Abstract
OBJECTIVE
Glucagon-like peptide (GLP)-1 lowers postprandial glycemia primarily through inhibition of gastric emptying. We addressed whether the GLP-1–induced deceleration of gastric emptying is subject to rapid tachyphylaxis and if so, how this would alter postprandial glucose control.
RESEARCH DESIGN AND METHODS
Nine healthy volunteers (25 ± 4 years old, BMI: 24.6 ± 4.7 kg/m2) were examined with intravenous infusion of GLP-1 (0.8 pmol · kg−1 . min−1) or placebo over 8.5 h. Two liquid mixed meals were administered at a 4-h interval. Gastric emptying was determined, and blood samples were drawn frequently.
RESULTS
GLP-1 decelerated gastric emptying significantly more after the first meal compared with the second meal (P = 0.01). This was associated with reductions in pancreatic polypeptide levels (marker of vagal activation) after the first but not the second meal (P < 0.05). With GLP-1, glucose concentrations declined after the first meal but increased after the second meal (P < 0.05). The GLP-1–induced reductions in postprandial insulin and C-peptide levels were stronger during the first meal course (P < 0.05). Likewise, glucagon levels were lowered by GLP-1 after the first meal but increased after the second test meal (P < 0.05).
CONCLUSIONS
The GLP-1–induced delay in gastric emptying is subject to rapid tachyphylaxis at the level of vagal nervous activation. As a consequence, postprandial glucose control by GLP-1 is attenuated after its chronic administration.
Therapeutic approaches based on the actions of the incretin hormone glucagon-like peptide (GLP)-1 have been widely established in the management of type 2 diabetes (1–3). Although the glucose-lowering effect of GLP-1 in the fasting state is primarily mediated by its glucose-dependent augmentation of insulin secretion and inhibition of glucagon release (4,5), the key mechanism driving the postprandial normalization of glycemia by GLP-1 is a marked deceleration of gastric emptying leading to a delayed entry of glucose into the circulation (6–9). In fact, when the deceleration of gastric emptying by GLP-1 is antagonized, the glucose-lowering effect of the incretin is largely abolished (10).
Although a longer duration of action covering the entire 24 h of the day is generally thought to confer improved glycemic control, constant activation of the GLP-1 receptor might also lead to the induction of tolerance against the incretin hormone, thereby possibly reducing its potency. In analogy, the development of tachyphylaxis is well known to alter the actions of nitrates or catecholamines during constant pharmacologic exposure (11,12). Whether the actions of GLP-1 are also subject to tachyphylaxis has not yet been examined. However, when GLP-1 was administered intravenously over a period of 7 days in patients with type 2 diabetes, postprandial glucose concentrations declined after serving the first meal, whereas an increment in postprandial glucose levels was observed after the subsequent meal courses (13). Unfortunately, gastric emptying measurements were not available from that study or any other study with chronic GLP-1 administration over repeated meal courses.
Therefore, the current study addressed whether the deceleration of gastric emptying by intravenous GLP-1 is subject to rapid tachyphylaxis and if so, whether this can lead to clinically relevant differences in postprandial glycemic control.
RESEARCH DESIGN AND METHODS
Study protocol.
The study protocol was approved by the ethics committee of the medical faculty of Ruhr University, Bochum, Germany (registration number 652 a) before study commencement. Written informed consent was obtained from all participants.
Subjects.
Nine healthy male volunteers were studied. They were 25 ± 4 years old with a BMI of 24.6 ± 4.7 kg/m2. All had a normal oral glucose tolerance according to World Health Organization criteria (fasting glucose 5.1 ± 0.4, 120-min value 5.0 ± 1.1 mmol/L). None had a family history of diabetes or a personal history of gastrointestinal disorders. Blood cell counts, serum transaminases, creatinine values, triglyceride, cholesterol, and HDL-cholesterol concentrations were in the normal range.
Study design.
