Abstract
OBJECTIVE
To investigate the effect of acute insulin-induced hypoglycemia on cerebral glucose metabolism in healthy humans, measured by 13C magnetic resonance spectroscopy (MRS).
RESEARCH DESIGN AND METHODS
Hyperinsulinemic glucose clamps were performed at plasma glucose levels of 5 mmol/L (euglycemia) or 3 mmol/L (hypoglycemia) in random order in eight healthy subjects (four women) on two occasions, separated by at least 3 weeks. Enriched [1-13C]glucose 20% w/w was used for the clamps to maintain stable plasma glucose labeling. The levels of the 13C-labeled glucose metabolites glutamate C4 and C3 were measured over time in the occipital cortex during the clamp by continuous 13C MRS in a 3T magnetic resonance scanner. Time courses of glutamate C4 and C3 labeling were fitted using a one-compartment model to calculate metabolic rates in the brain.
RESULTS
Plasma glucose 13C isotopic enrichment was stable at 35.1 ± 1.8% during euglycemia and at 30.2 ± 5.5% during hypoglycemia. Hypoglycemia stimulated release of counterregulatory hormones (all P < 0.05) and tended to increase plasma lactate levels (P = 0.07). After correction for the ambient 13C enrichment values, label incorporation into glucose metabolites was virtually identical under both glycemic conditions. Calculated tricarboxylic acid cycle rates (VTCA) were 0.48 ± 0.03 μmol/g/min during euglycemia and 0.43 ± 0.08 μmol/g/min during hypoglycemia (P = 0.42).
CONCLUSIONS
These results indicate that acute moderate hypoglycemia does not affect fluxes through the main pathways of glucose metabolism in the brain of healthy nondiabetic subjects.
Hypoglycemia is a major threat for brain function because the brain depends on a continuous glucose supply as principal source of energy. Thus, glucose counterregulatory responses are usually initiated when glucose levels fall below ∼3.8 mmol/L to quickly restore euglycemia and maintain sufficient glucose delivery to the brain (1). The glucose level at which cognitive function declines is not fixed but depends on the complexity of the cognitive task and the cognitive domain that is tested. Nevertheless, although simple motor functions may be sustained despite even quite severe degrees of hypoglycemia, many aspects of cognitive performance become impaired at glucose levels between 3.1 and 3.4 mmol/L (2). During complex cognitive tasks, such as with motor vehicle driving, deterioration can already be observed at glucose levels as high as ∼3.8 mmol/L (3).
Although the importance of maintaining sufficient glucose supply to the brain has been known for long, it is still unclear how hypoglycemia affects subsequent cerebral glucose metabolism. Various studies have indicated altered cerebral glucose handling during even mild symptomatic hypoglycemia. When the brain is supplied with an alternative energy source during hypoglycemia, such as lactate, the threshold level for initiation of glucose counterregulation shifts to lower glucose levels (4) and performance on cognitive function tests is maintained better (5). In accordance, upregulation of lactate transport into the brain during hypoglycemia has been associated with glucose counterregulatory defects (6). Using 1H magnetic resonance spectroscopy (MRS), Bischof et al. (7) reported discrete effects of moderate hypoglycemia (∼3.1 mmol/L glucose) on cerebral glucose-derived metabolite levels in healthy volunteers. Finally, positron emission tomography (PET) studies with fluor-18-fluorodeoxyglucose (FDG) and [11C]-O-methyl-d-glucose (CMG) have demonstrated regional, but not global, changes in cerebral glucose metabolism based on tracer uptake in the brain during hypoglycemia in patients with diabetes (8,9). Thus, many reports suggest that human brain glucose metabolism changes under hypoglycemic conditions, but the exact changes are unclear (10). In addition, neither with 1H MRS nor with PET can the cerebral metabolic rate of glucose conversion into its metabolites be determined.
With 13C MRS, it is possible to study the dynamics of glucose metabolism in vivo in the human brain. Because the natural abundance of 13C is only 1.1%, it can be applied as a nonradioactive magnetic resonance tracer. For this purpose, often 13C enriched glucose labeled at the C-1 position is used (11). With this method, the uptake of glucose in brain tissue, as well as its conversion into several downstream metabolites, can be followed over time. To optimize the intensity of the 13C signals of these metabolites, which occur at rather low concentration, most studies applying dynamic 13C MRS to the human brain have been performed under hyperglycemic conditions. We previously developed a specific protocol that has enabled us to apply 13C MRS with infusion of 13C-labeled glucose under both euglycemic and hypoglycemic conditions in human volunteers (12). This allows for mathematical modeling and calculation of metabolic fluxes of glucose metabolism (13,14). The aim of the current study was to compare human in vivo brain glucose metabolism under euglycemic and hypoglycemic conditions using 13C MRS.
