TUDCA treatment preserved ERG b-waves and the outer nuclear layer in Bardet-Biedl syndrome mice and prevented obesity assessed at P120. TUDCA treatment preserved ERG b-waves and the outer nuclear layer in rd10 mice to P30.
Abstract
Purpose.
To evaluate and compare the protective effect of tauroursodeoxycholic acid (TUDCA) on photoreceptor degeneration in different models of retinal degeneration (RD) in mice.
Methods.
BbsM390R/M390R mice were injected subcutaneously twice a week, from P40 to P120, and rd10 mice were injected every 3 days from P6 to P38 with TUDCA or vehicle (0.15 M NaHCO3). Rd1 and rd16 mice were injected daily from P6 to P30 with TUDCA or vehicle. Retinal structure and function were determined at multiple time points by electroretinography (ERG), optical coherence tomography (OCT), and histology.
Results.
The amplitude of ERG b-waves was significantly higher in TUDCA-treated Bbs1 and rd10 animals than in controls. Retinal thickness on OCT was slightly greater in treated Bbs1 animals than in the controls. Histologically, outer segments were preserved, and the outer nuclear layer was significantly thicker in the treated Bbs1 and rd10 mice than in the controls. Bbs1M390R/M390R mice developed less obesity than the control Bbs1M390R/M390R while receiving TUDCA. The Rd1 and rd16 mice showed no improvement with TUDCA treatment, and the rd1 mice did not have normal weight gain during treatment.
Conclusions.
TUDCA treatment preserved ERG b-waves and the outer nuclear layer in Bbs1M390R/M390R mice, and prevented obesity assessed at P120. TUDCA treatment preserved ERG b-waves and the outer nuclear layer in the rd10 mice to P30. TUDCA is a prime candidate for treatment of humans with retinal degeneration, especially those with Bardet-Biedl syndrome, whom it may help not only with the vision loss, but with the debilitating obesity as well.
Gene replacement therapy is currently the best hope for patients with progressive retinal degenerations due to genetic defects; however, at present only one subtype, RPE65-related Leber congenital amaurosis (LCA), has clinical gene therapy results reported.1–3 For patients who may benefit from gene therapy in the future or who exhibit only the earliest signs of retinal degeneration, there is a real need for treatments to slow or stop the progress of disease. In the absence of specific genetic information for a given patient, this could also be a temporizing measure until a genetic diagnosis can be found and a specific therapy devised and administered. An ideal treatment would ameliorate retinal degeneration from several different genetic causes. One group of agents that may have this property is antiapoptotic molecules. Apoptosis is the final pathway in programmed cell death. If this pathway can be aborted or delayed, photoreceptor cells may live and function longer.
Tauroursodeoxycholic acid (TUDCA) is the active component in bear bile, which has been used in traditional Chinese medicine for thousands of years. In 2006, Boatright et al.4 showed that systemic TUDCA decreased apoptosis and retinal degeneration in mice with either light-induced retinal damage or genetic retinal degeneration (RP due to a mutation in the Pde6beta gene in the rd10 mouse) at P18 and P30.5,6 TUDCA has also been found to disrupt apoptosis in animal models of neurodegenerative diseases, such as Alzheimer7,8 and Huntington Disease,9,10 and recently was reported to slow retinal degeneration in the Pro23His rat, a model of human autosomal dominant RP.11 We hypothesized that the antiapoptotic effect of TUDCA is also beneficial in treating ciliopathies, a different class of retinal degenerative disorder. Ciliopathies are characterized by having a primary dysfunction of the cilia, usually in several organ systems, including the connecting cilium of the photoreceptor cell. Disease may result from abnormal formation of the cilium, or abnormal transport within it. To test our hypothesis, we treated a mouse model of Bardet-Biedl syndrome (BBS) type 1, an autosomal recessive ciliopathy that causes severe retinal degeneration in humans. This retinal degeneration, which is caused by the most common BBS1 mutation in humans.12 is replicated in homozygous Bbs1M390R/M390R mice.