All participants were studied, in random order, on two occasions:
Liquid mixed meals (50 g sucrose plus amino acids, 400 mL Aminosteril N-Hepa 8%; Fresenius AG, Bad Homburg, Germany) were instilled intragastrically at time 0 and 240 min. Placebo (0.9% NaCl with 1% human serum albumin, Human-Albumin 20% Behring, salzarm, Behringwerke AG, Marburg, Germany) was infused intravenously from −30 to 480 min.
Liquid test meals were administered as described. In addition, a continuous intravenous administration of GLP-1 [7–36 amide], at a dose of 0.8 pmol · kg−1 · min−1, was started 30 min before the first meal (at −30 min) and continued until 480 min.
An interval of 1–4 weeks was kept in between the experiments to avoid carry-over effects.
Peptides.
Synthetic GLP-1 [7–36 amide] was purchased from Saxon Biochemicals GmbH (Hannover, Germany) and processed for infusion as described (10).
Experimental procedures.
The tests were performed in the morning after an overnight fast. Two forearm veins were punctured with a Teflon cannula (Moskito 123, 18 gauge, Vygon, Aachen, Germany) and kept patent using 0.9% NaCl (for blood sampling and GLP-1/placebo administration).
After drawing basal blood specimens, at −30 min an intravenous infusion of GLP-1 [7–36 amide], 0.8 pmol · kg−1 . min−1, or placebo (0.9% NaCl containing 1% human serum albumin) was started and continued for 510 min. This infusion rate was based on previous studies (5,14) and was selected to increase plasma GLP-1 concentrations into the pharmacologic range (approximately three- to fourfold higher concentrations in comparison with those measured after oral glucose) (15). The infusion was begun at −30 min to ensure elevated GLP-1 [7–36 amide] plasma concentrations already at the time point of administration of the first liquid meal. Blood was drawn at the time points indicated in Fig. 1, and plasma glucose was determined immediately.
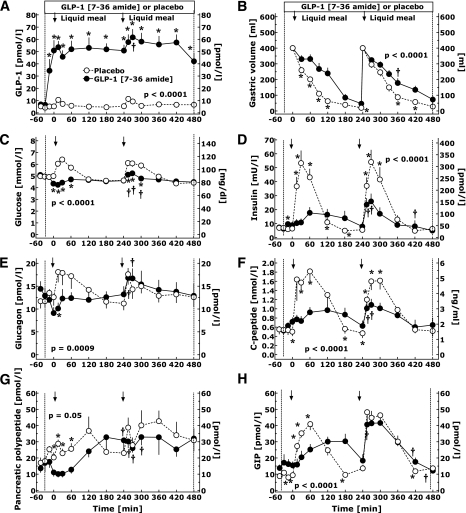
FIG. 1.
Plasma concentrations of GLP-1 (A), glucose (C), insulin (D), glucagon (E), C-peptide (F), PP (G), and GIP (H), and gastric emptying (B) after instilling two liquid test meals (amino acids and sucrose) via a nasogastric tube into the stomach at 0 and 240 min in nine healthy young volunteers during the intravenous infusion of GLP-1 (0.8 pmol · kg−1 ⋅ min−1) or placebo. Mean ± SEM. P values were calculated by repeated-measures ANOVA. *Significant (P < 0.05) differences at specific time points between experiments with placebo and GLP-1. †Significant difference from the corresponding time point after the first meal during the exogenous administration of GLP-1.
Gastric emptying.
Before the study, a nasogastric tube (Freka-Ernährungssonde, 120 cm, CH12, Fresenius AG) was placed and tape-fixed with the tip approximately 55 cm from the nostrils. Gastric juice was aspirated, and an acidic pH was ascertained using pH-sensitive Lackmus paper. The gastric lumen was washed with 100 mL water (37°C). The position of the tube was, if necessary, adjusted to allow a near-complete aspiration of instilled fluid. The subjects were lying on their back in a semirecumbent position with the upper half of the body 45 degrees upright. At 0 min, 400 mL (total volume) of the liquid test meal was instilled into their stomach. The liquid test meal was composed of 50 g sucrose dissolved in 400 mL of mixed amino acids.
