Abstract
OBJECTIVE
Adiponectin is an adipocyte-derived hormone that sensitizes insulin and improves energy metabolism in tissues. This study was designed to investigate the direct regulatory effects of adiponectin on lipid metabolism in adipocytes.
RESEARCH DESIGN AND METHODS
Basal and hormone-stimulated lipolysis were comparatively analyzed using white adipose tissues or primary adipocytes from adiponectin gene knockout and control mice. To further study the underlying mechanisms through which adiponectin suppresses lipolysis, cultured 3T3-L1 adipocytes and adenovirus-mediated gene transduction were used.
RESULTS
Significantly increased lipolysis was observed in both adiponectin gene knockout mice and primary adipocytes from these mice. Hormone-stimulated glycerol release was inhibited in adiponectin-treated adipocytes. Adiponectin suppressed hormone-sensitive lipase activation without altering adipose triglyceride lipase and CGI-58 expression in adipocytes. Moreover, adiponectin reduced protein levels of the type 2 regulatory subunit RIIα of protein kinase A by reducing its protein stability. Ectopic expression of RIIα abolished the inhibitory effects of adiponectin on lipolysis in adipocytes.
CONCLUSIONS
This study demonstrates that adiponectin inhibits lipolysis in adipocytes and reveals a novel function of adiponectin in lipid metabolism in adipocytes.
White adipose tissue (WAT) mass is mainly determined by adipocyte number and size. It is logical to assume that increase of adipocyte number and size expands adipose tissue mass and results in obesity. However, a study demonstrated that adipocyte populations are determined during the first 2 decades of life and are stable in adults (1), suggesting that lipid storage should play a key role in adult obesity. Lipid storage is dynamic and mainly controlled by two opposing processes: lipogenesis and lipolysis.
Adiponectin is an adipocyte-secreted hormone that enhances insulin sensitivity. Adiponectin gene expression and blood levels are decreased in obese adults and animal models (2). Adiponectin transgenic female FVB mice exhibit increased body weight and fat tissue mass (3). Furthermore, modestly increasing serum adiponectin completely rescued the diabetic phenotype in ob/ob mice with significant expansion of adipose tissue (4). These studies suggest that adiponectin may regulate lipid metabolism in adipocytes through a direct or indirect mechanism.
We describe a novel function of adiponectin in lipid metabolism in adipocytes. Our results show that adiponectin inhibits lipolysis by suppressing protein kinase A (PKA)–mediated hormone-sensitive lipase (HSL) activation. In addition, adiponectin reduces the PKA RIIα regulatory subunit protein level by increasing its protein degradation in adipocytes.
RESEARCH DESIGN AND METHODS
See detailed information on materials, virus construction, quantitative PCR, and adipocyte area measurement in the Supplementary Methods.
Experimental animals.
Adipoq−/− mice were created as previously described (5) on the 129/SvEv genetic background and had been back-crossed to C57BL/6 for five generations. Male mice were used. The experiments using mouse models were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval of the Animal Care and Use Committee. For some animal studies, 1 × 109 pfu of purified adenovirus vectors was injected into the mouse tail vein (6).
Cell culture.
3T3-L1 and 3T3-L1CARΔ1 cells and induction of adipocyte differentiation were described in a previous publication (7). OP9 adipocyte differentiation was induced using KnockOut SR (8). Although both 3T3-L1 and OP9 adipocytes secrete adiponectin into the medium, adiponectin in overnight cultured medium accumulated only to ∼0.1% of mouse serum adiponectin. Therefore, co-culture or conditioned medium was used to increase adiponectin protein levels. For co-culture, adipocytes were cultured in the outside well of the BD Biosciences (Franklin Lakes, NJ) transwell co-culture system, and FAO cells were grown on the protein and cytokine-permeable membrane of the insert well. FAO cells were transduced with adenovirus encoding mouse adiponectin (Ad-Acrp30) or green fluorescent protein (GFP) for 12 h in a regular 6-well plate, and then the inserts with transduced FAO cells were washed and transferred to the co-culture plates with differentiated adipocytes in the bottom wells. This system allows increase of medium adiponectin levels (∼40% of mouse serum adiponectin) without physical interaction between 3T3-L1 adipocytes and FAO cells. In a separate setting, conditioned medium from Ad-Acrp30–transduced FAO cells was collected and used for increasing adiponectin levels in some of the studies, as indicated in figure legends. For hormone-stimulated lipolysis, Bt2-cAMP (100 μmol/L) was added to culture medium and cells were incubated for 1 h. Total protein was used for certain protein or protein phosphorylation measurements using Western blots. For PKA RIIα protein half-life measurement, the adipocytes were pretreated with adiponectin overnight. Then, cycloheximide (100 μg/mL) was used to inhibit protein synthesis.
Lipolysis assay.