RESEARCH DESIGN AND METHODS
Eight healthy nondiabetic volunteers (four men and four women aged 23.2 ± 2.5 years with BMI 23.9 ± 4.5 kg/m2) were enrolled for this study. The study was approved by the institutional review board of the Radboud University Nijmegen Medical Centre, and all volunteers gave written informed consent before participation. All participants were examined on two occasions: once under euglycemic conditions and once under hypoglycemic conditions, scheduled in random order and separated by at least 3 weeks. Female subjects were tested at 4- or 8-week intervals to ensure that experiments took place during corresponding periods of the menstrual cycle.
Hyperinsulinemic glucose clamps.
Subjects came to the magnetic resonance research facility at 8:00 a.m. after an overnight fast and after having abstained from alcohol and caffeine-containing substances for 24 h. The brachial artery of the nondominant arm was cannulated under local anesthesia for frequent blood sampling. An intravenous catheter was inserted in the antecubital vein of the contralateral arm for administration of 13C-glucose and insulin. After a 30-min equilibration period, subjects were placed on the scanner bed in supine position with their heads positioned in the magnetic resonance coil. Arterial blood was sampled to obtain baseline variables, and reference spectra were obtained without administration of exogenous 13C-labeled material during the next 30 min. Subsequently, a hyperinsulinemic (60 mU/min/m2, equaling approximately 1.5 mU/min/kg) euglycemic (5.0 mmol/L) or hypoglycemic (3.0 mmol/L) glucose clamp was initiated with [1-13C]glucose 20% w/w, as described previously (12). Briefly, a bolus of 30 mL of 100% enriched [1-13C]glucose 20% w/w was infused over 10 min at the initiation of the clamp to rapidly increase plasma 13C enrichment. For the remainder of the experiments, variable infusions of 40 or 50% enriched [1-13C]glucose 20% w/w were infused during the euglycemic and hypoglycemic experiments, respectively, in order to maintain plasma glucose levels at predetermined target values. Blood was sampled every 5 min for immediate determination of plasma glucose by the glucose oxidation method (Beckmann Glucose Analyzer II; Beckman Coulter, Fullerton, CA) and later determination of plasma 13C isotopic enrichment of glucose and lactate by high-resolution proton nuclear magnetic resonance (1H NMR) at 500 MHz (15,16). Plasma lactate concentration was also determined from 1H NMR spectra. Every 30 min, additional blood was sampled for measurement of insulin and counterregulatory hormones as previously described (12).
MRS.
All studies were performed on a 3T magnetic resonance system (Magnetom Trio; Siemens, Erlangen, Germany) with a 1H volume coil and a circularly polarized 13C surface coil inserted into the 1H coil (17). A distortionless enhanced polarization transfer (DEPT) sequence for 1H to 13C polarization transfer combined with proton image-selected in vivo spectroscopy (1H ISIS) localization (18) was used for acquisition of 13C magnetic resonance spectra. Adiabatic 13C radiofrequency pulses were used in all sequences to ensure homogeneous excitation using the 13C surface coil, as well as wideband alternating phase low-power technique for zero residue splitting (WALTZ-16) proton decoupling (19) to simplify the spectra and enhance signal-to-noise ratio (SNR). A voxel of ∼125 mL was placed in occipital brain tissue. In all experiments, one spectrum consisted of 72 repetitions of 2 s, allowing for a time resolution of 2.5 min. Eight reference spectra were obtained before the start of [1-13C]glucose infusion, during which 13C MR spectra were acquired continuously. Visual stimulation was avoided by dimming the lights in the magnet room during the experiments.
13C MRS data processing and quantification.
The eight 13C MR reference spectra were averaged and subtracted from the dynamic spectra acquired during the 13C glucose clamp to correct for natural abundance 13C magnetic resonance signals. To enhance the SNR, the corrected spectra were added in running averages of 15 min. The resulting spectra were fitted with the advance magnetic resonance (AMARES) algorithm (20) in the java-based MR user interface (jMRUI) software package (21). The natural abundance signal of myo-inositol (mI) was used to quantify glutamate C4 and glutamate C3 in the spectra based on the premise that mI has a stable concentration of 6 μmol/g and is not labeled with 13C in the time frame of the experiment (22). The natural abundance signal of mI was quantified in jMRUI from the spectra obtained by adding all dynamic spectra before correction with reference spectra. DEPT 13C magnetic resonance spectra measured from a phantom were used to eliminate effects of the pulse sequence profile on the experimental spectra.