This composition of the meal was chosen because the solution had to be clear for the photometric measurement of phenol red (measurement of gastric emptying, see below) and should be similar in caloric and nutrient content to a normal mixed meal. The meal contained 32 g mixed amino acids (131 kCal = 40%) and 50 g sucrose (196 kCal = 60%); total energy content was 327 kCal (energy density: 0.82 kCal/mL).
Gastric emptying was measured as described (10) using a double-sampling dye dilution technique using phenol red (Merck AG, Darmstadt, Germany) according to George (16), with modifications introduced to reduce measurement error by Hurwitz (17). According to the expected rate of gastric emptying, gastric contents were determined at intervals shown in Fig. 1 over 480 min.
Blood specimens.
Blood was drawn into chilled tubes containing EDTA and aprotinin (Trasylol; 20,000 KIU/mL, 200 μL per 10 mL blood; Bayer AG, Leverkusen, Germany) and kept on ice. A sample (∼100 μL) was stored in NaF (Microvette CB 300; Sarstedt, Nümbrecht, Germany) for the measurement of glucose. After centrifugation at 4°C, plasma for hormone analyses was kept frozen at −30°C.
Laboratory determinations.
Glucose was measured using a glucose oxidase method, as described (10). Insulin was measured using an insulin microparticle enzyme immunoassay (IMx Insulin, Abbott Laboratories, Wiesbaden, Germany), as described (10). C-peptide was measured using C-peptide-antibody–coated microtiter wells (C-peptide MTPL EIA) from DRG Instruments GmbH, Marburg, Germany, as described (10). Plasma concentrations of total GLP-1 were determined in ethanol-extracted plasma as previously described (15,18), using antiserum 89 390 for the measurement of intact GLP-1 [7–36 amide] plus cleavage products. Pancreatic glucagon was assayed in ethanol-extracted plasma using antibody 4305 (19). Plasma gastric inhibitory polypeptide (GIP) was determined by RIA using antiserum R 65 as previously described (20). Plasma pancreatic polypeptide (PP) was determined by RIA as described (21). Each patient’s set of plasma samples was assayed at the same time to avoid errors due to interassay variation.
Phenol red in gastric contents was assayed photometrically after filtration through filter paper (100 μL in 2 mL Na2HPO4/NaH2PO4 buffer, 0.6 mol/L/L, pH 8.0) at a wavelength of 546 nm and read against a standard curve (phenol red in phosphate buffer).
Statistical analysis.
Results are reported as mean ± SEM. All statistical calculations were carried out using paired repeated-measures ANOVA using Statistica version 5.0 (Statsoft Europe, Hamburg, Germany). This analysis provides P values for the comparison between the two experiments, for changes over the time course, and for the interaction of experiment and time course. In case of a significant interaction between experiment and time course, values at individual time points were compared using Student t tests. To determine differences between the two test meals, the experiment was divided into two periods, whereby the first meal course was evaluated from t = −30 to 240 min, and the second meal course was evaluated from t = 210 to 480 min. Separate paired repeated-measures ANOVA analyses were performed to compare these periods during placebo and GLP-1 administration, respectively. A two-sided P value < 0.05 was taken to indicate significant differences. Linear regression analyses were performed to determine the relationship between gastric emptying and postprandial glycaemia, using GraphPad Prism, Version 4.0.
RESULTS
GLP-1 plasma concentrations.
During the placebo experiments, meal ingestion led to an increment in plasma GLP-1 concentrations from basal levels of 5 ± 1 pmol/L to peak levels of 11 ± 2 pmol/L and 11 ± 2 pmol/L after the first and second test meals, respectively (P < 0.05; Fig. 1A). Exogenous GLP-1 administration rapidly increased GLP-1 concentrations to steady-state levels of 50–55 pmol/L (P < 0.0001 vs. placebo), with superimposed small increments after the meal administrations. No major adverse outcome was reported during the experiments.