For the in vivo lipolysis assays, the selective β3-adrenergic agonist BRL37344 was injected i.p. into mice at 5 μg/g body wt (9). Blood samples were collected 0, 10, and 20 min after the injection. For the lipolysis assay of adipose explants, ∼20 mg of epididymal adipose tissue explants were cultured in Dulbecco’s modified Eagle’s medium with 0.5% fatty acid–free BSA (10). BRL37344 was added to the medium at 50 ng/mL. The culture tubes were shaken at 400 rpm. The medium samples were collected 30, 60, 120, and 180 min after adding BRL37344. The level of free fatty acid (FFA) or glycerol was normalized to the weight of adipose explants. For primary adipocyte lipolysis assays, adipocytes were prepared from epididymal fat using collagenase with an optimized procedure (11,12). Briefly, epididymal fat tissue was minced and digested in a Krebs-Ringer bicarbonate (KRB) buffer (pH 7.4) supplemented with 3% fatty acid–free BSA fraction V, 0.5 mmol/L adenosine, and 1 mg/mL type I collagenase. After 40-min digestion and three washes, isolated adipocytes were resuspended in KRB supplemented with 5 mmol/L glucose and 3% fatty acid–free BSA. Suspended adipocytes (∼6,000 cells) were used for a lipolysis assay in 400 μL KRB buffer plus 3% fatty acid–free BSA, 1 unit/mL adenosine deaminase, and 100 nmol/L (-)-N6-(2-phenyl-isopropyl)-adenosine (basal only) with or without BRL37344 (50 ng/mL). After 1-h incubation at 37°C, 200 μL of infranatant was removed and stored at −20°C. Bt2-cAMP (100 mmol/L) was used for stimulating 3T3-L1 adipocyte lipolysis. Serum free glycerol and glycerol released from fat explants were measured using a free-glycerol kit (Sigma-Aldrich Co., St. Louis, MO). Glycerol in the assay media of primary and 3T3-L1 adipocytes was measured using bioluminescence, which is very sensitive and especially well adapted when only small amounts of adipose tissue or cells are available (13,14).
Statistical analysis.
Data are expressed as mean ± SEM. Statistical analysis was performed using the Student t test or ANOVA, followed by contrast test. Differences were considered significant at P < 0.05.
RESULTS
Adiponectin knockout male mice have small fat mass and are resistant to high-fat diet–induced obesity.
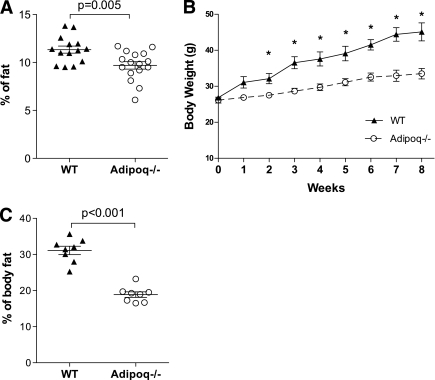
To study the effects of adiponectin on lipid metabolism in adipocytes, Adipoq−/− mice were used. Consistent with previous studies (5,15), there were no significant differences of body weights between Adipoq−/− and wild-type (WT) littermate controls when the mice were fed normal chow (data not shown). However, body fat percentage of Adipoq−/− mice was significantly lower than that of WT mice (Fig. 1A). Epididymal fat mass was also significantly smaller in Adipoq−/− male mice (0.26 ± 0.02 vs. 0.42 ± 0.04 g; vs. WT, P < 0.05, n = 10). Adipocyte cross-sectional areas of fixed epididymal fat of Adipoq−/− mice were 20% smaller than those of WT control mice (Supplementary Fig. 1A). When mice were fed a 60% (calorie) high-fat diet for 8 weeks, Adipoq−/− mice gained remarkably less body weight and adipose tissue mass and had smaller adipocyte area (Fig. 1B and C, Supplementary Fig. 1B). Similar to previous studies (5,16), high-fat diet induced more severe insulin resistance in Adipoq−/− mice (data not shown). Therefore, these studies clearly show that Adipoq−/− mice have less fat mass and are resistant to high-fat diet–induced obesity. Similar phenotypes have been reported by a group using an independent line of Adipoq−/− mice (17). Together, these data suggest that adiponectin may regulate lipid metabolism in adipocytes.
FIG. 1.
Adiponectin knockout mice have less body fat and are resistant to high-fat diet–induced obesity. Body composition of chow-fed 2-month-old male Adipoq−/− and WT mice was scanned using EchoMRI (A). Then the mice were fed a high-fat diet (60% of calories from fat). Body weight was measured weekly (B). Body fat was measured after 8 weeks of high-fat feeding (C). n = 8, *P < 0.05 vs. high-fat diet–fed Adipoq−/− male mice. Data are expressed as means ± SEM.
Triglyceride synthesis and breakdown determine the volume of lipid droplets and size of adipocytes. Our study shows that neither adiponectin gene knockout nor overexpression has any significant effect on gene expression of the key enzymes of triglyceride synthesis in epididymal fat (Supplementary Fig. 2), which suggests that adiponectin may regulate lipid metabolism in adipocytes through a pathway other than de novo lipogenesis.
Adiponectin inhibits lipolysis in mice and cultured adipocytes.
Previous animal studies have clearly demonstrated that adiponectin reduces circulating FFAs (16,18–20). Although serum FFA levels at the fed state were similar between chow-fed Adipoq−/− and WT control mice, prolonged fasting-induced increase of FFA was significantly higher in Adipoq−/− mice (Supplementary Fig. 3A). In addition, high-fat diet further increased fasting blood FFA in Adipoq−/− mice (Supplementary Fig. 3B). These results imply that adiponectin might suppress FFA release and prompted us to study the effects of adiponectin on lipolysis in adipocytes.
