Abstract
Background
Some studies have found worse prognosis among COX-2 expressing breast cancers. Aspirin and NSAIDs inhibit COX-2. Three studies, including ours, have reported a survival advantage among women with breast cancer who take either aspirin or NSAIDs. We hypothesized that in the Nurses’ Health Study (NHS), COX-2 expression would be associated with worse prognosis, and aspirin use would be associated with better survival particularly among women with COX-2 positive tumors.
Methods
We studied 2,001 women with invasive breast cancers stained for COX-2 by immunohistochemistry. Tumor prognostic factors were from medical records. Aspirin use was assessed at least 12 months after diagnosis and updated. Cause of death was from death certificates. Statistical analyses included logistic regression of prognostic factors with COX-2 status as the outcome, and proportional hazards regression with breast cancer death as the outcome.
Results
Tumor COX-2 expression was associated with higher diagnostic stage. Compared with stage I, the RR(95% CI) for stages II-IV were 1.16(0.93–1.45), 1.68(1.27–2.22), and 1.76(0.93–3.32). COX-2 expression was associated with lobular compared with ductal histology, (1.40[1.02–1.92]), and estrogen receptor positive compared with negative (2.22[1.66–2.95]). The RR(95% CI) of breast cancer death for current aspirin use was similar for women with COX-2 positive and negative tumors; 0.64(0.43–0.96) and 0.57(0.44–0.74) respectively.
Conclusions
In the NHS, COX-2 breast cancer expression was associated with higher stage at diagnosis. The survival benefit associated with aspirin use did not differ by COX-2 status.
Impact
COX-2 breast cancer expression is associated with worse prognosis. If aspirin truly impacts breast cancer survival, it is not solely via COX-2.
Keywords: breast neoplasms, cyclooxygenase-2, tumor markers, prognosis, survival, aspirin
INTRODUCTION
Non-steroidal anti-inflammatory drug (NSAID) or aspirin use may be associated with a survival benefit among women with breast cancer. [1,2] For example, in the Nurses’ Health Study (NHS) we recently reported a reduced risk of breast cancer death for current aspirin use among women with a history of stages I-III breast cancer. Compared to no use, current users had a relative risk (RR) and 95% confidence interval (CI) of breast cancer death equal to 0.51(0.41–0.65). Results were similar for recurrence. [3] This result is particularly notable because there is no association between aspirin and breast cancer incidence in the NHS. [4] However, most epidemiological studies of aspirin or NSAID use have found a 9–30% reduced risk of developing breast cancer with regular use.[5]
Also in the NHS, aspirin use was associated with improved survival among women with colorectal cancer; and the effect was limited to cyclooxygenase-2 (COX-2) positive tumors. Compared to non-users of aspirin, the RR(95% CI) of colorectal cancer death among aspirin users was 0.39(0.20–0.76) among those whose tumors are COX-2 positive and 1.25(0.37–4.22) among those whose tumors are COX-2 negative. [6]
The prevalence and clinical significance of COX-2 expression among breast cancers is not as well described as that in colonc cancer. In the largest study to date of 1, 576 breast cancers, COX-2 expression by immunohistochemistry was found in 40% of tumors and was associated with markers of worse prognosis, including larger tumor size, higher grade, high proliferation index, and lymph node metastasis, and also worse survival. Five-year distant disease-free survival (DFS) was 83% (95% CI 81–86) among those with COX-2 negative tumors and 73% (95% CI 70–77) among those with COX-2 positive tumors (p<0.0001) [7] Although most other studies have confirmed the association of COX-2 breast cancer expression with worse prognosis. [8–16], one found no association with prognosis [17] and another found an inverse association. [18] In terms of survival, results have been more inconsistent. Several studies have confirmed the association of COX-2 breast cancer expression with worse survival [9,11–14,16,17,19], while others have found no association with survival. [8,10,15,18]