Gastric empyting.
The velocity of gastric emptying was significantly retarded by exogenous GLP-1 compared with placebo, both after the first and second meals (P < 0.0001 for the comparison of GLP-1 vs. placebo over the time course; Fig. 1B). When gastric emptying was compared between the two test meals (period 1 vs. 2) during GLP-1 infusion, a significant interaction between period and time course was found (P = 0.010), and subsequent post hoc tests revealed significant differences in gastric volume 120 min after meal administration (Fig. 1B). On the basis of these analyses, GLP-1 significantly slowed gastric emptying after both test meals (periods 1 and 2), but the degree by which gastric emptying was slowed was less prominent 120 min after meal ingestion with the second meal. When only the experiments with placebo administration were analyzed, no significant differences in gastric emptying were found between the experiments (periods) over the time course (P = 0.38; Fig. 1B).
Postprandial concentrations of glucose, insulin, and C-peptide.
During placebo administration, there was a small increment in glycemia after the instillation of both meals (from 4.9 ± 0.1 mmol/L at t = −30 min to 6.4 ± 0.2 mmol/L at t = 30 min, and from 4.6 ± 0.1 mmol/L to 6.1 ± 0.3 mmol/L after the second meal, P < 0.05, respectively; Fig. 1C). Postprandial plasma glucose levels were lower with concomitant GLP-1 infusions over 60 min after administering the meals (P < 0.05; Fig. 1C).
However, although postprandial glucose concentrations were lowered by GLP-1 during the first hour after the first meal (from 5.0 ± 0.1 mmol/L at t = −30 min to 4.4 ± 0.2 mmol/L at t = 30 min), there was even a small increment in glycemia after the second meal (from 4.5 ± 0.1 mmol/L to 5.2 ± 0.2 mmol/L, respectively). The differences in postprandial glucose concentrations during GLP-1 infusion between the first meal and the second meal were statistically significant (P < 0.05).
Plasma insulin and C-peptide concentrations increased significantly after instillation of both the first and second test meals during the placebo experiments (P < 0.001, respectively; Fig. 1D and F). GLP-1 administration led to an increase in fasting insulin and C-peptide concentrations (P < 0.05 vs. placebo). However, the postprandial increases in insulin and C-peptide levels were ∼80 and ∼55% lower, respectively, during GLP-1 infusion compared with placebo (P < 0.001; Fig. 1D and F). The GLP-1–induced reduction in postprandial insulin and C-peptide concentrations was less pronounced during the second test meal (P < 0.05 vs. the first meal).
Pancreatic glucagon.
Instillation of the test meals resulted in a clear increase in plasma glucagon concentrations during the placebo experiments (Fig. 1E). GLP-1 administration led to a reduction in both fasting and postprandial glucagon levels (P = 0.0009). However, with GLP-1, glucagon concentrations were higher after the second test meal than after the first test meal (P < 0.05), whereas no differences in postprandial glucagon levels were observed in the placebo experiments.
GIP.
GIP plasma concentrations increased by approximately fourfold after the test meals during placebo administration (Fig. 1H). With exogenous GLP-1, the increments in GIP plasma levels were blunted and retarded after the first meal, whereas a rapid increment was observed after the second meal. The peak plasma levels of GIP were significantly lower after the first test meal compared with the second test meal during GLP-1 infusion (P < 0.05), whereas postprandial GIP levels were similar after both meals during placebo administration (Fig. 1H).
PP.
Meal ingestion elicited an increase in PP concentrations during placebo administration (Fig. 1G). GLP-1 infusion led to a significant reduction in postprandial PP concentrations after the first test meal (P < 0.05; Fig. 1G). However, PP levels after the second test meal were higher than after the first meal in the GLP-1 experiments, although there was still a significant difference in postprandial PP concentrations compared with the placebo experiments (P < 0.05).
Relationship between gastric emptying and postprandial glycemia.
