Abstract
Objective
Arginine deficiency may contribute to microvascular dysfunction, but previous studies suggest that arginine supplementation may be harmful in sepsis. Systemic arginine availability can be estimated by measuring the ratio of arginine to its endogenous inhibitors, asymmetric and symmetric dimethylarginine. We hypothesized that the arginine to dimethylarginine (Arg/DMA) ratio is reduced in patients with severe sepsis and associated with severity of illness and outcomes.
Design
Case-control and prospective cohort study
Setting
Medical and surgical intensive care units of an academic medical center
Patients and Subjects
109 severe sepsis and 50 control subjects
Measurements and Main Results
Plasma and urine were obtained in control subjects and within 48 hours of diagnosis in severe sepsis patients. The Arg/DMA ratio was higher in control subjects vs. sepsis patients ((median = 95 [inter-quartile range = 85 – 114]) vs. 34 [24 – 48], p < 0.001), and in hospital survivors vs. non-survivors ((39 [26 – 52]) vs. 27 [19 – 32], p = 0.004). The Arg/DMA ratio was correlated with Acute Physiology and Chronic Health Evaluation II score (Spearman’s correlation coefficient [rho] = − 0.40, p < 0.001) and organ-failure free days (rho = 0.30, p = 0.001). A declining Arg/DMA ratio was independently associated with hospital mortality (odds ratio =1.63 per quartile, 95% confidence interval [CI] = 1.00 – 2.65, p = 0.048) and risk of death over 6 months (hazard ratio = 1.41 per quartile, 95% CI = 1.01 – 1.98, p = 0.043). The Arg/DMA ratio was correlated with the urinary nitrate to creatinine ratio (rho = 0.46, p < 0.001).
Conclusions
The Arg/DMA ratio is associated with severe sepsis, severity of illness, and clinical outcomes. The Arg/DMA ratio may be a useful biomarker, and interventions designed to augment systemic arginine availability in severe sepsis may still be worthy of investigation.
Keywords: arginine, nitric oxide, amino acids, sepsis, septic shock, critical care
INTRODUCTION
Microvascular dysfunction in severe sepsis and septic shock may result from reduced bioavailability of nitric oxide (NO) (1). Nitric oxide is produced by nitric oxide synthases (NOS), converting arginine to NO and citrulline. Nitric oxide synthesis is inhibited by asymmetric and symmetric dimethylarginine (ADMA and SDMA, respectively), metabolites of arginine that are produced during protein catabolism and catalyzed by protein arginine methyltransferase (PRMT) (2, 3). SDMA inhibits NO synthesis by competitively inhibiting cellular arginine uptake via membrane cationic amino acid transporters (CAT) (3, 4), and ADMA inhibits NO synthesis primarily by direct competitive inhibition of NOS (2). Arginine is also metabolized by arginase, producing urea and ornithine. Based upon this biochemistry, systemic arginine availability has been estimated in cardiovascular and pulmonary diseases by calculating ratios of arginine to its enzymatic products (e.g., citrulline and ornithine) and arginine to its inhibitory metabolic byproducts (e.g., ADMA and SDMA) (5, 6).
Previous studies have shown that arginine levels are low in sepsis, at least in part because of impaired endogenous arginine synthesis and accelerated hepatic arginine clearance (7-10). However, these studies did not specifically link arginine deficiency with poor outcome or severity of illness. ADMA levels are independently associated with mortality in unselected critically ill patients (11, 12), but the relationship between ADMA and outcome specifically in septic patients has not been established (13). Experimental studies show that low systemic arginine availability occurs after endotoxemia, and also impairs vital organ blood flow and cardiovascular function (14, 15).
It is unknown whether the aforementioned ratios have prognostic significance in severe sepsis and septic shock. The goal of this study was to determine if these estimates of systemic arginine availability are reduced in patients with severe sepsis and septic shock and associated with severity of illness and outcomes. Some of the results of this study have been reported previously in abstract form (16).
MATERIALS AND METHODS
Study Design
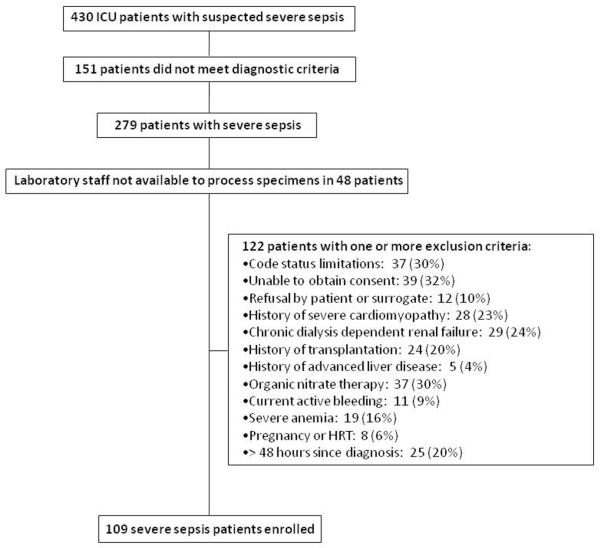
This was a prospective, single-center cohort study, described previously (17). Consecutive patients meeting diagnostic criteria for severe sepsis or septic shock (18) (collectively referred to hereafter as severe sepsis) admitted to the medical or surgical intensive care units at the University of Rochester Medical Center were screened for enrollment. The comprehensive list of prospectively-defined exclusion criteria are shown in Figure 1.
Figure 1.
Enrollment algorithm for severe sepsis / septic shock patients. Definitions: Code status limitations = patients with directives precluding common interventions used in the management of critically ill severe sepsis patients (e.g., mechanical ventilation or vasopressor agents). Patients were not excluded if their only specified directive was against electrical countershock or CPR; unable to obtain consent = inability to obtain written informed consent from patient or surrogate within 48 hours of diagnosis; severe cardiomyopathy = history of cardiomyopathy with left ventricular ejection fraction < 30%; transplantation = hematopoietic stem cell or solid organ transplantation; history of advanced liver disease = Child-Pugh grade C; severe anemia = hematocrit < 22% or < 25% while receiving vasopressor infusions; HRT = estrogen or testosterone hormone replacement therapy; > 48 hours since diagnosis = greater than 48 hours from when the patient first met diagnostic criteria for severe sepsis to screening for study enrollment.