Metabolic modeling.
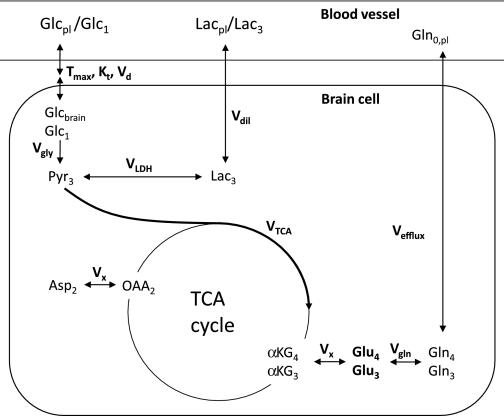
Rates of metabolic fluxes were determined using a standard one-compartment metabolic model (14,23,24) as depicted in Fig. 1. These rates (V) were assessed from the time courses of incorporation of 13C isotopes into different metabolites. Time courses of glutamate C4 and glutamate C3 concentrations and plasma values and isotopic enrichments of glucose were used as input factors for the model. In this specific situation, it was also decided to take plasma lactate values and isotopic enrichment into account as input variables for the modeling process. The model was implemented in Matlab R2008b (MathWorks, Natick, MA) and consisted of differential equations describing the inflow and outflow of label from each metabolite pool. These equations were solved numerically, and a nonlinear least-squares approach was used to fit the model to the experimental data by varying free flux parameters representing VTCA, the loss of label through exchange with unlabeled glutamine (Vefflux), and the exchange of intracellular lactate with plasma lactate (Vdil). VX, representing the exchange between α-ketoglutarate and glutamate, was assumed to be 5 μmol/g/min. This value was fixed to ensure stable fitting. In literature, there is no consensus on the value of VX; it is either determined to be much higher compared with VTCA (25) or to be in the same order of VTCA (23,26). However, for this study comparing two datasets, the choice of VX is not critical; it will influence absolute values but equally for both datasets and therefore will not compromise the comparison of the two glycemic conditions. Data of all individual volunteers were fitted separately and inspected for the goodness of fit by the cost value, a parameter that represents the difference between the measured concentrations and the corresponding estimates by the model. From these fits, flux parameters were calculated.
FIG. 1.
One-compartment model for uptake of [1-13C]glucose and its conversion into labeled metabolites. Brain glucose uptake from the blood was modeled by reversible Michaelis-Menten kinetics (40). Vgly represents the glycolysis rate and has a value of 0.5 × VTCA. The cycling of glutamate and glutamine between neurons and astroglia is approached by the parameter Vgln and assumed equal to VTCA. VTCA, Vdil, and Vefflux are the free parameters of the model, representing TCA cycle flux, exchange of plasma, and brain lactate and efflux of labeled glutamine, respectively. VLDH represents lactate dehydrogenase and was assumed to be 3 μmol/g/min; VX represents the exchange between α-ketoglutarate and glutamate over the mitochondrial membrane and was assumed to be 5 μmol/g/min. Furthermore, the following assumptions were made for total pool concentrations: [Glu]: 10 mmol/L; [Gln]: 2.5 mmol/L; [OAA]; 0.3 mmol/L; [αKG]: 0.25 mmol/L; [Asp]: 1.5 mmol/L; and [Pyr]: 0.15 mmol/L.
Statistical analysis.
All data are expressed as means ± SD unless otherwise indicated. Serial data (e.g., plasma hormone and lactate levels) were compared by repeated-measures ANOVA, and differences in means were tested by paired, two-tailed Student t tests. Graphpad Prism 4 (Graphpad, La Jolla, CA) was used for statistical analysis, and a P value < 0.05 was considered statistically significant.