BBS was first described in the 1920s by George Bardet, reporting two French girls with the triad of obesity, polydactyly, and RP.13 In 1922, Arthur Biedl reported similar cases.14 Because the syndrome was reminiscent of earlier cases described in 1866 by Laurence and Moon, in 1925 Solis-Cohen and Weiss coined the term Laurence-Moon-Bardet-Biedl syndrome. Later, Laurence and Moon were removed from the name, as their patients eventually developed paraplegia. To date at least 15 BBS genes have been identified. The protein products of seven of these genes associate in vivo to create the BBSome, a protein complex important to intracellular transport and intraflagellar trafficking.15,16 Three other known BBS proteins associate to form the BBS chaperone complex.17,18 Inactivation of any one of these BBS genes may adversely affect the BBSome and/or chaperone complex and therefore affect transport within the cell, explaining how mutations of many different genes can cause the same unusual findings as those in BBS—postaxial polydactyly, obesity, RP, renal and gonadal anomalies, and, in some cases, developmental delay. How this mistrafficking induces dysfunction and apoptosis of photoreceptor cells in the retina is not known. Since the retinal degeneration in rd10 mice has been reported to be ameliorated by TUDCA,4,5 we replicated the published protocol in this model as a positive control for our intervention, and in addition we observed the rd10 mice longer than previously reported. We also tested the same treatment protocol on rd1 and rd16 mice, which are models of very rapid retinal degeneration analogous to that in autosomal recessive (ar)RP and CEP290-related LCA, respectively, in humans.
The purpose of this study was to evaluate the effects of systemic TUDCA on the course of retinal degeneration in Bbs1M390R/M390R, rd10, rd1, and rd16 models by electroretinography (ERG), optical coherence tomography (OCT), and histology. We found that, compared to untreated controls and vehicle injected controls, the severity of retinal degeneration is lessened in two of the models tested. Treatment with TUDCA also attenuated the severity of obesity in Bbs1M390R/M390R mice.
Materials and Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa and conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animals were maintained in 12-hour light–dark cycles and fed standard mouse chow ad libitum.
Homozygous Bbs1M390R/M390R mice on a 129SVEV background were generated by homologous recombination in our laboratory, as described previously,12 and were selected by genotyping litters from heterozygous crosses. These mice have outer segment dysmorphology from at least P21, and the ERG is reduced by approximately 40% at P60 compared with WT or heterozygous mice of the same background. By 6 to 8 months of age, the ERG is essentially nonrecordable. The mice also become morbidly obese.12 This phenotype replicates the human BBS1 phenotype in which patients have rapidly progressive retinal degeneration that is usually diagnosed in the first or second decade, with blindness often resulting by age 20 to 30 years. These patients also have obesity caused by leptin resistance, usually from early childhood.18,19
Rd10 mice (Jackson Laboratories, Bar Harbor, ME) carrying a mutation in the β-subunit of rod cGMP-phosphodiesterase (Pde6b, rd10) were bred in our laboratory. These mice have photoreceptor degeneration starting at P14 to P16, with ERG approximately 50% of wild-type (WT) by P18 and progressing to an essentially nonrecordable ERG by P40 to P60,5,20 but no other systemic findings. Rd1 and rd16 mice (Jackson Laboratories) were bred in our laboratory. These mice never develop normal outer segments and have photoreceptor degeneration by P8 with an essentially extinguished ERG by approximately P21 for rd10 and P28 for rd16.5,21
Tauroursodeoxycholic Acid
TUDCA (EMD-Calbiochem, La Jolla, CA) was administered by subcutaneous injections of a 0.1-mL solution/10 g of mouse weight for a dose of 500 mg/kg in 0.15 M NaHCO3 (pH 7.4), freshly prepared daily according to the method of Boatright et al.4 Subcutaneous injection of TUDCA or vehicle (NaHCO3) was given twice a week (Thursday/Monday) for Bbs1 mice from P40 to P120; every 3 days (q3d) for the rd10 mice from P6 to P38, and daily for the rd1 and rd16 mice from P6 to P30. Body weight was measured and recorded immediately before each injection and used to calculate the TUDCA dose to be administered.