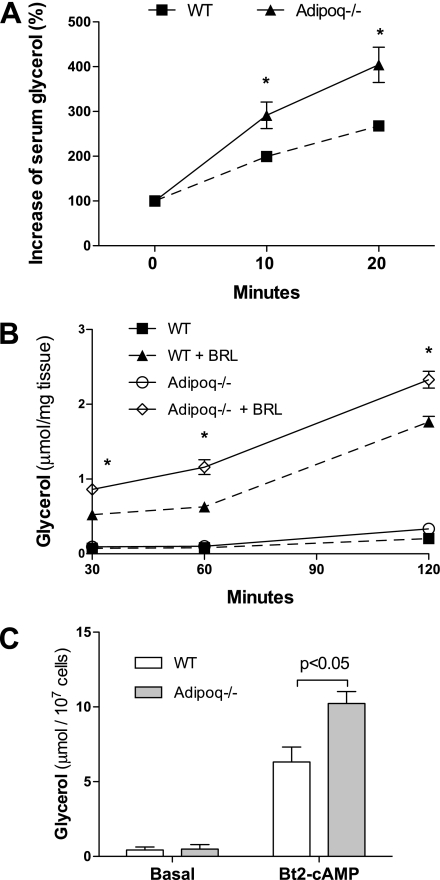
Initially, we used the selective β3-adrenergic receptor agonist BRL37344 and Adipoq−/− mice to carry out an in vivo lipolysis study, in which increases of serum free glycerol were measured to represent hormone-stimulated lipolysis. As expected, BRL37344 treatment robustly increased serum free glycerol and FFA levels in all mice. The magnitudes of increase of serum glycerol in Adipoq−/− mice were significantly higher than those in WT mice 10 or 20 min after BRL37344 injection (P < 0.05, Fig. 2A). Second, to avoid influence from other systems or hormones that may be rapidly altered by BRL37344 administration, in vitro lipolysis assays were conducted using epididymal adipose explants. Results showed that there were no significant differences in glycerol release between adipose explants from Adipoq−/− and WT control in a basal condition (Fig. 2B). Consistent with the in vivo lipolysis study, BRL37344-stimulated glycerol releases were remarkably higher in adipose explants from Adipoq−/− mice compared with WT control mice (P < 0.01, Fig. 2B). In the third study, lipolysis was assayed in primary mature adipocytes from Adipoq−/− and WT control mice. As shown in Fig. 2C, basal medium free glycerol levels were similar between adipocytes from WT control and Adipoq−/− mice. However, in Bt2-cAMP–treated cells, free glycerol levels in the medium of Adipoq−/− adipocytes were twofold higher than those of adipocytes from WT mice (Fig. 2C). To further verify the effects of adiponectin on lipolysis, mouse embryonic fibroblast-derived adipocytes from Adipoq−/− mice were treated with or without adiponectin using a transwell co-culture system. Results showed that cAMP-stimulated release of glycerol from adiponectin-treated adipocytes was significantly less than from control adipocytes (Supplementary Fig. 3C). Together, these studies demonstrate that adiponectin inhibits hormone-stimulated lipolysis in adipocytes.
FIG. 2.
Effects of adiponectin on hormone-stimulated lipolysis in fat and adipocytes. A: β3-Adrenergic receptor agonist BRL37344 was injected i.p. into 8-week-old male mice, which had been fasted overnight. Serum free glycerol was measured at the indicated times before and after the injection. Basal glycerol levels were normalized for the hormone-stimulated increment calculation. n = 6, *P < 0.05 vs. WT at the same time point. B: Epididymal adipose explants were incubated in Dulbecco’s modified Eagle’s medium with or without BRL37344 stimulation. Free glycerol in medium was measured. n = 8, *P < 0.05 vs. WT adipose explants with BRL37344 stimulation. C: Primary adipocytes were prepared from epididymal fat of Adipoq−/− or WT control mice. Adipocytes were treated with or without Bt2-cAMP (100 μmol/L) for 1 h. Glycerol levels in medium were measured. n = 4. Data are presented as mean ± SEM.
Adiponectin suppresses HSL activation in adipocytes.
Lipolysis is precisely regulated by hormones, such as glucagon and insulin. However, there was no significant difference in serum glucagon and insulin concentrations between chow-fed Adipoq−/− and WT mice (data not shown), which prompted us to look at the expression and activation state of the main lipases in WAT of Adipoq−/− mice.
HSL and adipose triglyceride lipase (ATGL) are two major lipases in adipocytes (21,22). CGI-58 acts as an ATGL coactivator for lipolysis (23). We examined the expression and activation status of these lipolytic proteins in epididymal adipose tissues from mice with adiponectin gene deletion or overexpression and from cultured adipocytes. For some animal studies, Ad-Acrp30 was used to reconstitute serum adiponectin in Adipoq−/− mice or to elevate serum adiponectin in C57BL/6 mice. As we previously reported (18), 3 days after virus injection, total serum adiponectin protein levels were increased ∼12-fold in C57BL/6 mice, whereas fasting FFA levels were significantly reduced (Supplementary Fig. 4A and B). Serum multimeric adiponectin levels were proportionally elevated, and there were no significant changes in insulin sensitivity, as measured by an insulin challenge test (data not shown) (18).
As shown in Fig. 3A, there were no significant differences of total protein levels of HSL, ATGL, or CGI-58 in epididymal fat from Adipoq−/−, adiponectin-overexpressing, or adiponectin-reconstituted mice compared with their corresponding controls. In addition, adiponectin treatment did not alter protein levels of HSL, ATGL, or CGI-58 in 3T3-L1 adipocytes (Fig. 3B). These results suggest that adiponectin-inhibited lipolysis is not through altering HSL, ATGL, or CGI-58 protein levels in adipocytes.
FIG. 3.