RESULTS
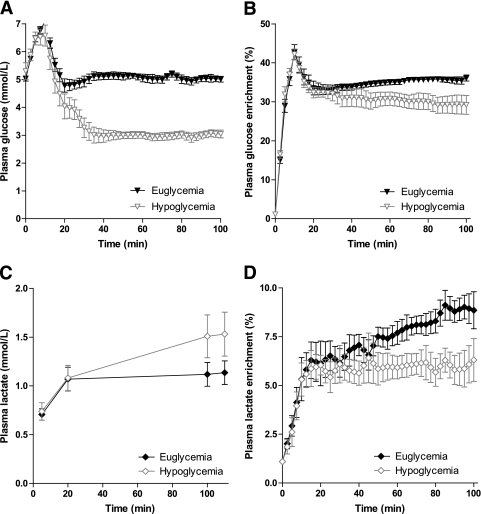
As a consequence of the [1-13C]glucose bolus, plasma glucose levels transiently increased to maximally 7.1 ± 0.4 mmol/L during the euglycemic experiment and to 6.6 ± 1.0 mmol/L during the hypoglycemic experiment. Thereafter, plasma glucose levels were allowed to fall and were maintained stable at 5.1 ± 0.3 mmol/L with a coefficient of variation (CV) of 4.1 ± 1.0% during the euglycemic clamp and at 3.0 ± 0.4 mmol/L (CV 5.9 ± 2.2%) during the hypoglycemic clamp (Fig. 2A). In response to hypoglycemia, plasma levels of glucagon, adrenaline, noradrenaline, cortisol, and growth hormone all significantly increased compared with baseline and compared with similar time points during the euglycemic clamp (Table 1). As expected, glucose infusion rates were approximately threefold lower during hypoglycemia than during euglycemia (2.2 ± 0.4 vs. 6.6 ± 0.9 μmol/kg/min; P < 0.001).
FIG. 2.
Plasma glucose concentration (A), plasma glucose 13C enrichment (B), plasma lactate concentration (C), and plasma lactate 13C enrichment (D) as a function of time during the euglycemic and hypoglycemic clamps. Data are shown as means ± SEM.
TABLE 1.
Counterregulatory hormone and insulin values during the euglycemic and hypoglycemic clamps
| Time after start of glucose infusion (min) |
P | ||||
|---|---|---|---|---|---|
| Baseline | 30 | 90 | 120 | ||
| Glucagon (pmol/L) | |||||
| Euglycemic clamp | 35.5 ± 6.9 | 30.5 ± 7.2 | 28.4 ± 7.3 | 41.4 ± 11.1 | |
| Hypoglycemic clamp | 27.6 ± 5.1 | 41.9 ± 19.8 | 53.6 ± 16.4 | 77.0 ± 41.9 | 0.0080 |
| Adrenaline (nmol/L) | |||||
| Euglycemic clamp | 0.22 ± 0.09 | 0.24 ± 0.08 | 0.42 ± 0.20 | 0.41 ± 0.12 | |
| Hypoglycemic clamp | 0.20 ± 0.10 | 0.82 ± 1.22 | 3.96 ± 2.10 | 4.84 ± 2.51 | <0.0001 |
| Noradrenaline (nmol/L) | |||||
| Euglycemic clamp | 0.91 ± 0.22 | 0.97 ± 0.21 | 1.07 ± 0.26 | 1.14 ± 0.31 | |
| Hypoglycemic clamp | 0.94 ± 0.4 | 1.15 ± 0.39 | 1.98 ± 1.06 | 1.96 ± 0.64 | 0.0316 |
| Cortisol (μmol/L) | |||||
| Euglycemic clamp | 0.44 ± 0.29 | 0.53 ± 0.13 | 0.42 ± 0.15 | 0.42 ± 0.13 | |
| Hypoglycemic clamp | 0.47 ± 0.18 | 0.47 ± 0.17 | 0.64 ± 0.33 | 0.89 ± 0.21 | 0.0048 |
| Growth hormone (mU/L) | |||||
| Euglycemic clamp | 5.91 ± 7.45 | 6.14 ± 7.84 | 14.86 ± 14.43 | 19.30 ± 18.34 | |
| Hypoglycemic clamp | 6.17 ± 7.16 | 14.44 ± 33.19 | 46.38 ± 47.96 | 74.09 ± 55.55 | 0.0462 |
| Insulin (pmol/L) | |||||
| Euglycemic clamp | 76.6 ± 44.1 | 777.8 ± 190.7 | 685.9 ± 114.9 | 694.1 ± 114.9 | |
| Hypoglycemic clamp | 63.4 ± 29.5 | 943.7 ± 548.11 | 493.7 ± 349.3 | 685.8 ± 107.1 | 0.6824 |
Data are means ± SD. Statistical tests performed with two-way ANOVA.
Isotopic enrichment of plasma glucose also peaked immediately after the bolus infusion. From t = 20 min onward, plasma 13C glucose labeling was stable during both the euglycemic and the hypoglycemic clamps, although the level was slightly lower during hypoglycemia (35.1 ± 1.8% [CV 3.6 ± 2.1] vs. 30.2 ± 5.5% [CV 6.8 ± 2.4]) (Fig. 2B).