ERG Recordings
Full field ERG was obtained (Espion V5 system; Diagnosys LLC, Lowell, MA). After overnight dark adaptation, the mice were anesthetized with an intraperitoneal injection of ketamine (87.5 mg/kg) and xylazine (2.5 mg/kg). ERGs were recorded simultaneously from the corneal surface of each eye after pupil dilation (1% tropicamide) using gold ring electrodes (Diagnosys) referenced to a needle electrode (Roland/LKC, Brandenburg, Germany) placed on the back of the head. Another needle electrode placed in the tail served as ground. A drop of methylcellulose (2.5%) was placed on the corneal surface to ensure electrical contact and to maintain corneal integrity. Body temperature was maintained at a constant temperature of 38°C using the system's heating pad. All stimuli were presented in a Ganzfeld (ColorDome; Diagnosys), and the mouse head and electrode positioning were monitored on the camera attached to the system. Dim red light was used for illumination until dark-adapted testing was completed. The a- and b-waves were recorded with an escalating 11-step protocol starting at 0.01 cd · s · m−2 and ending at 25 cd · s · m−2. The b-wave used for analysis was the highest amplitude b-wave registered, typically the ninth flash (4 cd · s · m−2), and the a-wave was calculated from the same flash.
The a-wave was measured from the baseline to the trough of the first negative wave. The b-wave was measured from the trough of the a-wave to the peak of the first positive wave, or from the baseline to the peak of the first positive wave if no a-wave was present.
Optical Coherence Tomography
OCT (Bioptigen, Research Triangle Park, NC) was performed after placing the animals under ketamine/xylazine anesthesia as described above. Tropicamide 1% was used to dilate pupils, and the retinas were scanned one eye at a time. Methylcellulose lubricant was placed on the corneas, and the noncontact probe was positioned near the cornea until the retinal image could be seen on the screen. This was then focused and oriented with the optic nerve (ON) in the middle of the scan as a landmark. Retinal OCT was performed using rectangular volume scan (volumetric acquisition made up of a series of B-scans) with a length of 1.40 mm at a width of 1.40 mm at a rate of 1000 A-scan/B-scan. An average of four repeated B scans (of the same region) centered on the ON was used for analysis.
The total retinal thickness was measured on the OCT image at two locations each 3 μm from the edge of the ON on either side.
Retinal Histology
Mice (treated and controls from all strains) were euthanized at predetermined time points by CO2 asphyxiation followed by cervical dislocation. The eyes were immersed in cold fixative (4% paraformaldehyde or one-half strength Karnovsky fixative: 2% formaldehyde and 2.5% glutaraldehyde in 100 mM cacodylate buffer [pH 7.4], containing 0.025% CaCl2) for 4 to 12 hours, while maintaining orientation, and then stored in PBS for later processing. The eyes were dissected and the anterior segment and the lens were removed. Samples were embedded in acrylamide solution22and frozen to obtain superior-to-inferior cross sections of the eye cup. Tissue was sectioned at 5 μm on a cryostat (Microm GmbH, Walldorf, Germany). Sections were processed for H&E histochemistry and were analyzed using light microscopy (BX41; Olympus, Tokyo, Japan). Overlapping images of the entire section were acquired with a 20× objective lens. Image management software was used to make montages of the eye cups (Photoshop; Adobe Systems, San Jose, CA). For treated and control eyes, three histologic sections per eye from the same retinal locations were photographed. Across each corresponding 5-mm2 area, five measurements of photoreceptor outer nuclear layer were made using the caliper on Image J and averaged as the representative value for that area (ImageJ software, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Statistical Analysis
The paired t-test was used to compare results of the ERG amplitudes and the outer nuclear layer thickness between the TUDCA-treated and control groups.
Results
Rd10 Mice
Electroretinogram.
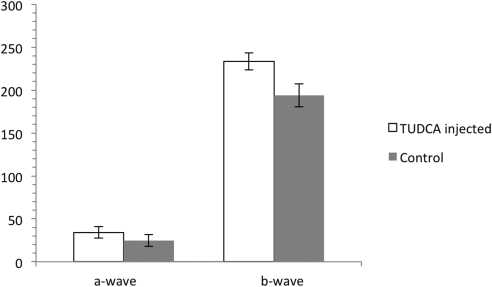
TUDCA injections partially preserved ERG function and retinal morphology in rd10 mice up to P30. Dark-adapted ERG b-wave amplitudes were significantly preserved in TUDCA-treated animals at P30 (P = 0.039) compared with those in vehicle-treated (NaHCO3) controls (Fig. 1). At P38 no statistically significant difference was seen between the groups due to one outlier in the control group with a large amplitude ERG. For other animals, TUDCA-treated mice had higher ERG amplitudes than vehicle-treated controls. At P30 the average ERG a-wave was also higher in TUDCA-treated eyes than vehicle-treated (36.7 μV vs. 24.7 μV); however, this failed to reach statistical significance (Fig. 1).