Adiponectin inhibits HSL phosphorylation at Ser660. A: Protein samples were prepared from epididymal fat of mice with indicated genotypes or treatment. Relative protein levels of HSL, ATGL, and CGI-58, and serine phosphorylation levels of HSL were measured by Western blot using specific antibodies. For adiponectin overexpression or reconstitution, Ad-Acrp30 or Ad-GFP adenovirus was injected into indicated mice through the tail vein. Adipose tissues were collected 3 days after injection. n = 8. B: Mature 3T3-L1 adipocytes were co-cultured overnight with FAO cells transduced with Ad-Acrp30 or Ad-GFP. The insert wells with FAO cells were removed, and adipocytes were treated with Bt2-cAMP for 1 h. n = 6. C: Differentiated 3T3-L1 adipocytes were treated with CC (10 μmol/L) and co-cultured with Ad-Acrp30 or Ad-GFP–transduced FAO cells overnight. Adipocytes were stimulated with Bt2-cAMP for 1 h. n = 4. D: Perilipin A protein levels in epididymal fat from Adipoq−/− and WT mice were measured using Western blotting. n = 4. E: For measuring PKA-phosphorylated perilipin A, perilipin A proteins were immunoprecipitated and then probed with an antibody against phospho-PKA substrate. n = 6. Data are expressed as means ± SEM. F: After overnight co-culture with adiponectin secreting FAO cells and wortmannin (200 nmol/L), 3T3-L1 adipocytes were stimulated with Bt2-cAMP and insulin (25 nmol/L) for 1 h. Glycerol in medium was measured. n = 4. Data are expressed as means ± SEM. *P < 0.05 vs. Bt2-cAMP–treated cells.
HSL is the first lipase that was found to respond to lipolytic hormones and hydrolyze triglycerides in adipocytes. PKA plays an essential role in mediating hormone-stimulated HSL activation and lipolysis in adipocytes. PKA directly phosphorylates several serine residues in HSL, including Ser563 and Ser660. However, only Ser660 phosphorylation activates HSL, whereas Ser563 phosphorylation does not correlate with HSL activation (24). By using phospho-HSL–specific antibodies, our study showed that the levels of HSL Ser660 phosphorylation in epididymal fat from Adipoq−/− mice were strikingly elevated compared with those in WT control mice (P < 0.001, Fig. 3A, bottom quantified graphs). In contrast, phosphorylation of HSL Ser660 was significantly suppressed in epididymal fat of the mice with adenovirus-mediated adiponectin overexpression and adiponectin reconstitution (P < 0.05, Fig. 3A), whereas a significantly reduced BRL-stimulated glycerol release was observed in epididymal adipose explants of mice with adiponectin overexpression (Supplementary Fig. 5A). In addition, significantly increased HSL Ser660 phosphorylation was observed in the epididymal fat of Adipoq−/− mice fed a high-fat diet (Supplementary Fig. 5B). These data indicate that HSL activity in WAT is increased in Adipoq−/− mice and suppressed in mice with elevated adiponectin. By using 3T3-L1 adipocytes and co-culture system, our study further revealed that overnight adiponectin treatment significantly decreased both basal and cAMP-stimulated HSL Ser660 phosphorylation (P < 0.05, Fig. 3B). Together, these results demonstrate that adiponectin suppresses HSL activation by reducing Ser660 phosphorylation. However, further studies are required to directly verify this.
It has been reported that Ser565 in HSL can be phosphorylated by AMP-activated protein kinase (AMPK), resulting in deactivation of HSL (21,25). Adiponectin activates AMPK in several tissues (2). We postulated that adiponectin might increase HSL Ser565 phosphorylation via AMPK to inhibit HSL. To our surprise, HSL Ser565 phosphorylation levels neither decreased in Adipoq−/− mice nor increased in adiponectin-overexpressing or -reconstituted mice compared with their controls (Fig. 3A). These results exclude the possibility that adiponectin inhibits HSL activation by increasing HSL Ser565 phosphorylation. To further determine the role of AMPK in adiponectin-suppressed lipolysis, selective AMPK inhibitor Compound C (CC) was used. Bt2-cAMP–induced glycerol release was significantly higher in CC-treated 3T3-L1 adipocytes (Fig. 3C). However, CC treatment did not abolish the inhibitory effects of adiponectin on glycerol release (Fig. 3C). Similar results were obtained in 3T3-L1CARΔ1 adipocytes transduced with adenovirus encoding dominant negative AMPKα (data did shown). These results do not support any key role of AMPK in adiponectin-suppressed lipolysis. However, our study does not completely exclude the involvement of AMPK in adiponectin-suppressed lipolysis. Apparently, the underlying mechanisms through which AMPK modulates lipolysis are far more complicated than a single serine site phosphorylation in HSL. Studies have shown that PKA or lipolysis itself induces AMPK activation in adipocytes (26,27). AMPK activation is required for maximal activation of lipolysis (26). Furthermore, a recent study reported that AMPK not only suppresses but also increases lipolysis and that the effects are dependent on duration of AMPK activation (28).
Perilipin A is a lipid droplet-binding protein that plays an important role in lipolysis in adipocytes (29,30). Upon lipolytic hormone stimulation, PKA-phosphorylated perilipin A recruits HSL to the lipid droplet surface (31). Our results showed that perilipin A protein levels in epididymal fat of Adipoq−/− mice were similar to those of WT control mice (Fig. 3D). There was slightly increased phosphorylation of perilipin A but without statistical significance (Fig. 3E). These results suggest that PKA-mediated perilipin A phosphorylation may be not responsible for adiponectin-inhibited lipolysis in adipocytes.
Adiponectin sensitizes insulin and insulin inhibits lipolysis (32). This raises the question whether adiponectin inhibits lipolysis through insulin and whether there is a synergistic effect. We used the insulin signaling blocker wortmannin, which abolishes the inhibitory effects of insulin on lipolysis in adipocytes (32). As expected, wortmannin treatment attenuated the inhibitory effects of insulin on glycerol release. However, wortmannin failed to attenuate adiponectin-suppressed glycerol release (Fig. 3F). In addition, adipocytes treated with both adiponectin and insulin did not exhibit a synergistic reduction in glycerol release. These results suggest that adiponectin inhibits lipolysis through a pathway other than insulin signaling.