After an initial rise, plasma lactate levels remained stable during euglycemia at 1.1 ± 0.3 mmol/L, but tended to increase further in response to hypoglycemia (from 1.1 ± 0.4 to 1.5 ± 0.6 mmol/L, P = 0.07 vs. euglycemia). Conversely, plasma lactate isotopic enrichment remained constant (after an initial increase) during hypoglycemia, whereas it increased further during euglycemia (from 6.1 ± 3.2 at t = 20 min to 8.9 ± 2.7% at t = 100 min), P < 0.0001, versus hypoglycemia (Fig. 2C and D).
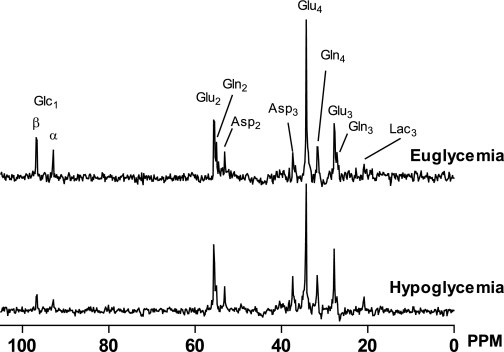
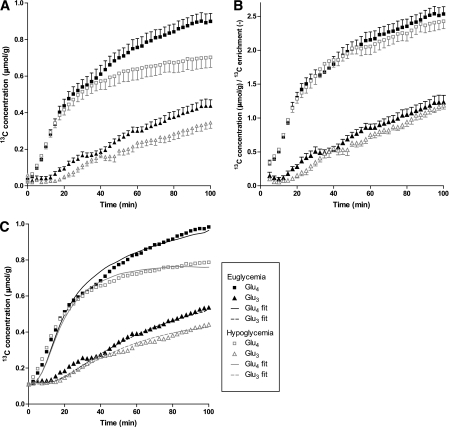
All 13C MR spectra were of similar spectral quality with sufficient SNR to analyze the signals of the metabolites of interest under both euglycemic and hypoglycemic conditions (Fig. 3). Under either condition, the 13C label was progressively incorporated into glutamate C4, C3, and C2; glutamine C4, C3, and C2; aspartate C3 and C2; and lactate C3. Figure 4A shows the time courses of glutamate C4 labeling, which appears during the first turn of the TCA cycle, and of glutamate C3 labeling, which appears during the second turn of the TCA cycle. The isotopic enrichment of the glutamate pool was lower under hypoglycemic conditions than under euglycemic conditions. However, after correction for the lower plasma glucose 13C enrichment level during hypoglycemia, the time courses for both these metabolites were virtually superimposable for the two glycemic conditions (Fig. 4B).
FIG. 3.
Representative 13C magnetic resonance spectra of the human brain measured at the end of a euglycemic experiment and a hypoglycemic experiment. PPM, parts per million.
FIG. 4.
A: Time courses of glutamate C4 and C3 labeling in brain tissue during euglycemic and hypoglycemic clamps. B: Time courses of glutamate C4 and C3 labeling in brain tissue corrected by plasma glucose 13C enrichment. C: Averaged time courses of glutamate C4 and C3 labeling in brain tissue with natural abundance signal added for modeling purposes together with averaged best fits of individual datasets.
The time courses of 13C label incorporation into glutamate C4 and C3 were fitted with a one-compartment metabolic model (Fig. 1) to compare metabolic fluxes under euglycemic and hypoglycemic conditions. The average of individually calculated TCA cycle rates (VTCA) under hypoglycemic clamp conditions was similar to the average values obtained under euglycemic conditions (Table 2). When the TCA cycle rates were calculated from averaged datasets (Fig. 4C; individual datasets of all participants can be found in Supplementary Fig. 1), similar values were obtained (0.48 and 0.43 μmol/g/min for euglycemia and hypoglycemia, respectively). There were also no differences between the two glycemic conditions with regard to other flux parameters. The absence of an effect of hypoglycemia on metabolic fluxes was similar among men and women in the study (data not shown).
TABLE 2.
Metabolic flux values during euglycemia and hypoglycemia
| Euglycemia | Hypoglycemia | P* | |
|---|---|---|---|
| VTCA (μmol/g/min) | 0.48 ± 0.03 | 0.43 ± 0.08 | 0.42 |
| Vdil (μmol/g/min) | 0.61 ± 0.25 | 0.61 ± 0.39 | 0.89 |
| Vefflux (μmol/g/min) | 0.41 ± 0.26 | 0.41 ± 0.20 | 0.83 |
Data are means ± SD.