Venous blood samples were collected within 48 hours of diagnosis and again 3-5 days later. Patients were not enrolled if laboratory technicians were unavailable to process specimens within 48 hours of diagnosis (Figure 1). Whole blood was centrifuged at 1,500 g for 10 minutes and the plasma supernatant was immediately snap frozen and stored at −80° C until analysis. Amino acids were measured using ion exchange chromatography, and dimethylarginines were measured using reversed phase high performance liquid chromatography (HPLC) coupled with positive-electron ionspray mass spectrometry (MS) in multiple reaction monitoring (MRM) mode (see the complete description of laboratory measurements in Supplemental Digital Content). The ratios of arginine to its inhibitory dimethylarginine metabolites ADMA and SDMA (Arg/DMA), and of arginine to its enzymatic by-products citrulline and ornithine (Arg/(Cit+Orn)) were calculated to estimate systemic arginine availability. Arginase activity was estimated by the arginine to ornithine ratio (Arg/Orn) (6). To provide relevant reference ranges for our measurements, control subjects without acute illness were recruited from the local community by age and gender strata approximating the severe sepsis cohort. Control subjects were excluded if they had conditions or used medications shown in Figure 1, or if they had active infection or used antibiotics within 6 weeks of sample collection. All subjects or their surrogates provided informed consent and the study protocol was approved by the University of Rochester Research Subjects Review Board.
In a post-hoc analysis we measured urinary nitrate concentrations in samples obtained simultaneous with the blood sample to assess relationships between estimates of systemic arginine availability and NO production. Urine nitrate was measured using reductive chemiluminescence (see description of laboratory measurements in Supplemental Digital Content). Urine nitrate concentrations were divided by the urine creatinine concentration, measured using a commercially available colorimetric assay (R & D Systems, Inc., Minneapolis, MN, USA), to adjust for urine dilution (19). Results are expressed as μM urine nitrate / mM urine creatinine. Values were not available in two septic subjects because one subject’s urine specimen was lost and another subject’s urine creatinine was below the lower detection limit.
Statistical Analyses
Results are expressed as mean ± standard deviation (SD) or median (inter-quartile range [IQR]), as appropriate. Student’s t-test, the Wilcoxon rank-sum test, or the Wilcoxon matched pairs signed-ranks test was used to compare continuous or discrete variables between groups of subjects and between measurements from the same subjects at different times, as appropriate. The chi-square test was used to compare categorical variables.
Spearman’s rank correlation coefficients (rho) were calculated between Arg/DMA or Arg/(Cit + Orn) and other continuous variables, including urinary nitrate concentrations, acute physiology and chronic health evaluation II (APACHE II) scores (using data from the first 24 hours of ICU admission), sepsis-related organ failure assessment (SOFA) scores, number of organ failure-free days from day 0-28, and number of ventilator-free days from day 0-28 (20-22). The Kaplan-Meier method was used to assess the relationship between Arg/DMA or Arg/(Cit+Orn) and 6 month survival (23).
Multivariable logistic regression was used to assess the independent associations between Arg/DMA or Arg/(Cit+Orn) ratios and severe sepsis while controlling for potential confounding variables. Multivariable logistic regression and Cox proportional hazards analyses were used to assess the independent relationship between these ratios and hospital mortality or risk of death over 6 months, respectively, while controlling for potential confounding variables (see statistical methods in Supplemental Digital Content).
Statistical analyses were performed using Stata Statistical Software: Release 9 (College Station, TX: StataCorp LP).
RESULTS
From February 2006 through May of 2008, 430 patients with clinical suspicion for severe sepsis were screened and 109 patients were enrolled (Figure 1). Fifty age- and gender-stratified control subjects without acute illness were recruited. Clinical characteristics of the study subjects are shown in Table 1. In severe sepsis subjects, the initial blood samples were obtained 30 (20 – 39) hours after diagnosis, and statistical analyses are based on these measurements unless otherwise specified. Thirty-one severe sepsis patients died during hospitalization: 25 (80%) during the initial sepsis episode, 3 (10%) during a recurrent sepsis episode, and 3 (10%) from cardiovascular causes after sepsis had clinically resolved.
Table 1.
Clinical characteristics of study subjects a
| Controls (n=50) |
Severe sepsis (n=109) |
p | Survivors (n=78) |
Non-survivors (n=31) |
p | |
|---|---|---|---|---|---|---|
| Age | 59 (53 – 66) | 62 (51 - 75) | 0.23 | 57 (49 – 69) | 72 (61 – 83) | < 0.001 |
| Male sex | 25/50 (50%) | 62/109 (57%) | 0.42 | 44/78 (56%) | 18/31 (58%) | 0.88 |
| Race | 0.21 | 0.14 | ||||
| Caucasian | 47 (94%) | 89 (82%) | -- | 65 (83%) | 24 (77%) | |