We studied tumor COX-2 expression among women diagnosed with invasive breast cancer in the NHS. We hypothesized that having a COX-2 positive tumor would be associated with worse breast cancer prognostic factors and a higher risk of dying of breast cancer. We further hypothesized that like colon cancer, aspirin intake after a breast cancer diagnosis would be associated with improved survival particularly among those women whose tumors were COX-2 positive.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of Brigham and Women’s Hospital, Boston MA. The NHS was established in 1976 when 121,700 female registered United States nurses, aged 30 to 55 years, answered a mailed questionnaire on cancer and cardiovascular risk factors. We have sent questionnaires every 2 years since. As of June 2010, follow-up of the entire cohort’s person-years is 95% complete. Collection of breast cancer tissue blocks and tissue microarray (TMA) construction have been described in detail previously.[20] Briefly, we collected archived formalin-fixed paraffin-embedded breast cancer blocks from participants with incident breast cancers over 20 years of follow-up (1976 to 1996). TMAs were constructed in the Dana Farber Harvard Cancer Center Tissue Microarray Core Facility, Boston, Massachusetts. Three cores 0.6 mm in diameter were obtained from each breast cancer sample and inserted into the recipient TMA blocks. We performed immunohistochemical staining for COX-2 on 5 μm paraffin sections cut from the TMA blocks. Immunostaining was performed in a single staining run on a Dako Autostainer (Dako Corporation, Carpinteria, CA). The primary monoclonal antibody COX-2 (Clone SP21 from LabVision) was applied to the sections and the slides were incubated for 30 minutes at room temperature followed by incubation with the HRP labeled polymer; DAB was used as the chromagen substrate. Visualization was performed using DAKO Envision automated detection system. Appropriate positive and negative controls were included in all staining runs. Immunostained TMA sections were reviewed under a microscope and visually scored for each individual tissue core. For COX-2, cytoplasmic staining for each core was scored as negative, 1+ (weak diffuse cytoplasmic staining), 2+ (moderate to strong cytoplasmic staining) or 3+ (>90% tumor cell stained with strong intensity). For this analysis, cases scored as negative (0) were considered negative and those scored as 1+,2+ or 3+ were considered positive. Overall scoring was as follows: if any one core was positive the case was scored as positive, and when all three cores were negative the case was scored as negative. Two examples of COX-2 positive staining tissue samples are shown in Figure 1. HER2neu status was measured in a similar fashion. There were TMA samples from 2,862 participants eligible for inclusion in this study. Participants were excluded for the following reasons: 148 were missing stage information, 263 had in situ tumors, 179 were missing COX-2 staining, 250 were missing information on aspirin use, 17 died in the same 2 year cycle as their breast cancer was diagnosed and thus they could not report on aspirin use after their diagnosis, and 5 were excluded for other reasons, leaving 2,001.
Figure 1.
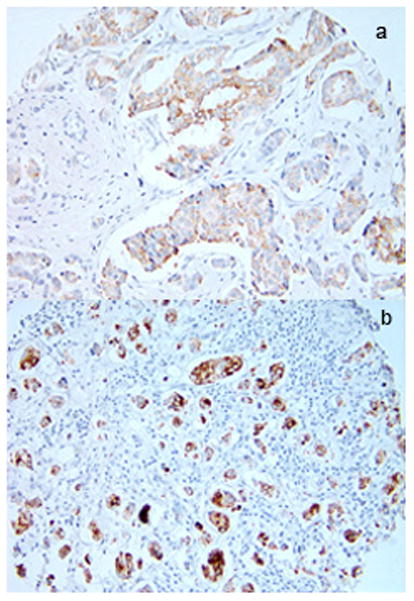
Two examples (a and b) of COX-2 positive staining breast cancers (magnified 20X)
We included 2,001 women in the NHS diagnosed with Stages I-IV invasive breast cancer between 1976 and 1996, from whom we were able to collect a tumor sample for TMA with COX2 data. For any report of breast cancer, participants gave written permission for physicians (blinded to exposure information) to review their medical records. Tumor prognostic factors including size, grade, histology, estrogen receptor (ER), progesterone receptor (PR), presence of metastatic lymph nodes and stage were extracted from the medical record. Initial treatment received (radiation, chemotherapy, and hormonal therapy) was self-reported by a supplemental questionnaire. Aspirin intake was assessed at least 12 months after diagnosis to avoid the period of active treatment when aspirin is often contraindicated. Date and cause of death were determined by physician review of death certificates.
Aspirin use was first assessed in 1980 and every two years thereafter except for 1986; 1984 use was carried forward for 1986. Aspirin use was characterized as current use versus never/past use. Aspirin use was first assessed using the questionnaire which occurred after the questionnaire in which the participant reported her breast cancer diagnosis, and subsequently updated until death or the end of follow-up.
In statistical analyses, we used logistic regression of prognostic factors with COX-2 status as the outcome. We also used Cox proportional hazards regression with time since diagnosis as the underlying time variable and death from breast cancer as the outcome; other causes of death were censored. Women were followed from diagnosis until death or June 2008, whichever came first. Simple models were adjusted for time since diagnosis and age at diagnosis. Multivariable models were additionally adjusted for disease stage at diagnosis, treatment, and the other factors shown in the footnote to Table 2.