*Student t test.
DISCUSSION
In this study, we used 13C MRS to investigate the effect of moderate hypoglycemia on glucose handling by the human brain in vivo. Despite the fact that hypoglycemia considerably stimulated glucose counterregulation, which is under control of the central nervous system, metabolism of glucose in the occipital tissue of the brain was remarkably similar under hypoglycemic and euglycemic conditions. Indeed, the time courses of 13C labeling of both glutamate C4 and glutamate C3 during hypoglycemia and during euglycemia were superimposable, when corrected for plasma isotopic enrichment, and the rate of TCA cycle flux was ~0.5 μmol/g/min on both occasions. These calculated fluxes are in line with previously reported values for VTCA under hyperglycemic conditions (27–29).
This is the first study to investigate and quantify cerebral glucose metabolism under hypoglycemic conditions in humans using 13C MRS. The lack of a difference in cerebral glucose metabolism between hypo- and euglycemia contrasts with a 1H MRS study, which suggested slowing of the TCA cycle rate during hypoglycemia based on a reduction of the glutamate-to-creatine ratio (7). However, snapshot 1H magnetic resonance spectra provide steady-state levels of metabolites and cannot be used to quantify cerebral glucose metabolic fluxes, as is possible with dynamic 13C MRS. Moreover, our findings are in line with a 13C MRS study in rats (30) in which overall cerebral metabolism in control animals was not affected by hypoglycemia.
Mathematical modeling of the data was performed to quantify and compare metabolic processes under the two glycemic conditions. Our model showed that the rates for free flux parameters (VTCA, Vdil, and Vefflux) were about similar during hypoglycemia and euglycemia. The VTCA values were within the published range (27–29), but Vdil and Vefflux were higher than previously reported (31,32). Because several assumptions for Vgln, VLDH, and VX had to be made to derive the rates for free flux parameters, these data need to be interpreted with caution. There is ongoing debate as to which estimated values are appropriate. In the literature, for VX values below 1 and as high as 57 μmol/g/min have been used (23,25). In the current study, the data were fitted assuming VX = 5 μmol/g/min to optimize the fitting. Refitting the data with VX = 1 μmol/g/min resulted in slightly lower values for VTCA of 0.43 μmol/g/min during euglycemia and 0.38 μmol/g/min during hypoglycemia, but the difference between the two groups remained nonsignificant. We cannot completely exclude the possibility that Vgln, VLDH, and VX are modulated by the level of glycemia itself, but there are no data available to support such a notion. Thus, identical assumptions for the fixed model parameters were used for both glycemic conditions.
There are several potential mechanisms that could maintain normal cerebral glucose metabolism during hypoglycemia. A plausible explanation would be a compensatory increase in cerebral uptake of lactate. In agreement with previous observations (33), plasma lactate increased by approximately 50% in response to hypoglycemia. Lactate can be used by the brain as an alternative energy source and may reduce the cerebral need for glucose (34–36). After conversion to pyruvate, lactate carbons may enter the TCA cycle as the carbons of glucose do (37). A study by Mason et al. (6) has indicated that brain transporter activity for monocarboxylic substrates such as lactate can be increased twofold during hypoglycemia, which would support the hypothesis of increased lactate consumption during hypoglycemia. Moreover, even under resting conditions, increases in lactate availability stimulate consumption of lactate by the brain at the cost of reductions in glucose utilization (34). Our model does not include net lactate uptake because this flux is small and can be neglected under physiological conditions. However, it is possible that the increased plasma lactate level during hypoglycemia could result in increased net lactate uptake in the brain. Therefore, to assess the potential contribution of net lactate uptake, we performed additional modeling incorporating net lactate uptake (0.2 μmol/g/min, estimated from lactate Michaelis-Menten kinetics through the blood-brain barrier) into the model during hypoglycemia and found that it did not affect the values of VTCA significantly (<5% change). Thus, this is unlikely to explain our findings.
Alternatively, we cannot exclude the entrance of unlabeled carbons into the TCA cycle via glycogen breakdown as was suggested by an in vivo study by Oz et al. (38). Breakdown of glycogen located in astrocytes can then provide neurons with lactate to maintain energy metabolism (39).
This study provides no evidence for a link between a decline in cognitive function and moderate hypoglycemia. As 13C MRS of the brain was usually performed in a limited area of the occipital cortex, we cannot exclude the possibility that regional variation in cerebral glucose metabolism occurs. It is also possible that increased cortical activation in response to hypoglycemia stimulated glucose uptake so that any fall in glucose metabolism was sufficiently compensated for. PET studies have demonstrated increased cortical activation during hypoglycemia (∼2.6 mmol/L) in various regions of the brain in diabetic men (8,9) but not in the occipital cortex, where reduced rather than increased activation was observed.