| African – American | 3 (6%) | 17 (16%) | -- | 12 (15%) | 5 (16%) | |
| Asian | 0 (0%) | 1 (1%) | -- | 1 (1%) | 0 | |
| Hispanic/ Latino | 0 (0%) | 2 (2%) | -- | 0 | 2 (6%) | |
| History of Hypertension | 10 (20%) | 69 (63%) | <0.001 | 43 (55%) | 26 (84%) | 0.005 |
| Charlson score | 0 (0-1) | 3 (1-6) | <0.001 | 2.5 (1-5) | 4 (2 – 8) | 0.05 |
| Creatinine (mg/dL) | 0.7 (0.6-0.8) | 1.3 (0.9 – 2.1) | <0.001 | 1.1 (0.8 – 1.6) | 1.8 (1.3 – 2.6) | 0.001 |
| Surgical patient | -- | 11 (10%) | -- | 5 (6%) | 6 (19%) | 0.043 |
| Medical patient | -- | 98 (90%) | -- | 73 (94%) | 25 (81%) | |
| APACHE II score | -- | 24 (± 9) | -- | 22 (± 7) | 31 (± 9) | < 0.001 |
| Dysfunctional organs b | 0.008 | |||||
| 1 | -- | 19 (17%) | -- | 18 (23%) | 1 (3%) | -- |
| 2 | -- | 35 (32%) | -- | 28 (36%) | 7 (22%) | -- |
| 3 | -- | 25 (23%) | -- | 16 (21%) | 9 (29%) | -- |
| ≥ 4 | -- | 55 (28%) | -- | 16 (20%) | 14 (45%) | -- |
| Site of Infection | 0.84 | |||||
| Pulmonary | -- | 62 (57%) | -- | 42 (54%) | 20 (64%) | -- |
| Intra-abdominal | -- | 18 (16%) | -- | 14 (18%) | 4 (13%) | -- |
| Urinary | -- | 13 (12%) | -- | 10 (13%) | 3 (10%) | -- |
| Other | -- | 9 (8%) | -- | 6 (8%) | 3 (10%) | -- |
| Skin/ catheter | -- | 5 (4%) | -- | 4 (5%) | 1 (3%) | -- |
| Bloodstream | -- | 2 (2%) | -- | 2 (2%) | 0 (0%) | -- |
| Infection type | 0.057 | |||||
| Gram + bacteria | -- | 34 (31%) | -- | 22 (28%) | 12 (39%) | -- |
| Gram − bacteria | -- | 16 (15%) | -- | 10 (13%) | 6 (19%) | -- |
| Unknown | -- | 31 (28%) | -- | 28 (36%) | 3 (10%) | -- |
| Other | -- | 28 (26%) | -- | 18 (23%) | 10 (32%) | -- |
| Blood culture + | -- | 46 (42%) | -- | 18 (23%) | 12 (39%) | 0.64 |
| Septic shock c | -- | 90 (83%) | -- | 60 (77%) | 30 (97%) | 0.01 |
| Vasopressor use | -- | 77 (71%) | -- | 47 (60%) | 30 (97%) | < 0.001 |
Definition of abbreviations: Charlson score = Charlson comorbidity index (24) APACHE II = acute physiology and chronic health evaluation II (21); survivors = hospital survivors, non-survivors = hospital non-survivors
Values are median (interquartile range), number (percentage), or mean (± SD)
organ dysfunctions as defined previously (49) with slight modification, including: cardiovascular (hypotension [systolic blood pressure < 90 mm Hg or mean arterial pressure < 60 mm Hg], vasopressor requirement, or clinical evidence of hypoperfusion); acid-base (metabolic acidosis and plasma lactate concentration > 2 mmol/L); renal (urine output < 0.5 mL/kg/hour despite fluid resuscitation); neurologic (altered mental status without other causes); respiratory (P:F ratio < 250, or < 200 if lungs are only dysfunctional organ); hematologic (platelet count < 80,000 or > 50% decrease from baseline)
shock = hypotension or vasopressor dependence that persisted for ≥ 3 hours despite fluid challenge
Severe sepsis vs. control subjects
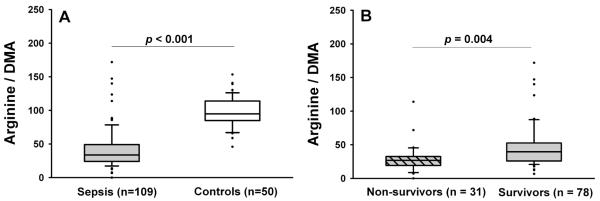
Severe sepsis patients had lower arginine, citrulline, and ornithine levels and higher ADMA and SDMA levels than control subjects (Table 2). Compared to control subjects, severe sepsis patients had lower Arg/DMA (Figure 2A), but similar Arg/(Cit+Orn) and Arg/Orn ratios (Table 2). The Arg/DMA ratio was independently associated with sepsis after adjustment for baseline differences in creatinine and Charlson comorbidity score (24) between control and severe sepsis subjects (OR = 5.91 per quartile decrease in Arg/DMA ratio, 95% CI = 2.45 – 14.26, p < 0.001, Table 3).
Table 2.
Arginine metabolites and bioavailability ratios a
| Control subjects vs. severe sepsis subjects |
Severe sepsis, hospital survivors vs. non-survivors |
|||||
|---|---|---|---|---|---|---|
| Variable | Control subjects (n=50) | Sepsis patients (n=109) | p value | Survivors (n=78) | Non-survivors (n=31) | p value |
| Arginine | 81 (73 – 97) | 48 (34 – 64) | < 0.001 | 48 (34 – 64) | 49 (36 – 71) | 0.719 |
| Citrulline | 32 (24 – 36) | 10 (7 – 15) | < 0.001 | 10 (7 – 14) | 12 (7 – 19) | 0.134 |
| Ornithine | 47 (39 – 60) | 29 (21 – 49) | < 0.001 | 28 (20 – 49) | 33 (21 – 70) | 0.407 |
| ADMA | 422 (395 – 475) | 468 (355 – 619) | 0.052 | 460 (352 – 554) | 558 (408 – 802) | 0.024 |
| SDMA | 442 (377 – 495) | 824 (549 – 1200) | < 0.001 | 729 (522 – 984) | 1180 (903 - 1550) | <0.001 |
| Arg/DMA | 95 (85 – 114) | 34 (24 – 48) | < 0.001 | 39 (26 – 52) | 27 (19 – 32) | 0.004 |
| Arg/(Cit+Orn) | 1.05 (0.98 – 1.17) | 1.04 (0.86 – 1.48) | 0.770 | 1.09 (0.89 – 1.53) | 0.95 (0.70 – 1.44) | 0.030 |
| Arg/Orn | 1.72 (1.58 – 1.86) | 1.57 (1.11 – 2.08) | 0.153 | 1.57 (1.17 – 2.18) | 1.70 (0.90 – 1.96) | 0.152 |
Values refer to median (interquartile range). Amino acid concentrations are micromolar, methylarginine concentrations are nanomolar, and methylarginine concentrations were converted to micromolar before calculation of the Arg/DMA ratio.
Figure 2.
Arg/DMA ratio in severe sepsis patients vs. control subjects (A) and severe sepsis non-survivors vs. survivors (B). Box plots the median (horizontal line), 25th and 75th percentiles (lower and upper limits of the box). The dots represent outliers beyond the whiskers that designate the 10th and 90th percentiles. Comparisons made with the Wilcoxon rank-sum test.
Table 3.