Figure 1.
ERG a- and b-wave amplitude results for rd10 mice treated with TUDCA versus rd10 mice treated with vehicle (NaHCO3). Rd10 injected with TUDCA every 3 days from P6 to P38 (n = 4). Rd10 controls injected with vehicle on the same schedule (n = 5). The difference in b-wave amplitudes is statistically significant at P30 (P = 0.039) by paired t-test. The difference in a-wave amplitude did not reach statistical significance. Error bars, SEM.
Histology.
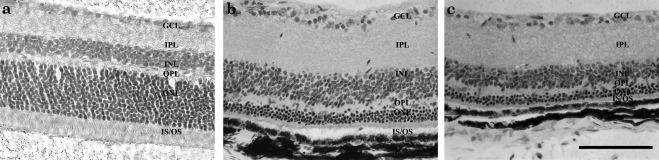
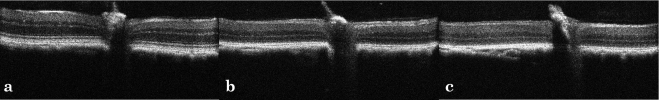
Overall morphology of the photoreceptor cells was better preserved in the TUDCA-treated rd10 mice (Fig. 2) at P30 and P38 than in controls. Outer segments were longer and ONL was thicker in TUDCA-treated rd10 mouse eyes compared with the NaHCO3-treated controls. ONL thickness averaged 9.2 μm in controls versus 11.3 μm in the treated animals. The difference in ONL thickness was significant at P = 0.0006 (paired t-test).
Figure 2.
Histology of rd10 mice retinas treated with TUDCA or control at P38. Outer nuclear layer thickness averaged 9.2 μm in controls versus 11.3 μm in the treated animals. The difference in ONL thickness was significant at P = 0.0006 (paired t-test). H&E-stained photomicrographs depict (a) C57Bl6, (b) TUDCA-treated rd10, and (c) vehicle-treated rd10 mice. Scale bar, 100 μm.
Bbs1 Mice
ERG.
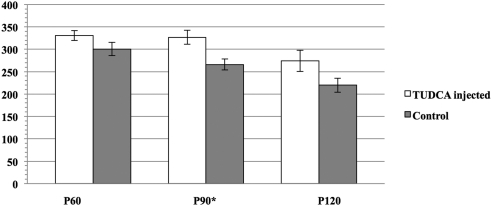
Bbs1 mice treated with systemic TUDCA demonstrated better photoreceptor function as measured by ERG dark-adapted b-wave at every time point tested (Fig. 3). This was statistically significant at P = 0.012 at P90. The ERG a-wave was not significantly better in the treated group.
Figure 3.
ERG b-wave for Bbs1M390R/M390R (Bbs1) mice (n = 6) treated with TUDCA 2 days a week versus Bbs1 control animals (n = 5) treated with vehicle NaHCO3 2 days a week or untreated. Error bars, SEM. *P < 0.05.
Histology.
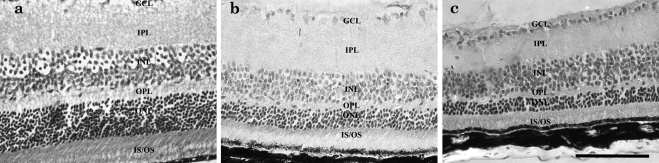
Outer nuclear layer thickness was greater in the eyes of TUDCA-treated Bbs1 mice than in NaHCO3-treated or untreated animals (Fig. 4). The entire retina was thicker in TUCDA-treated retinas, with the ONL and the inner segment/outer segment layers most prominently preserved. The average ONL thickness in TUDCA-treated eyes was 24.3 μm compared to 17.5 for vehicle-treated eyes, with P < 0.05. Average ONL thickness for an unaffected animal of the same age and strain was 49.5 μm.
Figure 4.