Several adiponectin receptors have been reported (33,34). AdipoR1 is ubiquitously expressed and is the main adiponectin receptor in white adipocytes (33). To verify whether adiponectin inhibits HSL activation through the adiponectin receptor, AdipoR1 or siRNA oligo against AdipoR1 was expressed using adenovirus vectors in 3T3-L1CARΔ1 adipocytes, which express adenovirus receptor to facilitate adenovirus-mediated gene transduction in mature adipocytes (35). Phosphorylation levels of HSL Ser660 at both basal level and after cAMP stimulation were significantly reduced in Ad-AdipoR1–treated cells but were remarkably increased in AdipoR1 siRNA–treated adipocytes (Supplementary Fig. 6). These results suggest that adiponectin inhibits HSL Ser660 phosphorylation through AdipoR1.
Adiponectin inhibits HSL activation through PKA RIIα.
The coordinate changes of HSL Ser660 phosphorylation in Adipoq−/− mice and adiponectin-treated adipocytes suggest that adiponectin might suppress HSL activation in adipocytes through reducing PKA activity. With the use of an antibody that recognizes the consensus of phosphorylated PKA substrate, our study showed that there are significant increases of PKA substrate phosphorylation of proteins in epididymal fat from Adipoq−/− mice (Supplementary Fig. 7A), which further supports the notion that adiponectin inhibits PKA activation in adipocytes. However, adiponectin gene deletion has no significant impact on total cAMP levels in WAT (P > 0.05, Supplementary Fig. 7B).
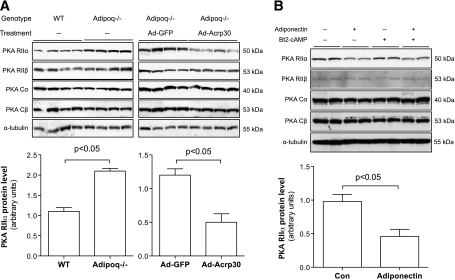
PKA is a holoenzyme containing two regulatory subunits and two catalytic subunits. PKA type II regulatory subunit (RII) has two distinct isoforms, RIIα and RIIβ. RIIα is ubiquitously expressed, but RIIβ is predominantly expressed in adipose, brain, and adrenal tissues. RIIβ gene knockout mice are lean and resistant to high-fat diet–induced obesity (36). We measured the gene expression levels of PKA catalytic subunits and regulatory subunits RI and RII at both mRNA and protein levels in epididymal fat from Adipoq−/− and adiponectin-restored mice. There were no significant changes of mRNA levels of the two catalytic and four regulatory subunits of PKA in Adipoq−/− mice (data not shown). Protein levels of two catalytic subunits, PKA Cα and Cβ, were also similar between Adipoq−/− and WT controls (Fig. 4A). RIIα but not RIIβ protein levels were significantly elevated in epididymal fat of Adipoq−/− mice (P < 0.05, Fig. 4A). In contrast, adiponectin reconstitution in Adipoq−/− mice remarkably reduced RIIα in the epididymal fat (P < 0.05, Fig. 4A). A similar inhibitory effect of adiponectin on RIIα protein level was observed in 3T3-L1 adipocytes (P < 0.05, Fig. 4B). These results led us to hypothesize that reduced RIIα mediates the inhibitory effect of adiponectin on HSL activation.
FIG. 4.
Adiponectin reduces PKA RIIα protein levels in adipose tissues and adipocytes. A: Total protein samples were prepared using epididymal fat from Adipoq−/− or Ad-Acrp30–treated mice. B: Fully differentiated 3T3-L1 adipocytes were co-cultured with Ad-Acrp30–transduced FAO cells, which secrete adiponectin. Protein samples were prepared after 24-h co-culture. Protein levels of PKA subunits were measured by Western blot. n = 8 for animal study, n = 6 for adipocyte study. Data are expressed as means ± SEM.
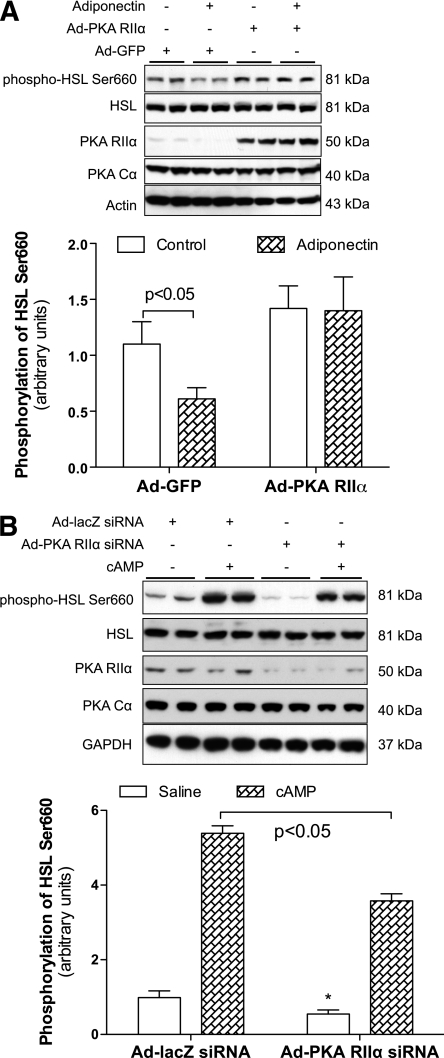
To verify this hypothesis, we overexpressed RIIα in differentiated mature adipocytes. The levels of HSL Ser660 phosphorylation were significantly increased in Ad-PKA RIIα–treated 3T3-L1CARΔ1 adipocytes (P < 0.05, Fig. 5A) or OP9 adipocytes (Supplementary Fig. 8). Overexpression of RIIβ even slightly decreased HSL Ser660 phosphorylation without statistical significance (P > 0.05, Supplementary Fig. 8). Most important, overexpression of RIIα abolished adiponectin-induced inhibition of HSL Ser660 phosphorylation in 3T3-L1CARΔ1 adipocytes (Fig. 5A). Furthermore, knocking down RIIα in 3T3-L1CARΔ1 adipocytes significantly reduced both basal and cAMP-stimulated HSL Ser660 phosphorylation (Fig. 5B). These results indicate that adiponectin suppresses HSL activation by reducing RIIα.