Multivariable analyses evaluating role of Arg/DMA ratio in predicting sepsis and outcomes
| Association between Arg/DMA ratio and severe sepsis | |||
| Dependent variable | Independent variables a | OR (95% CI) b | p value |
| Severe sepsis | Arg/DMA ratio | 5.91 (2.45 – 14.26) | <0.001 |
| Charlson score | 3.46 (1.64 – 7.30) | 0.001 | |
| Creatinine concentration | 1.72 (0.87 – 3.38) | 0.118 | |
| Association between Arg/DMA ratio and hospital mortality | |||
| Dependent variable | Independent variables c | OR (95% CI) b | p value |
|
| |||
| Hospital mortality | Arg/DMA ratio | 1.63 (1.00 – 2.65) | 0.048 |
| Age | 1.57 (1.01 – 2.44) | 0.046 | |
| Creatinine concentration | 1.58 (1.00 – 2.48) | 0.049 | |
| Association between Arg/DMA ratio and risk of death over 6 months | |||
| Dependent variable | Independent variables d | HR (95% CI) | p value |
|
| |||
| 6 – month mortality risk | Arg/DMA ratio | 1.41 (1.01 – 1.97) b | 0.043 |
| History of hypertension | 3.91 (1.51 – 10.14) | 0.005 | |
| Creatinine concentration | 1.22 (0.90 – 1.67) b | 0.198 | |
Definition of abbreviations: Arg/DMA ratio = Arginine/(asymmetric + symmetric dimethylarginine); OR = odds ratio; CI = confidence interval; HR = hazard ratio.
The initial model also included presence or absence or pre-existing hypertension, removed without significant deterioration in model fit as described in Methods.
These odds ratios and hazard ratios refer to a one quartile change in the value of the covariate.
The initial model also included Charlson comorbidity score and presence or absence or pre-existing hypertension. Both were removed without significant deterioration in model fit as described in Methods.
The initial model also included age and Charlson comorbidity score. Both were removed without significant deterioration in model fit as described in Methods.
Relationship of Arg/DMA and Arg/(Cit+Orn) to clinical outcomes and severity of illness in severe sepsis
Comparison of clinical variables between severe sepsis hospital survivors and non-survivors are shown in Table 1. Hospital non-survivors had similar amino acid levels but higher dimethylarginine levels compared to survivors (Table 2). The Arg/DMA ratio and to a lesser extent the Arg/(Cit+Orn) ratio were reduced in non-survivors vs. survivors (Table 2 and Figure 2B). The Arg/DMA ratio was negatively correlated with APACHE II score (Spearman’s rho = − 0.404, p < 0.001) and maximal SOFA score from day 0 – 7 (Spearman’s rho = −0.319, p < 0.001), and positively correlated with numbers of organ failure free days (Spearman’s rho = 0.302, p = 0.001) and ventilator-free days (Spearman’s rho = 0.262, p = 0.006). These illness severity measures and outcomes were not significantly correlated with the Arg/(Cit+Orn) or Arg/Orn ratios.
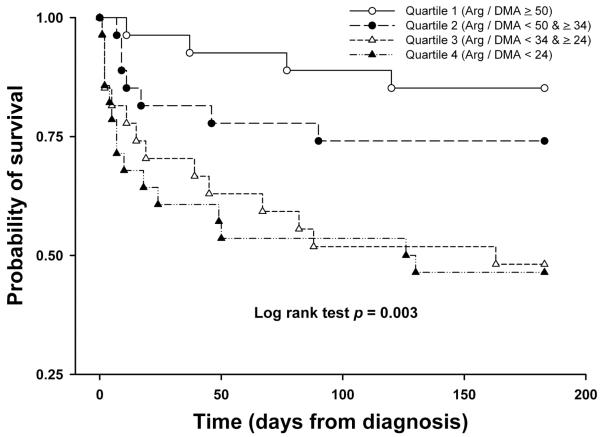
In univariate analysis, the odds ratio of hospital death increased progressively with decreasing Arg/DMA ratio (odds ratio [OR] = 2.13 per quartile decline of Arg/DMA ratio, 95% confidence interval [C.I.] = 1.38 – 3.29, p = 0.001). Six-month survival also decreased as the Arginine/DMA ratio declined (hazard ratio [HR] = 1.68 per quartile decline of Arg/DMA ratio, 95% CI = 1.25 – 2.26, p = 0.001, log rank test chi-square = 13.72, p = 0.003, Figure 3). The Arg/(Cit+Orn) ratio was associated with hospital mortality in univariate analysis (OR = 1.47 per quartile decline in Arg/(Cit+Orn) ratio, 95% C.I. = 1.00 – 2.16, p = 0.049) but did not predict 6 month survival (HR = 1.26, 95% CI = 0.95 -1.67, p = 0.105, log rank test chi-square = 2.89, p = 0.41). The Arg/Orn ratio was not associated with either hospital mortality or 6 month survival.
Figure 3.
Kaplan-Meier survival probability plot for quartiles of Arg/DMA ratio in the 6 months after severe sepsis diagnosis. There were no subjects lost to follow-up. Quartile 1 (Arg / DMA ≥ 50) = open circles; quartile 2 (Arg / DMA < 50 & ≥ 34) = closed circles; quartile 3 (Arg / DMA < 34 & ≥ 24) = open triangles; quartile 4 (Arg / DMA < 24) = closed triangles.
Multivariable analysis
Multivariable logistic regression showed that the Arg/DMA ratio was a significant independent predictor of hospital mortality (OR =1.63, 95% CI = 1.00 – 2.65 per quartile decrease in Arg/DMA ratio, p = 0.048, Table 3) after controlling for baseline differences in age and creatinine concentration (see Table 1 for baseline differences between groups and Supplemental Digital Content for details of multivariable modeling). Likewise, multivariable Cox proportional hazards analysis showed that the Arg/DMA ratio was a significant independent predictor of the risk of death over 6 months after controlling for a history of hypertension and creatinine concentration (HR = 1.41, 95% CI = 1.01 – 1.98 per quartile decrease in Arg/DMA ratio, p = 0.043, Table 3).
The Arg/(Cit+Orn) was not an independent predictor of hospital mortality (OR = 1.23 per quartile decrease in ratio, 95% CI = 0.81 – 1.87, p = 0.330), after controlling for age and creatinine in the final model.