Histology at 40× magnification of Bbs1M390R/M390R TUDCA-treated versus control all at P120. (a) Untreated 129SVEV control; (b) TUDCA-treated Bbs1M390R/M390R; and (c) untreated Bbs1M390R/M390R. Inner and outer segments were better preserved, and ONL was thicker in TUDCA-treated eyes. Scale bar, 100 μm.
Optical Coherence Tomography
OCT of representative animals showed a total retinal thickness of 0.177 mm on average in both the treated group and control groups at 2 months of age. Over time, the retinal thickness as measured on OCT decreased in both groups, with the TUDCA-treated eyes ultimately showing a trend toward less thinning of the retinas compared with the control group (Fig. 5).
Figure 5.
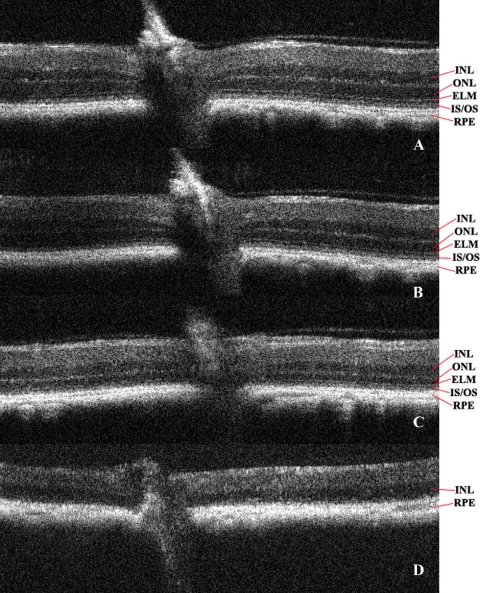
OCT of Bbs1M390R/+ control (no phenotype, SVEV129 background), versus Bbs1M390R/M390R TUDCA-treated, versus Bbs1M390R/M390R untreated control mice, all at age P120. (a) Control and (b) TUDCA-treated Bbs1M390R/M390R and (c) untreated Bbs1M390R/M390R mice. The retina was slightly thicker (0.161 mm vs. 0.152 mm), and the inner and outer nuclear layers were slightly wider and better defined in the OCT of the TUDCA-treated retina. On high magnification, the bright line representing the external limiting membrane (ELM) was better preserved in the TUDCA-treated retina.
It was hoped that OCT, with its near histologic representation of the retinal layers, would obviate the need for histologic examination at different stages, but this proved not to be true. As shown in Figure 5, the differences on OCT at P120 between TUDCA- and vehicle-treated Bbs1 animals are much more subtle than the differences on ERG or histology. However, a difference in OCT over time as the retina degenerates can be seen in the Bbs1M390R/M390R mouse. Figure 6 shows OCT of the retinas of vehicle-treated Bbs1 mice at four time points: 2, 3, 5, and 9 months of age. There was a steady shrinking of the ONL and loss of the external limiting membrane signal over time, with ONL and inner segments/outer segments absent at 9 months. This correlates with nonrecordable ERG being noted at 6 to 8 months.
Figure 6.
Evolution of OCT image of retina in vehicle-treated Bbs1M390R/M390R mice over time: (a) 2-, (b) 3-, (c) 5-, and (d) 9-month-old mice.
Body Weight
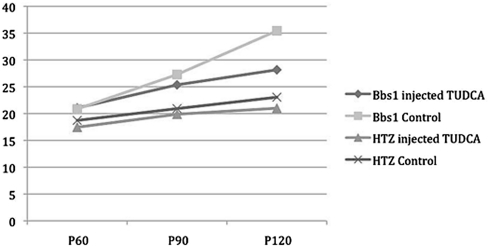
Bbs1 mice develop obesity, just as humans with BBS1 mutations do. It was noted during the experiment that TUDCA-treated Bbs1M390R/M390R mice could not be visually identified on the basis of extreme body weight as they can be in other experiments. Analysis of body weight data over time showed that the average weight of an untreated or vehicle-treated Bbs1M390R/M390R mouse at age P120 was 35.4 g, compared with the average weight of TUDCA-treated Bbs1M390R/M390R mice of the same age (28.2 g). Heterozygous controls treated with NaHCO3 or untreated had an average weight of 23 g. All the animals' weights started out in a similar range, but the Bbs1M390R/M390R mice gained more weight over time, and at age P120, there was a significant difference (P = 0.04) in weight between TUDCA-treated Bbs1M390R/M390R mice and those with vehicle or no treatment (Fig. 7).