FIG. 5.
PKA RIIα overexpression attenuates the inhibitory effects of adiponectin on HSL Ser660 phosphorylation. 3T3-L1CARΔ1 cells were differentiated into adipocytes. Adenovirus vectors encoding GFP, PKA RIIα (A), or siRNA against PKA RIIα (B) were added to the medium. A: Cells were co-cultured 12 h later with Ad-Acrp30–transduced FAO cells, which secrete adiponectin. B: Some cells were treated with Bt2-cAMP for 1 h. Protein samples were prepared, and the phosphorylation levels of HSL Ser660 and other proteins were detected using specific antibodies. n = 6. Data are expressed as means ± SEM. *P < 0.05 vs. Ad-lacZ siRNA–treated cells at basal state.
Adiponectin reduces PKA RIIα protein level via proteasomal degradation.
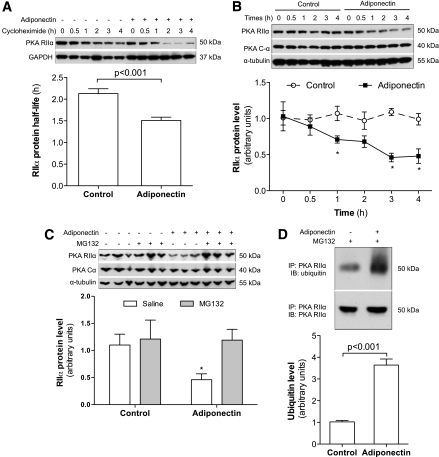
The above studies show that adiponectin reduces RIIα protein levels without altering RIIα mRNA level. Therefore, we compared the protein half-life of RIIα between 3T3-L1 adipocytes treated with or without adiponectin. As shown in Fig. 6A, adiponectin treatment significantly decreases RIIα protein stability. RIIα protein levels were reduced by 30% after just 1 h of adiponectin treatment in the presence of cycloheximide (Fig. 6B), which suggests that adiponectin enhances RIIα protein degradation.
FIG. 6.
Adiponectin reduces PKA RIIα protein stability. 3T3-L1 cells were differentiated into adipocytes. A: Adipocytes were co-cultured overnight with Ad-Acrp30–transduced FAO cells. Cycloheximide (100 μg/mL) was added to the medium, and the insert wells with FAO cells were removed. Protein samples of 3T3-L1 adipocytes were prepared at the indicated time points. B: 3T3-L1 adipocytes were treated with adiponectin-enriched conditioned medium from Ad-Acrp30–transduced FAO cells. C: Proteasome inhibitor MG132 (25 μmol/L) was added to the medium 30 min before adiponectin treatment. Protein samples were prepared 2 h later. D: Mature 3T3-L1 adipocytes were co-cultured with adiponectin-expressing FAO cells for 4 h. The Pierce Crosslink IP kit (Thermo Scientific Pierce Protein Research Products, Rockford, IL) was used for immunoprecipitation to eliminate nonspecific bands. All studies were repeated 4–6 times. The quantified data are expressed as means ± SEM. *P < 0.05 vs. control cells at the same time point (B) or without MG132 treatment (C).
The ubiquitin-dependent proteasome is the most common system for protein degradation. To further investigate how adiponectin reduces RIIα, the proteasome-specific inhibitor MG-132 was added into medium 30 min before adiponectin treatment. MG-132 completely rescued adiponectin-induced RIIα reduction in 3T3-L1 adipocytes (Fig. 6C), which indicates that adiponectin enhances RIIα protein degradation through the ubiquitin-proteasome system. Then, we measured ubiquitin levels of immunoprecipated RIIα protein from adiponectin-treated and control 3T3-L1 adipocytes. As Fig. 6D shows, the levels of ubiquitinated RIIα protein were significantly elevated in adiponectin-treated 3T3-L1 adipocytes. Together, these results indicate that adiponectin reduces RIIα protein levels by enhancing ubiquitin-proteasome–mediated protein degradation. It should be noted that overall protein ubiquitin levels were not clearly increased in adiponectin-treated cells (Supplementary Fig. 9). Similar effects of adiponectin on overall protein ubiquitination have also been observed in adiponectin-treated C2C12 myotubes (data not shown). These results suggest that adiponectin-enhanced RIIα ubiquitination and protein degradation occur in a protein-specific manner. The underlying mechanism through which adiponectin enhances RIIα ubiquitination is under investigation.