Relationship Between Arg/DMA and Urinary Nitrate Concentrations
Urinary nitrate concentrations were significantly higher in controls (92.8 [IQR = 68.0 – 151.2] μM nitrate/mM creatinine) vs. sepsis patients (24.9 [6.6 – 67.0] μM nitrate/mM creatinine, p < 0.001), and non-significantly higher in sepsis survivors (30.8 [9.2 – 82.5] μM nitrate/mM creatinine) vs. non-survivors (16.5 [5.4 – 54.1] μM nitrate/mM creatinine, p = 0.077). Analyzing all paired plasma and urine measurements (n = 157), the Arg/DMA ratio was directly correlated with urinary nitrate concentration (Spearman’s rho = 0.449, p < 0.001, see Figure E2 in Supplemental Digital Content). This significant correlation was not evident, however, when separately analyzing the severe sepsis cases (Spearman’s rho = 0.132, p = 0.174) or the control subjects (Spearman’s rho = − 0.179, p = 0.214).
Subsequent measurements
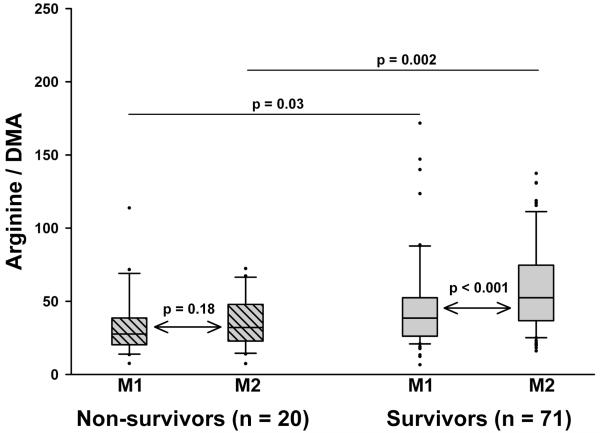
Ninety-one sepsis subjects had repeat plasma samples (Measurement 2) obtained 67 (48 – 72) hours after the initial measurements and 90 (79 – 110) hours after diagnosis. Eighteen subjects did not have repeat measurements because of death (n=10), insufficient sample (n=5), or hospital discharge (n=3). Over time, arginine, citrulline, ornithine, and ADMA levels increased in both survivors and non-survivors, while SDMA levels did not change (Table 4). At Measurement 2, ornithine, ADMA, and SDMA were significantly higher in non-survivors compared to survivors. The Arg/DMA ratio increased over time in survivors but did not change significantly in non-survivors, and remained lower in non-survivors vs. survivors at Measurement 2 (Figure 4). These results suggest improving systemic arginine availability in the survivors but persistent impairment in the non-survivors. While the Arg/(Cit+Orn) ratio did not change significantly over time in either survivors or non-survivors, the ratio remained lower in non-survivors vs. survivors at Measurement 2 (Table 4). The Arg/Orn ratio, with lower values suggesting higher arginase activity, trended upward in survivors but downward in non-survivors, such that this ratio was significantly lower in non-survivors vs. survivors at Measurement 2 (Table 4).
Table 4.
Measurement 1 vs. Measurement 2 in survivors and non-survivors a
| Hospital survivors (n = 71) |
Hospital non-survivors (n = 20) |
|||||
|---|---|---|---|---|---|---|
| Variable | Measurement 1 b | Measurement 2 c | p value | Measurement 1 b | Measurement 2 c | p value |
| Arginine | 48 (33 – 64) | 64 (50 – 89) | <0.001 | 49 (40.5 – 75.5) | 70 (61.5 – 78.5) | 0.008 |
| Ornithine | 28 (19 – 48) | 45 (30 – 61) | <0.001 | 39.5 (23 – 80) | 76.5 (55 – 92) d | 0.015 |
| Citrulline | 9 (7-13) | 14 (10-19) | <0.001 | 12 (4.5 – 18) | 18.5 (9.5 – 27.5) | 0.001 |
| ADMA | 461 (354 – 560) | 539 (433 – 681) | <0.001 | 596 (395 – 881.5) | 706.5 (577 – 842.5) e | 0.003 |
| SDMA | 735 (519 – 735) | 647 (457 – 1000) | 0.476 | 1125 (963.5 – 1555) | 1125 (925.5 – 2265) d | 0.093 |
| Arg/ DMA | 38 (26 – 52) | 52 (37 – 75) | <0.001 | 28 (21 – 38) | 32 (23 – 48) e | 0.179 |
| Arg/(Cit+Orn) | 1.12 (0.94 – 1.55) | 1.17 (0.85 – 1.47) | 0.125 | 0.95 (0.74 – 1.40) | 0.80 (0.58 – 1.13) e | 0.179 |
| Arg/ Orn | 1.61 (1.20 – 2.19) | 1.65 (1.11 – 1.96) | 0.062 | 1.31 (0.92 – 1.83) | 1.05 (0.74 – 1.51) e | 0.247 |
Values refer to median (interquartile range). Amino acid concentrations are micromolar, methylarginine concentrations are nanomolar, and methylarginine concentrations were converted to micromolar before calculation of the Arg/DMA ratio.
Samples for Measurement 1 were obtained 30 (20 – 39) hours after diagnosis
Samples for Measurement 2 were obtained 67 (48 – 72) hours after Measurement 1 and 90 (79 – 110) hours after diagnosis
p < 0.001 compared to corresponding Measurement 2 value in hospital survivors
p < 0.01 compared to corresponding Measurement 2 value in hospital survivors
Figure 4.
Arg/DMA in non-survivors and survivors at Measurement 1 (M1) and Measurement 2 (M2). Samples for M1 were obtained 30 (20 – 39) hours after diagnosis and samples for M2 were obtained 67 (48 – 72) hours after M1 and 90 (79 – 110) hours after diagnosis. This figure excludes patients without paired M1 and M2 measurements. Box plots the median (horizontal line), 25th and 75th percentiles (lower and upper limits of the box). The dots represent outliers beyond the whiskers that designate the 10th and 90th percentiles. Paired comparisons between M1 and M2 made with the Wilcoxon matched-pairs signed-ranks test. Comparisons between non-survivors and survivors at each time point were made with the Wilcoxon rank-sum test.
DISCUSSION
The main finding of our study is that the ratio of arginine to its endogenous metabolic inhibitors, ADMA and SDMA, is associated with severe sepsis, severity of illness, and independently predicts both hospital mortality and risk of death over the 6 months after diagnosis. Our results indicate that this estimate of systemic arginine availability could be a prognostically useful and pathophysiologically significant biomarker in severe sepsis, with potential therapeutic implications. Similar measurements and ratios have been calculated to estimate systemic arginine availability in a variety of cardiopulmonary disorders (5, 6, 25, 26), but to our knowledge such estimates have not been reported in septic patients.