It is possible that the relatively mild hypoglycemic condition imposed during this study was only strong enough to stimulate hormonal counterregulation but that deeper hypoglycemia is required to induce impairments in cerebral glucose metabolism. However, a clamp at much lower glucose levels may be hard to achieve while maintaining stable glucose infusion at a sufficient rate, which is required for administration of the 13C isotope. In addition, more severe hypoglycemia would expose participants to significantly more discomfort and risk.
In conclusion, our results indicate that acute moderate hypoglycemia does not affect cerebral glucose metabolism in healthy human volunteers. Thus, the healthy human brain appears sufficiently resilient to withstand moderate drops in plasma glucose, potentially as a consequence of increased lactate availability. Our study provides new insights into mechanisms that protect the brain from substrate deprivation, which may have important clinical implications if this finding can be reproduced in patients with type 1 diabetes, especially those suffering from repeated hypoglycemic episodes.
ACKNOWLEDGMENTS
This work was financially supported by the Dutch Diabetes Research Foundation (grant 2004.00.012), the National Institutes of Health (grants DK-069881, P41-RR-08079, and R01-NS-38672), the framework of the Center for Translational Molecular Medicine (www.ctmm.nl), project PREDICCt grant 01C-104, and the Netherlands Heart Foundation and Dutch Kidney Foundation.
No potential conflicts of interest relevant to this article were reported.
K.C.C.v.d.V. analyzed data, drafted the manuscript, contributed to interpreting data and to editing the content, and approved the final version of the paper. B.E.d.G. analyzed data, drafted the manuscript, designed the study, contributed to interpreting data and to editing the content, and approved the final version of the paper. M.v.d.G. analyzed data, designed the study, contributed to interpreting data and to editing the content, and approved the final version of the paper. A.A.S. and P.-G.H. contributed to interpreting data and to editing the content and approved the final version of the paper. C.J.J.T. and A.H. designed the study, contributed to interpreting data and to editing the content, and approved the final version of the paper.
The authors are indebted to Karin Saini, Radboud University Nijmegen Medical Centre, for assistance during the hyperinsulinemic clamps and to Dennis Klomp, Radboud University Nijmegen Medical Centre, for sequence implementation, coil development, and further technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1592/-/DC1.
REFERENCES
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friers BM, Fisher BM. (Eds.). Hypoglycemia in Clinical Diabetes. New York, Wiley, 1999 [Google Scholar]
- 3.Cox DJ, Gonder-Frederick LA, Kovatchev BP, Julian DM, Clarke WL. Progressive hypoglycemia’s impact on driving simulation performance: occurrence, awareness and correction. Diabetes Care 2000;23:163–170 [DOI] [PubMed] [Google Scholar]
- 4.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes 1993;42:1233–1237 [DOI] [PubMed] [Google Scholar]
- 5.Maran A, Crepaldi C, Trupiani S, et al. Brain function rescue effect of lactate following hypoglycaemia is not an adaptation process in both normal and type I diabetic subjects. Diabetologia 2000;43:733–741 [DOI] [PubMed] [Google Scholar]
- 6.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 2006;55:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof MG, Brehm A, Bernroider E, et al. Cerebral glutamate metabolism during hypoglycaemia in healthy and type 1 diabetic humans. Eur J Clin Invest 2006;36:164–169 [DOI] [PubMed] [Google Scholar]
- 8.Bingham EM, Dunn JT, Smith D, et al. Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-D-glucose PET study. Diabetologia 2005;48:2080–2089 [DOI] [PubMed] [Google Scholar]
- 9.Cranston I, Reed LJ, Marsden PK, Amiel SA. Changes in regional brain (18)F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes 2001;50:2329–2336 [DOI] [PubMed] [Google Scholar]
- 10.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes 2010;59:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed 2003;16:339–357 [DOI] [PubMed] [Google Scholar]
- 12.van de Ven KC, van der Graaf M, Tack CJ, Klomp DW, Heerschap A, de Galan BE. Optimized [1-(13)C]glucose infusion protocol for 13C magnetic resonance spectroscopy at 3T of human brain glucose metabolism under euglycemic and hypoglycemic conditions. J Neurosci Methods 2010;186:68–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason GF, Rothman DL. Basic principles of metabolic modeling of NMR (13)C isotopic turnover to determine rates of brain metabolism in vivo. Metab Eng 2004;6:75–84 [DOI] [PubMed] [Google Scholar]