DISCUSSION
Adipose tissue is the main energy depot in humans and some animals. During fasting and exercise, triglycerides are hydrolyzed to release FFAs, which are oxidized by skeletal muscle, heart, and other tissues as fuel. With the discovery of adipocyte-derived hormones, the regulatory role of adipose-derived hormones on lipid metabolism has emerged as an important system in maintaining energy homeostasis. Our current study provides strong evidence demonstrating that adiponectin suppresses hormone-stimulated lipolysis in mouse adipocytes. During the revision of this article, the same effects of adiponectin on lipolysis were reported in human adipocytes (37). Therefore, we propose that adiponectin plays an important role in maintaining energy homeostasis by suppressing lipolysis in WAT.
It has been reported that leptin, another adipose-derived hormone, directly increases lipolysis and enhances lipid depletion from adipocytes (38,39). Although it is uncertain whether leptin and adiponectin regulate lipolysis through the same pathway, these studies suggest that adiponectin and leptin exert opposite regulatory effects on lipolysis in adipocytes. In addition, the results from cultured adipocytes clearly indicate that adiponectin suppresses lipolysis through an autocrine loop.
The discovery of the inhibitory effects of adiponectin on lipolysis will help us to understand how adiponectin improves circulating lipid profiles. However, it is premature to claim the importance of adiponectin-inhibited lipolysis in the development of obesity. There is a decrease of adiponectin gene expression in obesity (2), which should attenuate the inhibitory effect of adiponectin on lipolysis, resulting in less lipid storage in fat. Thus, obesity-associated low adiponectin expression may be beneficial in the context of obesity. In addition, despite lipolytic defects that have been observed in obese human and animal models (10,40), the role of lipolytic defects in obesity is still uncertain. Therefore, more studies are required to elucidate the role of adiponectin-suppressed lipolysis in the development of obesity and in energy metabolism under physiologic conditions.
Plasma FFA inversely associates with insulin sensitivity, especially in obese subjects (41,42). Our current study demonstrates that adiponectin inhibits lipolysis in WAT, which should decrease the level of circulating FFA. Therefore, we postulate that inhibition of lipolysis and reduction of FFA is a mechanism through which adiponectin enhances insulin sensitivity. On the other hand, the insulin-sensitizing effect of adiponectin may explain how adiponectin suppresses lipolysis. However, our study demonstrated that insulin signaling is not responsible for adiponectin-inhibited lipolysis in adipocytes. Furthermore, although high-fat feeding induces more severe insulin resistance in Adipoq−/− mice compared with WT control mice (16), the inhibitory effects of adiponectin on lipolysis are still well preserved. Therefore, these studies indicate that adiponectin inhibits hormone-stimulated adipocyte lipolysis through a mechanism(s) other than insulin sensitizing.
The essential role of PKA in mediating hormone-stimulated lipolysis in adipocytes has been well established. It has been reported that RIIβ gene knockout mice are lean and resistant to high-fat diet–induced obesity because of increased locomotor activity, energy expenditure, and lipolysis in WAT (43–45). Our study showed that adiponectin has no effect on RIIβ expression and that RIIβ overexpression even slightly reduces HSL Ser660 phosphorylation in adipocytes. The current study does not provide any evidence to support the involvement of RIIβ in adiponectin-inhibited lipolysis in adipocytes. In contrast, we found that the protein level of RIIα was elevated in adiponectin gene knockout mice. Ectopic expression of RIIα abolished the inhibitory effects of adiponectin on HSL Ser660 phosphorylation and lipolysis. Conversely, knocking down RIIα reduced both basal and cAMP-stimulated HSL Ser660 phosphorylation in adipocytes. These results led us to conclude that reduced RIIα mediates the inhibitory effects of adiponectin on lipolysis in adipocytes. It should be pointed out that by comparing the PKA substrate phosphorylation profile and PKA protein levels between Adipoq−/− and WT mice in different tissues (data not shown), we found that the effects of adiponectin on PKA subunit expression and PKA activation occur in a tissue-specific manner.
The mechanism by which RIIα mediates the inhibitory effect of adiponectin on lipolysis remains unknown. It is still incompletely understood how RIIα and RIIβ regulate PKA activity. Because of the large number of substrates and diverse downstream signaling pathways of PKA, precise localization of PKA to its specific substrate by A kinase-anchoring proteins (AKAPs) has been demonstrated to be an important mechanism to restrict PKA action (46). Recent studies revealed that RIIα binds tightly with some AKAPs and plays an important role in compartmentalizing cAMP/PKA signal transduction (46–48). We postulate that adiponectin suppresses RIIα expression, which may shift AKAPs/PKA away from the compartment where HSL localizes, resulting in decreased HSL phosphorylation and activation. The absence of effect of adiponectin on perilipin A phosphorylation also supports this postulation.
In conclusion, our study demonstrates that adiponectin inhibits lipolysis and directly regulates lipid metabolism in adipocytes. Our data also suggest that adiponectin suppresses triglyceride hydrolysis by inhibiting PKA-induced HSL activation.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DK-080418 (to J.Sh.) and DK-077643 (to J.Sh.), and American Diabetes Association grant 7-07-CD23 (to J.Sh.).
No potential conflicts of interest relevant to this article were reported.
L.Q. contributed research data. B.K. contributed research data. J.Sc. contributed to viral vectors and discussion. J.Sh. conceived the study and wrote the article.
The authors thank Dr. Philipp Scherer (University of Texas Southwestern Medical Center) for providing adiponectin gene knockout mice, Dr. Susan Taylor (University of California San Diego) for helpful discussion, and Dr. Perry Bickel (University of Texas Health Science Center at Houston) for advice and providing OP9 cells.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1017/-/DC1.