The relationship between lower Arg/DMA and higher mortality suggests that impaired capacity for NO synthesis is a clinically relevant and detrimental feature of sepsis pathophysiology. This concept is supported by the direct correlation between Arg/DMA and urine nitrate shown in our post-hoc analysis and previous experimental studies. For example, Mittermayer and colleagues showed that arginine and the arginine/ADMA ratio decline within 4 hours of intravenous endotoxin in humans (14). Human endothelial cells exposed to lipopolysaccharide (LPS) and tumor necrosis factor-α (TNF-α) in vitro develop increased arginase activity and decreased endothelial NOS activity, suggesting diversion of arginine away from NO production (27). Similar experiments showed that TNF-α decreases endothelial dimethyl-aminohydrolase (DDAH) activity, the enzyme responsible for ADMA breakdown, resulting in higher ADMA concentrations (28). The hemodynamic consequences of these biochemical perturbations has been shown in rats treated with arginase (diverting arginine away from NO synthesis) and ADMA (blocking NO synthesis), causing decreased cardiac output, increased systemic vascular resistance, and decreased blood flow to liver, kidneys, and spleen (15). Additional detrimental effects of low systemic arginine availability may include immune deficiency, impaired wound healing, and oxidative stress (29, 30).
We find that arginine levels are lower in sepsis patients than in healthy subjects, consistent with several previous studies (7, 31). Previous kinetic studies show that arginine decrements in sepsis are related to both impaired production (8) and accelerated clearance (10). Importantly, enhanced arginine clearance appears to result from enhanced protein synthesis in sepsis (9), not augmented NO production (8, 9). Like our study, none of these papers report a significant relationship between arginine concentration and severity of illness or outcome.
ADMA levels have been associated with severity of illness in septic patients (13) and poor outcome in unselected critically ill patients (11, 12). Prior measurements of ADMA and SDMA in critical illness have used either an enzyme-linked immunoassay (ELISA) or HPLC. To our knowledge, our study is the first to measure methylarginines using HPLC-MS in sepsis. This method has significant advantages in separating and specifically quantifying ADMA and SDMA (32).
Unlike ADMA, SDMA does not inhibit NOS (2). Until recently SDMA was thought to have little role in NO physiology and was largely ignored except as a novel measure of renal function (33). However, recent studies show that SDMA inhibits NO synthesis and increases reactive oxygen species production in a dose-dependent fashion in endothelial cells (3, 34). The inhibitory effect of SDMA on NO production occurs through competitive inhibition of cellular arginine uptake via the cationic amino acid transporter system (4). SDMA also independently predicts coronary artery disease severity, cardiovascular events, and dialysis-associated hypotension (35, 36). These data show that SDMA is metabolically active and independently associated with cardiovascular disease. Importantly, the association we observed between the Arg/DMA ratio and mortality persisted even after controlling for serum creatinine concentration.
We found that the Arg/(Cit+Orn) ratio was similar in sepsis patients vs. controls. While this estimate of systemic arginine availability was lower in non-survivors compared to survivors, it was not an independent predictor of outcomes in multivariable analysis. This ratio of reactant (arginine) to enzymatic products (citrulline and ornithine) has been used previously to estimate systemic arginine availability because a lower ratio should reflect lower reactant availability. However, citrulline is metabolized back to arginine under physiological conditions (9, 37). This inter-conversion between product and reactant probably undermines the ability of this ratio to estimate systemic arginine availability.
Another limitation of the Arg/(Cit+Orn) ratio is that it will not reflect diversion of arginine metabolism from NOS to arginase, since products of both enzymes are in the denominator. Notably, ornithine was the only amino acid significantly different in non-survivors vs. survivors at Measurement 2 (higher in non-survivors). The Arg/Orn ratio has previously been validated as an estimate of arginase activity (6). Although there was no difference in this ratio at the first measurement, the rising ornithine concentration was greater over time in the non-survivors, resulting in a lower Arg/Orn ratio in non-survivors vs. survivors at Measurement 2. These results suggest a link between arginase activity and worsening sepsis pathophysiology in non-survivors.
The therapeutic implications of our findings are worthy of consideration. Arginine supplementation has been studied in critically ill patients with mixed and controversial results (29, 37, 38), some studies suggesting harm particularly in patients with severe sepsis (39, 40). Although these clinical trials are limited by co-administration of several immunonutrients, insufficient power, and methodological weaknesses (37), they are supported by experimental sepsis studies showing that arginine therapy is harmful (41). Published nutritional guidelines recommend against arginine supplementation in critically ill septic patients (42).
Our results confirm low arginine levels in severe sepsis but indicate that outcomes are predicted only by the Arg/DMA ratio. Moreover, although we found that arginine levels rise over time during the course of sepsis, the Arg/DMA ratio remains low in non-survivors, while it improves in survivors. These findings suggest the potential for novel therapeutic interventions that could specifically increase systemic arginine availability in severe sepsis, without administering exogenous arginine. For example, oxidative stress decreases DDAH activity, slows ADMA clearance, and increases ADMA production (43, 44). These effects are prevented by antioxidant therapy. Anti-oxidant therapy has been studied in sepsis and a compilation of the evidence suggests benefit (45). The Arg/DMA ratio could be used to specifically identify patients for enrollment in clinical trials of anti-oxidant therapy. Sepsis is a heterogeneous syndrome, and biomarker-based identification of patients eligible for tailored therapies offers promise (1). In addition, the declining Arg/Orn ratio in non-survivors could warrant consideration of arginase inhibition as a novel therapeutic strategy that could be offered later in the course of sepsis (46).
Study Limitations
Our study shares the limitation of all observational research in that it determines associations, not causality. Therefore, the possible detrimental effects of low Arg/DMA discussed herein are speculative and must be further evaluated.
It is important to emphasize that the Arg/DMA ratio only estimates systemic arginine availability. The Arg/DMA ratio does not include monomethylarginine (MMA), another PRMT-catalyzed arginine metabolite that inhibits NOS (47) and was included in the denominator of the ratio reported by Lara, et al (5). However, MMA concentrations are approximately one-tenth those of either ADMA or SDMA, so its exclusion has only a small effect on the magnitude of the ratio. The Arg/DMA ratio also does not provide precise insights about the metabolic fate of arginine in severe sepsis. The latter requires radio-isotope studies that were beyond the scope of this study. However, such studies have been performed previously in smaller cohorts and support the concept that arginine bioavailability and nitric oxide production are reduced in sepsis (8, 10). Likewise, we also could not determine whether DMA concentrations were correlated with the extent of protein breakdown because we did not measure nitrogen balance or other indices of protein catabolism.