- 14.Henry PG, Adriany G, Deelchand D, et al. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn Reson Imaging 2006;24:527–539 [DOI] [PubMed] [Google Scholar]
- 15.Serlie MJ, de Haan JH, Tack CJ, et al. Glycogen synthesis in human gastrocnemius muscle is not representative of whole-body muscle glycogen synthesis. Diabetes 2005;54:1277–1282 [DOI] [PubMed] [Google Scholar]
- 16.Van Den Bergh AJ, Tack CJ, Van Den Boogert HJ, Vervoort G, Smits P, Heerschap A. Assessment of human muscle glycogen synthesis and total glucose content by in vivo 13C MRS. Eur J Clin Invest 2000;30:122–128 [DOI] [PubMed] [Google Scholar]
- 17.Klomp DW, Renema WK, van der Graaf M, de Galan BE, Kentgens AP, Heerschap A. Sensitivity-enhanced 13C MR spectroscopy of the human brain at 3 Tesla. Magn Reson Med 2006;55:271–278 [DOI] [PubMed] [Google Scholar]
- 18.Klomp DW, Kentgens AP, Heerschap A. Polarization transfer for sensitivity-enhanced MRS using a single radio frequency transmit channel. NMR Biomed 2008;21:444–452 [DOI] [PubMed] [Google Scholar]
- 19.Shaka AJ, Keeler J, Frenkiel T, Freeman R. An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson 1983;52:335–338 [Google Scholar]
- 20.Vanhamme L, van den Boogaart A, Van Huffel S, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35–43 [DOI] [PubMed] [Google Scholar]
- 21.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001;12:141–152 [DOI] [PubMed] [Google Scholar]
- 22.Ross B, Lin A, Harris K, Bhattacharya P, Schweinsburg B. Clinical experience with 13C MRS in vivo. NMR Biomed 2003;16:358–369 [DOI] [PubMed] [Google Scholar]
- 23.Henry PG, Lebon V, Vaufrey F, Brouillet E, Hantraye P, Bloch G. Decreased TCA cycle rate in the rat brain after acute 3-NP treatment measured by in vivo 1H-[13C] NMR spectroscopy. J Neurochem 2002;82:857–866 [DOI] [PubMed] [Google Scholar]
- 24.Henry PG, Criego AB, Kumar A, Seaquist ER. Measurement of cerebral oxidative glucose consumption in patients with type 1 diabetes mellitus and hypoglycemia unawareness using (13)C nuclear magnetic resonance spectroscopy. Metabolism 2010;59:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab 1995;15:12–25 [DOI] [PubMed] [Google Scholar]
- 26.Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab 2002;22:1343–1351 [DOI] [PubMed] [Google Scholar]
- 27.Mason GF, Falk Petersen K, de Graaf RA, et al. A comparison of (13)C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-(13)C]glucose. Brain Res Brain Res Protoc 2003;10:181–190 [DOI] [PubMed] [Google Scholar]
- 28.Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab 2001;281:E100–E112 [DOI] [PubMed] [Google Scholar]
- 29.Boumezbeur F, Mason GF, de Graaf RA, et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab 2010;30:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Herzog RI, Mason GF, et al. Recurrent antecedent hypoglycemia alters neuronal oxidative metabolism in vivo. Diabetes 2009;58:1266–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan JW, Stein DT, Telang F, et al. Spectroscopic imaging of glutamate C4 turnover in human brain. Magn Reson Med 2000;44:673–679 [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Petersen KF, Behar KL, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA 1999;96:8235–8240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abi-Saab WM, Maggs DG, Jones T, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab 2002;22:271–279 [DOI] [PubMed] [Google Scholar]
- 34.van Hall G, Strømstad M, Rasmussen P, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 2009;29:1121–1129 [DOI] [PubMed] [Google Scholar]
- 35.Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab 2003;23:658–664 [DOI] [PubMed] [Google Scholar]
- 36.Boumezbeur F, Petersen KF, Cline GW, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 2010;30:13983–13991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher CN, Carpenter KL, Grice P, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 2009;132:2839–2849 [DOI] [PubMed] [Google Scholar]
- 38.Oz G, Kumar A, Rao JP, et al. Human brain glycogen metabolism during and after hypoglycemia. Diabetes 2009;58:1978–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia 2007;55:1263–1271 [DOI] [PubMed] [Google Scholar]
- 40.Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem 1998;70:397–408 [DOI] [PubMed] [Google Scholar]