Table 2.
RR(95%CI) of breast cancer death for women with COX2+ tumors, adjusted for time since diagnosis (TSD) and the following additional factors.
| Adjustment factors | RR | 95% CI |
|---|---|---|
| TSD + age | 1.35 | 1.11 – 1.64 |
| Multivariable, without including stage at diagnosis* | 1.37 | 1.13 – 1.67 |
| Multivariable, including stage at diagnosis** | 1.19 | 0.97 – 1.45 |
Adjusted for the following factors at the time of diagnosis: age (continuous), calendar year, smoking status (never, current, past), body mass index in kg/m2 (<21, 21–22.9, 23–24.9, 25–28.9, ≥29), age at first birth and parity (nulliparous, <25 years and 1–2 births, <25 years and ≥3 births, ≥ 25 years and 1–2 births, ≥ 25 years and ≥ 3 births), oral contraceptive use (never, ever), menopausal status and use of hormone replacement (premenopausal, unknown, postmenopausal never-user, postmenopausal past user, postmenopausal current user), radiation treatment (yes/no), systemic treatment with chemotherapy and/or hormonal therapy (chemo no and hormonal no, chemo yes and hormonal no, chemo no and hormonal yes, chemo yes and hormonal yes) They are additionally adjusted for the following factors after diagnosis and treatment: protein and energy intake (quintiles), physical activity (quintiles), weight change (loss ≥0.5 kg/m2, gain ≥0.5 kg/m2, maintained weight),
Additionally adjusted for disease stage at diagnosis (I, II, III, IV)
RESULTS
Table 1 shows the odds ratio of COX-2 positive tumors compared to COX-2 negative tumors by selected breast cancer prognostic factors. Of the 2,001 samples, 28.0% were COX-2 positive and 72.0% were COX-2 negative. COX-2 positive tumors were associated with higher stage at diagnosis, larger tumor size, more nodal metastases, lobular histology, ER positive status, and PR positive status. COX-2 status was not associated with tumor grade or HER2neu status.
Table 1.
Odds ratio of COX-2 positive expression by selected breast cancer prognostic factors
| Prognostic factor | COX-2 positive | COX-2 negative | OR (95% CI) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Overall | 561 | 28.0 | 1440 | 72.0 | |
|
| |||||
| Stage | |||||
| 1 | 252 | 25.2 | 748 | 74.8 | 1.00 |
| 2 | 186 | 28.1 | 476 | 71.9 | 1.16(0.93–1.45) |
| 3 | 107 | 36.2 | 189 | 63.9 | 1.68(1.27–2.22) |
| 4 | 16 | 37.2 | 27 | 62.8 | 1.76(0.93–3.32) |
|
| |||||
| Grade | |||||
| 1 | 48 | 35.3 | 88 | 64.7 | 1.00 |
| 2 | 115 | 27.1 | 309 | 72.9 | 0.68(0.45–1.03) |
| 3 | 104 | 26.7 | 285 | 73.3 | 0.67(0.44–1.02) |
| missing | 294 | 28.0 | 758 | 72.1 | --- |
|
| |||||
| Tumor size (cm) | |||||
| ≤1 | 127 | 25.5 | 372 | 74.6 | 1.00 |
| 1.1–2.0 | 203 | 26.5 | 564 | 73.5 | 1.05(0.82–1.36) |
| 2.1–4.0 | 162 | 29.9 | 380 | 70.1 | 1.25(0.95–1.64) |
| >4.0 | 67 | 35.5 | 122 | 64.6 | 1.61(1.12–2.31) |
| missing | 2 | 50.0 | 2 | 50.0 | --- |
|
| |||||
| Lymph nodes | |||||
| 0 | 337 | 25.3 | 994 | 74.7 | 1.00 |
| 1–3 | 120 | 31.9 | 256 | 68.1 | 1.38(1.08–1.78) |
| 4–9 | 52 | 34.0 | 101 | 66.0 | 1.52(1.06–2.17) |
| ≥10 | 36 | 36.7 | 62 | 63.3 | 1.71(1.12–2.63) |
| Metastatic at diagnosis | 16 | 37.2 | 27 | 62.8 | 1.75(0.93–3.28) |
|
| |||||
| Histology | |||||
| Ductal | 462 | 27.2 | 1236 | 72.8 | 1.00 |
| Lobular | 67 | 34.4 | 128 | 65.6 | 1.40(1.02–1.92) |
| missing, other | 32 | 29.6 | 76 | 70.4 | --- |
|
| |||||
| ER status | |||||
| Negative | 68 | 17.4 | 323 | 82.6 | 1.00 |
| Positive | 414 | 31.8 | 888 | 68.2 | 2.22(1.66–2.95) |
| missing | 79 | 25.7 | 229 | 74.4 | --- |
|
| |||||
| PR status | |||||
| Negative | 130 | 22.4 | 450 | 77.6 | 1.00 |
| Positive | 320 | 32.1 | 676 | 67.9 | 1.64(1.29–2.08) |
| missing | 111 | 26.1 | 314 | 73.9 | --- |
|
| |||||
| HER2 status | |||||