It is also important to note that urine nitrate concentrations only roughly estimate systemic NO production (48). We were unable to determine which of the nitric oxide synthase isoforms were most active in our septic patients, and whether they may have been affected differently by altered concentrations of arginine and its metabolites. In addition, the urine nitrate measurements were performed post-hoc, so these findings serve only to generate hypotheses and require further study.
Finally, because we chose a relatively healthy control group, we cannot rule out the possibility that our findings are applicable to non-septic critically ill subjects. This healthy control group was necessary to establish expected ranges of arginine metabolites in subjects with age and gender distributions similar to our severe sepsis patients. The possibility that the Arg/DMA ratio is a useful predictor of illness severity and outcomes in non-septic critically ill patients warrants additional study.
CONCLUSIONS
The Arg/DMA ratio is reduced in severe sepsis and associated with illness severity. A low Arg/DMA ratio independently predicts higher hospital mortality and lower 6-month survival. Novel interventions that augment systemic arginine availability but do not require arginine supplementation should be studied in severe sepsis.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the trust and generosity of our patients and their families.
Grant Funding: NIH K23 HL080077, NCRR 1 UL1 RR024160-01, T32 HL066988
Footnotes
Reprints will not be ordered
Dr. Bottiglieri received honoraria/speaking fees and a grant from PamLab LLC. Dr. Bottiglieri also holds stock options with MSI Inc. The remaining authors have not disclosed any potential conflicts of interest.
LIST OF SUPPLEMENTAL DIGITAL CONTENT Supplemental Digital Content contains text describing laboratory measurements, text, tables, and a figure showing relevant statistical analyses, and a supplemental results figure.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Trzeciak S, Cinel I, Dellinger P, Shapiro NI, Arnold RC, Parrillo JE, Hollenberg SM. Resuscitating the microcirculation in sepsis: The central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15:399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallance P, Leiper J, Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:Dimethylarginine dimethylaminohydrolase pathway. Arteriosclerosis, Thrombosis & Vascular Biology. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 3.Bode-Boger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H, Bode-Boger SM, Scalera F, Kielstein JT, et al. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128–1134. doi: 10.1681/ASN.2005101119. [DOI] [PubMed] [Google Scholar]
- 4.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of l-arginine analogues with l-arginine transport mediated by the y+ carrier hcat-2b. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 5.Lara A, Khatri SB, Want Z, Cornhair SAA, Xu W, Dweik RA, Bodine M, Levison BS, Hammel J, Bleecker E, et al. Alterations of the arginine metabolome in asthma. Am J Respir Crit Care Med. 2008;178:673–681. doi: 10.1164/rccm.200710-1542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund H, Atamian S, Holroyde J, Fischer JE. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg. 1979;190:571–576. doi: 10.1097/00000658-197911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalpando S, Gopal J, Balasubramanyam A, Bandi VP, Guntupalli K, Jahoor F. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am J Clin Nutr. 2006;84:197–203. doi: 10.1093/ajcn/84.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Luiking YC, Poeze M, Ramsay G, Deutz NEP. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142–152. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 10.Druml W, Heinzel G, Kleinberger G. Amino acid kinetics in patients with sepsis. Am J Clin Nutr. 2001;73:908–913. doi: 10.1093/ajcn/73.5.908. [DOI] [PubMed] [Google Scholar]
- 11.Nijveldt RJ, Teerlink T, van der Hoven B, Siroen MPC, Kuik DJ, Rauwerda JA, van Leeuwen PAM. Asymmetrical dimethylarginine (adma) in critically ill patients: High plasma adma concentration is an independent risk factor of icu mortality. Clin Nutr. 2003;22:22–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- 12.Siroen MPC, van Leeuwen PAM, Nijveldt RJ, Teerlink T, Wouters PJ, Van den Berghe G. Modulation of asymmetric dimethylarginine in critically ill patients receiving intensive insulin treatment: A possible explanation of reduced morbidity and mortality? Crit Care Med. 2005;33:504–510. doi: 10.1097/01.ccm.0000155784.59297.50. [DOI] [PubMed] [Google Scholar]
- 13.O’Dwyer M, Dempsey F, Crowley V, Kelleher DP, McManus R, Ryan T. Septic shock is correlated with asymmetrical dimethylarginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase ii gene: A prospective observational study. Crit Care. 2006;10:R139. doi: 10.1186/cc5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittermayer F, Namiranian K, Pleiner J, Schaller G, Wolzt M. Acute escherichia coli endotoxaemia decreases the plasma l-arginine/ asymmetrical dimethylargine ratio in humans. Clin Sci. 2004;106:577–581. doi: 10.1042/CS20030363. [DOI] [PubMed] [Google Scholar]
- 15.Richir MC, van Lambalgen AA, Teerlink T, Wisselink W, Bloemena E, Prins HA, de Vries TP, van Leeuwen PA, Richir MC, van Lambalgen AA, et al. Low arginine/asymmetric dimethylarginine ratio deteriorates systemic hemodynamics and organ blood flow in a rat model. Crit Care Med. 2009;37:2010–2017. doi: 10.1097/CCM.0b013e31819ffdaf. [DOI] [PubMed] [Google Scholar]
- 16.Gough MS, Morgan MA, Mack CM, Darling DC, Frasier LM, Doolin KP, Apostolakos MJ, Arning E, Bottiglieri T, Mooney R, et al. Reduced arginine bioavailability in sepsis [abstract] Am J Respir Crit Care Med. 2009;179:A1157. [Google Scholar]
