google Scholar]
- 16.Atherton HJ, Gulston MK, Bailey NJ, et al. Metabolomics of the interaction between PPAR-alpha and age in the PPAR-alpha-null mouse. Mol Syst Biol 2009;5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98:115–124 [DOI] [PubMed] [Google Scholar]
- 18.Kurowska EM, Manthey JA. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J Agric Food Chem 2004;52:2879–2886 [DOI] [PubMed] [Google Scholar]
- 19.Kurowska EM, Spence JD, Jordan J, et al. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr 2000;72:1095–1100 [DOI] [PubMed] [Google Scholar]
- 20.Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes 2005;54:1676–1683 [DOI] [PubMed] [Google Scholar]
- 21.Allister EM, Mulvihill EE, Barrett PH, Edwards JY, Carter LP, Huff MW. Inhibition of apoB secretion from HepG2 cells by insulin is amplified by naringenin, independent of the insulin receptor. J Lipid Res 2008;49:2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borradaile NM, de Dreu LE, Huff MW. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes 2003;52:2554–2561 [DOI] [PubMed] [Google Scholar]
- 23.Kurowska EM, Manthey JA. Regulation of lipoprotein metabolism in HepG2 cells by citrus flavonoids. Adv Exp Med Biol 2002;505:173–179 [DOI] [PubMed] [Google Scholar]
- 24.Mulvihill EE, Allister EM, Sutherland BG, et al. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009;58:2198–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YS, Cha BY, Saito K, et al. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem Pharmacol 2010;79:1674–1683 [DOI] [PubMed] [Google Scholar]
- 26.Do GM, Kwon EY, Kim HJ, et al. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun 2008;374:55–59 [DOI] [PubMed] [Google Scholar]
- 27.Loke WM, Proudfoot JM, Hodgson JM, et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol 2010;30:749–757 [DOI] [PubMed] [Google Scholar]
- 28.Mulvihill EE, Assini JM, Sutherland BG, et al. Naringenin decreases progression of atherosclerosis by improving dyslipidemia in high-fat-fed low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol 2010;30:742–748 [DOI] [PubMed] [Google Scholar]
- 29.Borradaile NM, Carroll KK, Kurowska EM. Regulation of HepG2 cell apolipoprotein B metabolism by the citrus flavanones hesperetin and naringenin. Lipids 1999;34:591–598 [DOI] [PubMed] [Google Scholar]
- 30.Patel H, Truant R, Rachubinski RA, Capone JP. Activity and subcellular compartmentalization of peroxisome proliferator-activated receptor alpha are altered by the centrosome-associated protein CAP350. J Cell Sci 2005;118:175–186 [DOI] [PubMed] [Google Scholar]
- 31.Huss JM, Torra IP, Staels B, Giguère V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 2004;24:9079–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borradaile NM, de Dreu LE, Barrett PH, Behrsin CD, Huff MW. Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry 2003;42:1283–1291 [DOI] [PubMed] [Google Scholar]
- 33.Beyea MM, Heslop CL, Sawyez CG, et al. Selective up-regulation of LXR-regulated genes ABCA1, ABCG1, and APOE in macrophages through increased endogenous synthesis of 24(S),25-epoxycholesterol. J Biol Chem 2007;282:5207–5216 [DOI] [PubMed] [Google Scholar]
- 34.Siri P, Candela N, Zhang YL, et al. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity, and insulin resistance. J Biol Chem 2001;276:46064–46072 [DOI] [PubMed] [Google Scholar]
- 35.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 2006;55:390–397 [DOI] [PubMed] [Google Scholar]
- 36.Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes 2010;59:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD. Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol 1999;19:1223–1230 [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Middleton A, Chambers TC, Mehta KD. Differential roles of extracellular signal-regulated kinase-1/2 and p38(MAPK) in interleukin-1beta- and tumor necrosis factor-alpha-induced low density lipoprotein receptor expression in HepG2 cells. J Biol Chem 1998;273:15742–15748 [DOI] [PubMed] [Google Scholar]
- 39.Singh RP, Dhawan P, Golden C, Kapoor GS, Mehta KD. One-way cross-talk between p38(MAPK) and p42/44(MAPK). Inhibition of p38(MAPK) induces low density lipoprotein receptor expression through activation of the p42/44(MAPK) cascade. J Biol Chem 1999;274:19593–19600 [DOI] [PubMed] [Google Scholar]
- 40.Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol 2005;25:2170–2176 [DOI] [PubMed] [Google Scholar]
- 41.Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 2004;10:1344–1351 [DOI] [PubMed] [Google Scholar]
- 42.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10:268–274 [DOI] [PubMed] [Google Scholar]
- 43.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 1997;46:983–988 [DOI] [PubMed] [Google Scholar]
- 44.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 2006;119(Suppl. 1):S10–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen KF, Shulman GI. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14(Suppl. 1):34S–40S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ide T, Tsunoda M, Mochizuki T, Murakami K. Enhancement of insulin signaling through inhibition of tissue lipid accumulation by activation of peroxisome proliferator-activated receptor (PPAR) alpha in obese mice. Med Sci Monit 2004;10:BR388–BR395 [PubMed] [Google Scholar]
- 47.Kalaany NY, Gauthier KC, Zavacki AM, et al. LXRs regulate the balance between fat storage and oxidation. Cell Metab 2005;1:231–244 [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 2009;4:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin N, Sato T, Takayama Y, et al. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol 2003;65:2065–2071 [DOI] [PubMed] [Google Scholar]
- 50.Whitman SC, Kurowska EM, Manthey JA, Daugherty A. Nobiletin, a citrus flavonoid isolated from tangerines, selectively inhibits class A scavenger receptor-mediated metabolism of acetylated LDL by mouse macrophages. Atherosclerosis 2005;178:25–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.