| Negative | 498 | 28.6 | 1229 | 71.4 | 1.00 |
| Positive | 58 | 26.2 | 163 | 73.8 | 0.89(0.65–1.22) |
Table 2 shows the relative risk of breast cancer death for women with COX-2 positive compared to COX-2 negative tumors, adjusted for different covariates. In the simple model adjusted only for time since diagnosis and age, having a COX-2 positive tumor was associated with a 35% increased risk of breast cancer death; RR(95% CI) = 1.35(1.11–1.64). The RR(95% CI) for a model adjusted for all covariates except disease stage at diagnosis was nearly identical to that of the simple model: 1.37(1.13–1.67). However, this elevated risk of death from breast cancer was attenuated with the addition of disease stage at diagnosis to the multivariable model: RR(95% CI) = 1.19 (0.97–1.45). Thus the higher risk of breast cancer death among women with COX-2 positive tumors was fully accounted for by worse stage at diagnosis.
Table 3 shows the relative risk of breast cancer death according to current aspirin use, stratified by COX-2 expression of the tumor. The decreased risk of breast cancer death associated with aspirin use did not differ by whether the tumor was COX-2 positive or negative.
Table 3.
RR(95% CI) of breast cancer death according to current aspirin use, among all women, and stratified by COX-2 expression
| Among | N/# breast cancer deaths | Type of adjustment | RR | 95% CI |
|---|---|---|---|---|
| All women | 2001/473 | simple* | 0.58 | 0.48–0.71 |
| multivariable** | 0.61 | 0.50–0.75 | ||
| COX2+ | 561/160 | simple* | 0.60 | 0.42–0.85 |
| multivariable** | 0.64*** | 0.43–0.96 | ||
| COX2− | 1440/313 | simple* | 0.56 | 0.44–0.72 |
| multivariable** | 0.57*** | 0.44–0.74 |
Adjusted for time since diagnosis and age
Adjusted for the same factors as the multivariable model in Table 2 including disease stage at diagnosis
p-value for the interaction = 0.44
DISCUSSION
To our knowledge this is the largest series to date to examine breast cancer COX-2 status with breast cancer prognostic factors and death from breast cancer. Similar to most other reports, we found that COX-2 positive tumors were associated with markers of worse breast cancer prognosis: higher stage, larger tumors, and more nodal metastases. We also found that COX-2 positive tumors were associated with ER+, and PR+, but not HER2neu+ tumors. Also similar to most other reports, we found that COX-2 positive tumors were associated with a 35% increased risk of breast cancer death in simple models. However, this association appeared to be mediated by advanced stage as adjustment for stage attenuated the risk such that it was no longer statistically significant.
Unlike the NHS results for colon cancer, [6] the apparent survival benefit for aspirin use in breast cancer did not differ by the COX-2 status of the tumor. Compared with colorectal cancers which seem to be strongly related to COX-2, both COX-1 and COX-2 activity may be more important in breast carcinogenesis.[21] Aspirin binds covalently to COX-1 and COX-2 and inhibits both. Results from our study imply that if aspirin truly is beneficial for breast cancer survival, its mechanism of action is not solely or primarily by inhibiting COX-2. Therefore, studies aimed at understanding pathways linking aspirin and breast cancer will need to expand beyond COX-2.
Acknowledgments
This work was supported by the National Institutes of Health grant CA87969 and a grant from the Breast Cancer Research Fund and GlaxoSmithKline (WE234 (EPI40307)). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no further financial relationship with the funders. The authors have full control of all primary data and the Journal may review the data if requested.