To determine the impact of gastric emptying on postprandial glycemia, linear regression analyses between the parameters of gastric emptying and the respective glucose increments after the liquid meal were performed. Across all experiments (with GLP-1 and placebo, analyzing both the first and the second periods), the percentage of initial gastric volume that had emptied within 60 min correlated significantly with the increment in glucose concentrations (peak minus basal concentrations; r2 = 0.194, P = 0.0044). Likewise, 60-min emptying correlated significantly with peak glucose concentrations (r2 = 0.327, P < 0.0001) and the time of the glucose peak after the meal (r2 = 0.265, P = 0.0007), but not with the integrated incremental glucose area (above baseline; r2 = 0.022, P = 0.37). Qualitatively similar (highly significant) results were obtained when the gastric emptying half-time, the lag time, or the 30-min emptying were used instead to characterize the velocity of gastric emptying (details not shown; an example is shown in Supplementary Figure 1).
DISCUSSION
The current study was designed to examine whether the deceleration of gastric emptying by GLP-1 is subject to rapid tachyphylaxis and how this would affect postprandial glucose homeostasis. We report that the GLP-1–induced deceleration of gastric emptying is significantly diminished already after 5 h of continuous infusion compared with its initial effects. This attenuation of GLP-1 efficacy leads to increased postprandial concentrations of glucose, insulin, and glucagon, as well as changes in the concentration time pattern of GIP.
A deceleration of gastric emptying by GLP-1 has been demonstrated in patients with type 2 diabetes and healthy individuals (5,8,14). In line with these studies, the GLP-1–induced delay in gastric emptying not only prevented the postprandial increase in glycemia but also led to a marked reduction in insulin and C-peptide levels. The GLP-1–induced reduction in postprandial glycemia and insulin secretion was less pronounced during the second meal course, when the delay in gastric emptying was largely attenuated. This finding is consistent with previous studies showing that the reduction in postprandial glycemia and insulin secretion is largely diminished, when the effects of the incretin on the stomach are antagonized (10). Taken together, these studies underline the importance of delayed gastric emptying as the primary factor driving the reduction in postprandial glycemia during acute GLP-1 administration.
What are the mechanisms conferring the induction of tolerance against the GLP-1 effects on gastric emptying? One possibility would be a downregulation or desensitization of the GLP-1 receptor in response to chronically elevated GLP-1 levels. Indeed, receptor desensitization has been described for GIP (22), which shares many intracellular signaling steps with GLP-1. For GLP-1, chronic exposure to a receptor antagonist in mice in vivo did not result in a significant receptor downregulation or attenuation of its glucoregulatory effects (23), thereby rendering this explanation less likely.
The alternative hypothesis would be tachyphylaxis to the effects of GLP-1 at the level of the vagal nerve. Such a mechanism would explain why the effects of GLP-1 on gastric emptying are obviously more affected by the induction of tolerance than the islet hormone responses (24). Furthermore, chronic adaptation of the autonomous nervous system is a well-recognized principle, which is also responsible for many other phenomena, such as hypoglycemia unawareness (25), tolerance to β-adrenergic agonists (26), and opioid tolerance (27). Finally, the time kinetics of tolerance induction observed in the current study (within ∼5 h) are more typical for the mechanism of tachyphylaxis than for receptor downregulation, which usually requires longer periods of exposure. The hypothesis of vagal nervous tachyphylaxis is further supported by the plasma concentrations of PP, which are thought to mirror the systemic level of vagal nervous activation (10,28). In fact, PP levels were markedly suppressed by GLP-1 during the first meal course, but slightly elevated during the second test meal, suggesting a more pronounced inhibition of parasympathetic outflow during the first test meal. Thus, GLP-1 seems to inhibit gastric emptying primarily through inhibition of the vagal nerve (28), but this mechanism is subject to rapid tachyphylaxis after chronic GLP-1 exposure.