REFERENCES
- 1.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–451 [DOI] [PubMed] [Google Scholar]
- 3.Combs TP, Pajvani UB, Berg AH, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 2004;145:367–383 [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006;281:2654–2660 [DOI] [PubMed] [Google Scholar]
- 6.Qiao L, Maclean PS, Schaack J, et al. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 2005;54:1744–1754 [DOI] [PubMed] [Google Scholar]
- 7.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 2006;281:39915–39924 [DOI] [PubMed] [Google Scholar]
- 8.Wolins NE, Quaynor BK, Skinner JR, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res 2006;47:450–460 [DOI] [PubMed] [Google Scholar]
- 9.Kinney BP, Qiao L, Levaugh JM, Shao J. B56alpha/protein phosphatase 2A inhibits adipose lipolysis in high-fat diet-induced obese mice. Endocrinology 2010;151:3624–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinney BP, Qiao L, Levaugh JM, Shao J. B56alpha/protein phosphatase 2A inhibits adipose lipolysis in high-fat diet-induced obese mice. Endocrinology 2010;151:3624–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanadha S, Londos C. Optimized conditions for measuring lipolysis in murine primary adipocytes. J Lipid Res 2006;47:1859–1864 [DOI] [PubMed] [Google Scholar]
- 12.Viswanadha S, Londos C. Determination of lipolysis in isolated primary adipocytes. Methods Mol Biol 2008;456:299–306 [DOI] [PubMed] [Google Scholar]
- 13.Björkhem I, Arner P, Thore A, Ostman J. Sensitive kinetic bioluminescent assay of glycerol release from human fat cells. J Lipid Res 1981;22:1142–1147 [PubMed] [Google Scholar]
- 14.Mauriège P, Imbeault P, Langin D, et al. Regional and gender variations in adipose tissue lipolysis in response to weight loss. J Lipid Res 1999;40:1559–1571 [PubMed] [Google Scholar]
- 15.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 2002;277:25863–25866 [DOI] [PubMed] [Google Scholar]
- 16.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731–737 [DOI] [PubMed] [Google Scholar]
- 17.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007;6:55–68 [DOI] [PubMed] [Google Scholar]
- 18.Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes 2008;57:1824–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–946 [DOI] [PubMed] [Google Scholar]
- 20.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 2001;98:2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem 1990;191:245–250 [DOI] [PubMed] [Google Scholar]
- 22.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006;312:734–737 [DOI] [PubMed] [Google Scholar]
- 23.Lass A, Zimmermann R, Haemmerle G, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 2006;3:309–319 [DOI] [PubMed] [Google Scholar]
- 24.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 1998;273:215–221 [DOI] [PubMed] [Google Scholar]
- 25.Daval M, Diot-Dupuy F, Bazin R, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 2005;280:25250–25257 [DOI] [PubMed] [Google Scholar]
- 26.Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem 2003;278:43074–43080 [DOI] [PubMed] [Google Scholar]
- 27.Gauthier MS, Miyoshi H, Souza SC, et al. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 2008;283:16514–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaidhu MP, Fediuc S, Anthony NM, et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res 2009;50:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyoshi H, Perfield JW, 2nd, Souza SC, et al. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem 2007;282:996–1002 [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi H, Souza SC, Zhang HH, et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 2006;281:15837–15844 [DOI] [PubMed] [Google Scholar]
- 31.Sztalryd C, Xu G, Dorward H, et al. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 2003;161:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi SM, Tucker DF, Gross DN, et al. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol 2010;30:5009–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–769 [DOI] [PubMed] [Google Scholar]
- 34.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A 2004;101:10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlicky DJ, DeGregori J, Schaack J. Construction of stable coxsackievirus and adenovirus receptor-expressing 3T3-L1 cells. J Lipid Res 2001;42:910–915 [PubMed] [Google Scholar]
- 36.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature 1996;382:622–626 [DOI] [PubMed] [Google Scholar]
- 37.Hnevkovska Z, Dietrich J, Siklova-Vitkova M, et al. Adiponectin inhibits spontaneous and catecholamine-induced lipolysis in human adipocytes of non-obese subjects through AMPK-dependent mechanisms. Physiol Res. 15 October 2010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Frühbeck G, Aguado M, Martínez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun 1997;240:590–594 [DOI] [PubMed] [Google Scholar]
- 39.Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest 1997;100:2858–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langin D, Dicker A, Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 2005;54:3190–3197 [DOI] [PubMed] [Google Scholar]
- 41.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 1995;44:863–870 [DOI] [PubMed] [Google Scholar]
- 42.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czyzyk TA, Sikorski MA, Yang L, McKnight GS. Disruption of the RIIbeta subunit of PKA reverses the obesity syndrome of Agouti lethal yellow mice. Proc Natl Acad Sci U S A 2008;105:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newhall KJ, Cummings DE, Nolan MA, McKnight GS. Deletion of the RIIbeta-subunit of protein kinase A decreases body weight and increases energy expenditure in the obese, leptin-deficient ob/ob mouse. Mol Endocrinol 2005;19:982–991 [DOI] [PubMed] [Google Scholar]
- 45.Planas JV, Cummings DE, Idzerda RL, McKnight GS. Mutation of the RIIbeta subunit of protein kinase A differentially affects lipolysis but not gene induction in white adipose tissue. J Biol Chem 1999;274:36281–36287 [DOI] [PubMed] [Google Scholar]
- 46.Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol 2007;19:192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold MG, Lygren B, Dokurno P, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell 2006;24:383–395 [DOI] [PubMed] [Google Scholar]
- 48.Kinderman FS, Kim C, von Daake S, et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell 2006;24:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]