- 17.Morgan MA, Frasier LM, Stewart JC, Mack CM, Gough MS, Graves BT, Apostolakos MJ, Doolin KP, Darling DC, Frampton MW, et al. Artery-to-vein differences in nitric oxide metabolites are diminished in sepsis. Crit Care Med. 2010;38:1069–1077. doi: 10.1097/CCM.0b013e3181d16a3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 19.McClintock DE, Starcher B, Eisner MD, Thompson BT, Hayden DL, Church GD, Matthay MA. Higher urine desmosine levels are associated with mortality in patients with acute lung injury. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2006;291:L566–571. doi: 10.1152/ajplung.00457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE, The antioxidant in ards study group A trial of antioxidants n-acetylcysteine and procysteine in ards. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache ii: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The sofa (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 23.Woodward M. Epidemiology: Study design and data analysis. Chapman & Hall/ CRC Press; Boca Raton: 2005. [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Wanby P, Teerlink T, Brudin L, Brattstrom L, Nilsson I, Palmqvist P, Carlsson M. Asymmetric dimethylarginine (adma) as a risk marker for stroke and tia in a swedish population. Atherosclerosis. 2006;185:271–277. doi: 10.1016/j.atherosclerosis.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Bae SW, Stuhlinger MC, Yoo HS, Yu KH, Park HK, Choi BY, Lee YS, Pachinger O, Choi YH, Lee SH, et al. Plasma asymmetric dimethylarginine concentrations in newly diagnosed patients with acute myocardial infarction or unstable angina pectoris during two weeks of medical treatment. Am J Cardiol. 2005;95:729–733. doi: 10.1016/j.amjcard.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Bachetti T, Comini L, Francolini G, Bastianon D, Valetti B, Cadei M, Grigolato P, Suzuki H, Finazzi D, Albertini A, et al. Arginase pathway in human endothelial cells in pathophysiological conditions. J Mol Cell Cardiol. 2004;37:515–523. doi: 10.1016/j.yjmcc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 29.Barbul A, Uliyargoli A, Barbul A, Uliyargoli A. Use of exogenous arginine in multiple organ dysfunction syndrome and sepsis. Crit Care Med. 2007;35:S564–567. doi: 10.1097/01.CCM.0000279188.97421.FE. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vente JP, von Meyenfeldt MF, van Eijk HMH, van Berlo CLH, Gouma DJ, van der Linden CJ, Soeters PB. Plasma amino acid profiles in sepsis and stress. Ann Surg. 1989;209:57–62. doi: 10.1097/00000658-198901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martens-Lobenhoffer J, Bode-Boger SM. Measurement of asymmetric dimethylarginine (adma) in human plasma: From liquid chromatography estimation to liquid chromatography-mass spectrometry quantification. Eur J Clin Pharmacol. 2006;62:61–68. [Google Scholar]
- 33.Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D, Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (sdma) as endogenous marker of renal function--a meta-analysis. Nephrol Dial Transplant. 2006;21:2446–2451. doi: 10.1093/ndt/gfl292. [DOI] [PubMed] [Google Scholar]
- 34.Schepers E, Glorieux G, Dhondt A, Leybaert L, Vanholder R, Schepers E, Glorieux G, Dhondt A, Leybaert L, Vanholder R. Role of symmetric dimethylarginine in vascular damage by increasing ros via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24:1429–1435. doi: 10.1093/ndt/gfn670. [DOI] [PubMed] [Google Scholar]
- 35.Kiechl S, Lee T, Santer P, Thompson G, Tsimikas S, Egger G, Holt DW, Willeit J, Xu Q, Mayr M, et al. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis. 2009;205:261–265. doi: 10.1016/j.atherosclerosis.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 36.Mangoni AA, Hewitson CL, Woodman RJ, Whiting MJ, McAteer-Carr B, Barbara JA, Mangoni AA, Hewitson CL, Woodman RJ, Whiting MJ, et al. Symmetric dimethylarginine is an independent predictor of intradialytic hypotension. Am J Hypertens. 2008;21:955–959. doi: 10.1038/ajh.2008.214. [DOI] [PubMed] [Google Scholar]
- 37.Kalil AC, Danner RL, Kalil AC, Danner RL. L-arginine supplementation in sepsis: Beneficial or harmful? Curr Opin Crit Care. 2006;12:303–308. doi: 10.1097/01.ccx.0000235206.92697.bf. [DOI] [PubMed] [Google Scholar]
- 38.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–953. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 39.Bower RH, Cerra FB, Bershadsky B, Licari JJ, Hoyt DB, Jensen GL, Van Buren CT, Rothkopf MM, Daly JM, Adelsberg BR. Early enteral administration of a formula (impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: Results of a multicenter, prospective, randomized, clinical trial. Crit Care Med. 1995;23:436–449. doi: 10.1097/00003246-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, Zanforlin G, Tognoni G, Bertolini G, Iapichino G, et al. Early enteral immunonutrition in patients with severe sepsis: Results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003;29:834–840. doi: 10.1007/s00134-003-1711-5. [DOI] [PubMed] [Google Scholar]
- 41.Kalil AC, Sevransky JE, Myers DE, Esposito C, Vandivier RW, Eichacker P, Susla GM, Solomon SB, Csako G, Costello R, et al. Preclinical trial of l-arginine monotherapy alone or with n-acetylcysteine in septic shock. Crit Care Med. 2006;34:2719–2728. doi: 10.1097/01.CCM.0000242757.26245.03. [DOI] [PubMed] [Google Scholar]
- 42.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P, Canadian Critical Care Clinical Practice Guidelines C. Heyland DK, Dhaliwal R, Drover JW, Gramlich L, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. Jpen: Journal of Parenteral & Enteral Nutrition. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 43.Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW, Li YJ, Jia S-J, Jiang D-J, Hu C-P, Zhang X-H, et al. Lysophosphatidylcholine-induced elevation of asymmetric dimethylarginine level by the nadph oxidase pathway in endothelial cells. Vascul Pharmacol. 2006;44:143–148. doi: 10.1016/j.vph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Xiao HB, Jun F, Lu XY, Chen XJ, Chao T, Sun ZL, Xiao H-B, Lu X-Y, Chen X-j, Sun Z-L. Protective effects of kaempferol against endothelial damage by an improvement in nitric oxide production and a decrease in asymmetric dimethylarginine level. Eur J Pharmacol. 2009;616:213–222. doi: 10.1016/j.ejphar.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Heyland DK, Dhaliwal R, Suchner U, Berger MM, Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: A systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 46.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Chamption HC, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 47.Vallance P, Leiper J, Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:Dimethylarginine dimethylaminohydrolase pathway. Arterioscl Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 48.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;1411:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 49.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steinbrub JS, Garber GE, Helterbrand JD, Ely W, et al. Efficacy and safety of recombinant human activated protein c for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.