References
- 1.Kwan ML, Habel LA, Slattery ML, Caan B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control. 2007;18 (6):613–620. doi: 10.1007/s10552-007-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair CK, Sweeney C, Anderson KE, Folsom AR. NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast Cancer Res Treat. 2007;101 (2):191–197. doi: 10.1007/s10549-006-9277-x. [DOI] [PubMed] [Google Scholar]
- 3.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.22.7918. JCO.2009.22.7918 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan KM, Stampfer MJ, Giovannucci E, Rosner BA, Colditz GA. Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst. 1996;88 (14):988–993. doi: 10.1093/jnci/88.14.988. [DOI] [PubMed] [Google Scholar]
- 5.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100 (20):1439–1447. doi: 10.1093/jnci/djn324. djn324 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302 (6):649–658. doi: 10.1001/jama.2009.1112. 302/6/649 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62 (3):632–635. [PubMed] [Google Scholar]
- 8.Zeeneldin AA, Mohamed AM, Abdel HA, Taha FM, Goda IA, Abodeef WT. Survival effects of cyclooxygenase-2 and 12-lipooxygenase in Egyptian women with operable breast cancer. Indian J Cancer. 2009;46 (1):54–60. doi: 10.4103/0019-509x.48597. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SM, Cook NR, Manson JE, Lee IM, Buring JE. Low-dose aspirin and breast cancer risk: results by tumour characteristics from a randomised trial. Br J Cancer. 2008;98 (5):989–991. doi: 10.1038/sj.bjc.6604240. 6604240 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis G, Cohen C. COX-2 expression in invasive breast cancer: correlation with prognostic parameters and outcome. Appl Immunohistochem Mol Morphol. 2007;15(3):255–259. doi: 10.1097/01.pai.0000213130.63417.b3. 00129039-200709000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Park K, Han S, Shin E, Kim HJ, Kim JY. Cox-2 expression on tissue microarray of breast cancer. Eur J Surg Oncol. 2006;32 (10):1093–1096. doi: 10.1016/j.ejso.2006.05.010. S0748-7983(06)00217-4 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Surowiak P, Materna V, Matkowski R, Szczuraszek K, Kornafel J, Wojnar A, Pudelko M, Dietel M, Denkert C, Zabel M, Lage H. Relationship between the expression of cyclooxygenase 2 and MDR1/P-glycoprotein in invasive breast cancers and their prognostic significance. Breast Cancer Res. 2005;7 (5):R862–870. doi: 10.1186/bcr1313. bcr1313 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Bossuyt V, Ponn T, Lannin D, Haffty BG. Cyclooxygenase-2 expression in postmastectomy chest wall relapse. Clin Cancer Res. 2005;11 (14):5199–5205. doi: 10.1158/1078-0432.CCR-05-0524. 11/14/5199 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, Kristiansen G, Reles A, Siegert A, Guski H, Hauptmann S. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97 (12):2978–2987. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 15.Wulfing P, Diallo R, Muller C, Wulfing C, Poremba C, Heinecke A, Rody A, Greb RR, Bocker W, Kiesel L. Analysis of cyclooxygenase-2 expression in human breast cancer: high throughput tissue microarray analysis. J Cancer Res Clin Oncol. 2003;129 (7):375–382. doi: 10.1007/s00432-003-0459-1. [DOI] [PubMed] [Google Scholar]
- 16.Costa C, Soares R, Reis-Filho JS, Leitao D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55 (6):429–434. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witton CJ, Hawe SJ, Cooke TG, Bartlett JM. Cyclooxygenase 2 (COX2) expression is associated with poor outcome in ER-negative, but not ER-positive, breast cancer. Histopathology. 2004;45 (1):47–54. doi: 10.1111/j.1365-2559.2004.01898.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakopoulou L, Mylona E, Papadaki I, Kapranou A, Giannopoulou I, Markaki S, Keramopoulos A. Overexpression of cyclooxygenase-2 is associated with a favorable prognostic phenotype in breast carcinoma. Pathobiology. 2005;72 (5):241–249. doi: 10.1159/000089418. PAT2005072005241 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Spizzo G, Gastl G, Wolf D, Gunsilius E, Steurer M, Fong D, Amberger A, Margreiter R, Obrist P. Correlation of COX-2 and Ep-CAM overexpression in human invasive breast cancer and its impact on survival. Br J Cancer. 2003;88 (4):574–578. doi: 10.1038/sj.bjc.6600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, Collins LC. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10 (4):R67. doi: 10.1186/bcr2128. bcr2128 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe LR, Lippman SM. Modulation of breast cancer risk by nonsteroidal anti-inflammatory drugs. J Natl Cancer Inst. 2008;100 (20):1420–1423. doi: 10.1093/jnci/djn347. djn347 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]