The present data give rise to consider which of the multiple GLP-1 effects are subject to rapid tachyphylaxis and which are not. Clearly, the GLP-1–induced inhibition of gastric emptying was less pronounced during the second meal course compared with the first meal. In contrast, the quantitative amount of insulin released after the second test meal was even greater than after the first test meal, thereby arguing against an attenuation of the insulinotropic effect of GLP-1 during continuous exposure. For glucagon, an opposite picture was found, with a marked suppression after the first meal and higher levels after the second meal. However, because these postprandial changes in insulin and glucagon levels were primarily driven by the changes in gastric emptying, it is impossible to draw firm conclusions regarding a rapid tachyphylaxis to the GLP-1 effects on islet hormone secretion from this study. By taking into account the results obtained from the various long-term studies with GLP-1 analogs (24,29,30), there is little reason to assume the presence of such a mechanism. Thus, although the GLP-1 actions on the stomach seem to critically depend on the time kinetics of GLP-1 levels, its insulinotropic and glucagon static effects are likely less dependent on the fluctuations in GLP-1 levels.
Of note, glucagon concentrations increased after the ingestion of the test meal. This is in contrast with previous studies showing a significant decline in glucagon levels after intravenous or oral glucose ingestion (31,32). This apparent discrepancy is most likely due to the high amino acid content of the test meal, which is known to stimulate rather than suppress glucagon secretion (33).
Another interesting finding from this study is the alteration in GIP secretion by GLP-1. During the first test meal, the GLP-1–induced retardation of gastric emptying led to a significant delay in GIP secretion. Given the importance of GIP as a physiologic incretin hormone (15,34), this might theoretically impair postprandial insulin responses and thus worsen glucose control. In the present experiments, the concentration time patterns of insulin and C-peptide seemed to closely mirror the respective GIP levels, thereby emphasizing the role of GIP as a physiologic enhancer of postprandial insulin release. In addition, GIP has also been shown to augment glucagon secretion in healthy humans (35). It is therefore possible that the differences in the postprandial concentrations of glucagon between the two test meals were also partly secondary to the changes in GIP release. However, given the complexity of these interactions among vagal nervous activation, gastrointestinal motility, gut hormone release, and islet hormone secretion, it is difficult to fully elucidate these relationships on the basis of the present experiments.
Although the present studies in healthy individuals do not allow for firm conclusions regarding the therapeutic use of GLP-1–based drugs, it would be tempting to speculate that the development of tachyphylaxis might be involved in the unequal susceptibility of patients to develop nausea when treated with either short- or long-acting GLP-1 analogs. It would therefore be of interest to examine the induction of tachyphylaxis in patients with type 2 diabetes during the chronic treatment with different GLP-1 analogs.
In conclusion, the delay in gastric emptying by GLP-1 is markedly attenuated during continued exposure to high GLP-1 concentrations. Most likely, this is due to the induction of tachyphylaxis at the level of vagal nervous activation. As a consequence, postprandial glucose control by GLP-1 is attenuated after its chronic administration.
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (grant Na 203/2-2) and the Danish Medical Research Council.
No potential conflicts of interest relevant to this article were reported.
M.A.N. designed the study and contributed to data analysis and article preparation. G.K. researched data and reviewed the article. J.J.H. contributed to data collection, performed the hormone measurements, and discussed and edited the article. J.J.M. contributed to data analysis and article preparation.
The authors thank S. Richter, Th. Gottschling, K. Faust (Central Laboratory, Department of Medicine, Knappschaftskrankenhaus Bochum Langendreer, Ruhr University Bochum, Germany), L. Rabenhøj, and L. Albæk (Department of Medical Physiology, Panum Institute, University of Copenhagen, Copenhagen, Denmark) for technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0474/-/DC1.
REFERENCES
- 1.Meier JJ, Nauck MA. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab Res Rev 2005;21:91–117 [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003;26:2929–2940 [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ. Treatment of type 2 diabetes mellitus with agonists of the GLP-1 receptor or DPP-IV inhibitors. Expert Opin Emerg Drugs 2004;9:155–166 [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–744 [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003;88:2719–2725 [DOI] [PubMed] [Google Scholar]
- 6.Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008;151:123–129 [DOI] [PubMed]
- 7.Little TJ, Pilichiewicz AN, Russo A, et al. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrinol Metab 2006;91:1916–1923 [DOI] [PubMed]
- 8.Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 1993;38:665–673 [DOI] [PubMed] [Google Scholar]
- 9.Willms B, Werner J, Holst JJ, Ørskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 1996;81:327–332 [DOI] [PubMed] [Google Scholar]
- 10.Meier JJ, Kemmeries G, Holst JJ, Nauck MA. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes 2005;54:2212–2218 [DOI] [PubMed] [Google Scholar]
- 11.Lönnqvist F, Wahrenberg H, Hellström L, Reynisdottir S, Arner P. Lipolytic catecholamine resistance due to decreased beta 2-adrenoceptor expression in fat cells. J Clin Invest 1992;90:2175–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 2005;97:618–628 [DOI] [PubMed] [Google Scholar]
- 13.Larsen J, Hylleberg B, Ng K, Damsbo P. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 2001;24:1416–1421 [DOI] [PubMed] [Google Scholar]
- 14.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol (Endocrinol Metab) 1997;273:E981–E988 [DOI] [PubMed] [Google Scholar]
- 15.Nauck MA, Bartels E, Ørskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab 1993;76:912–917 [DOI] [PubMed] [Google Scholar]
- 16.George JD. New clinical method for measuring the rate of gastric emptying: the double sampling test meal. Gut 1968;9:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurwitz A. Measuring gastric volume by dye dilution. Gut 1981;22:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994;43:535–539 [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem J 1982;207:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krarup T, Madsbad S, Moody A, et al. Diminished immunoreactive gastric inhibitory polypeptide response to a meal in newly diagnosed type I (insulin-dependent) diabetics. J Clin Endocrinol Metab 1983;56:1306–1312 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz T, Holst JJ, Fahrenkrug J, et al. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest 1978;61:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng CC, Boylan MO, Jarboe LA, Usdin TB, Wolfe MM. Chronic desensitization of the glucose-dependent insulinotropic polypeptide receptor in diabetic rats. Am J Physiol (Endocrinol Metab) 1996;270:E661–E666 [DOI] [PubMed] [Google Scholar]
- 23.Baggio LL, Kim JG, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes 2004;53(Suppl. 3):S205–S214 [DOI] [PubMed] [Google Scholar]
- 24.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 2005;54:3592–3601 [DOI] [PubMed] [Google Scholar]
- 26.Taylor DR, Sears MR, Cockcroft DW. The beta-agonist controversy. Med Clin North Am 1996;80:719–748 [DOI] [PubMed] [Google Scholar]
- 27.Willette RN, Sapru HN. Peripheral versus central cardiorespiratory effects of morphine. Neuropharmacology 1982;21:1019–1026 [DOI] [PubMed] [Google Scholar]
- 28.Wettergren A, Wøjdemann M, Holst JJ. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am J Physiol (Gastroenterol Liver) 1998;275:G984–G992 [DOI] [PubMed] [Google Scholar]
- 29.Garber A, Henry R, Ratner R, et al. ; LEAD-3 (Mono) Study Group Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 30.Buse JB, Rosenstock J, Sesti G, et al. ; LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 31.Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992;326:22–29 [DOI] [PubMed] [Google Scholar]
- 32.Meier JJ, Deacon CF, Schmidt WE, Holst JJ, Nauck MA. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 2007;50:806–813 [DOI] [PubMed] [Google Scholar]
- 33.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 1985;28:574–578 [DOI] [PubMed] [Google Scholar]
- 34.Miyawaki K, Yamada Y, Yano H, et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A 1999;96:14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meier JJ, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 2003;46:798–801 [DOI] [PubMed] [Google Scholar]