Figure 7.
Body weight of Bbs1M390R/M390R and heterozygous (no phenotype) mice of the same SVEV129 background treated with TUDCA or NaHCO3 vehicle over time.
Rd1 and rd16 Mice
The Rd16 (n = 3 treated with TUDCA versus n = 3 treated with vehicle) did not show any difference in ERG amplitudes compared with controls when treated with TUDCA. All animals had essentially nonrecordable ERGs at the end of the study period; therefore, no statistics were possible. The Rd1 mice (n = 6 treated with TUDCA, n = 5 treated with vehicle, n = 8 untreated) also showed no demonstrable improvement in ERG; however, the data were sparse, since these mice also failed to gain weight, and most were not able to complete the TUDCA protocol.
Discussion
This study demonstrates that systemic TUDCA administration can delay apoptosis of photoreceptor cells and slow the diminution of electroretinogram amplitudes in two murine models of RP with completely distinct pathobiology, one a ciliopathy and the other a primary dysfunction of phosphodiesterase β. Of interest, TUDCA also appears to ameliorate the obesity that accompanies retinal degeneration in the BBS mouse.
The two models in which TUDCA was beneficial, Bbs1M390R/M390R and rd10, have in common a relatively slow course with nonrecordable ERG at approximately P210 and P40 to P60, respectively. This allows for injections twice weekly in the Bbs1 and every 3 days in the rd10 mice. The two unsuccessful models, rd1 and rd16, exhibit an essentially nonrecordable ERG by P21 and P28, respectively. Because of this, TUDCA was administered daily. TUDCA has been reported to have a beneficial effect in rd1 mice with this regimen in one report,5 but in the present study, the rd1 mice did not gain weight well, and the few that completed the protocol had no benefit on ERG. The Rd16 mice have a mutation in the Cep290 gene, which is important in cilia just as is the Bbs1 gene. These mice tolerated daily injections better than the rd1 mice did, but still showed no difference in ERG from the controls. This suggests that site of action of the abnormal protein is not as important as the rapidity of disease course in determining TUDCA efficacy or tolerability. A significant difference in both mice and humans between BBS and CEP290 disease is the age at which the ERG becomes nonrecordable. It occurs much earlier in CEP290 disease. Thus TUDCA appears to be a better candidate as a treatment for degenerations with later onset and slower course. It may also be a good candidate for treatment of Alstrom disease in which obesity develops with RP, but this has not been tested in an animal model.
Apoptosis, or programmed cell death, is an important physiologic pathway. Both embryogenesis and tumorigenesis depend in part on a skewing of the balance between apoptosis and maintenance. Apoptosis can be a biological defense to rid the body of unwanted or unneeded cells; and, in normal retinal development, considerable apoptotic pruning occurs past the first postnatal week; however, disorders of apoptosis can also cause disease. There is evidence that excess or dysregulated apoptosis plays an important role in neurodegenerative and ischemic neurologic disorders.
The mechanism by which TUDCA delays or stops apoptosis is becoming better understood. TUDCA acts via a phosphatidylinositol 3-kinase (PI3K)-dependent signaling pathway to block neuronal death triggered by amyloid-β peptide.23 TUDCA modulates amyloid-β-induced apoptosis, E2F-1 induction, p53 stabilization and Bax expression. In one study, it protected PC12 cells against p53- and Bax-dependent apoptosis induced by E2F-1 and p53 overexpression.24 Boatright et al.4 have demonstrated that it appears to block the caspase pathway. There is also evidence that it ameliorates endoplasmic reticulum stress and the unfolded protein response.25
Ursodeoxycholic acid (UDCA), a similar endogenous bile acid that lacks the taurine moiety, has been shown to inhibit apoptosis at a mitochondrial level.26 Both TUDCA and UDCA have been shown to reduce endoplasmic reticulum stress and inflammation in models of liver disease and diabetes27,28 and to stabilize the mitochondrial membrane and prevent TGF-β1-induced apoptosis in hepatocytes. They inhibit mitochondrial membrane depolarization and channel formation, production of reactive oxygen species, and release of cytochrome c, as well as inhibiting caspase activation. However UDCA has also been shown to modulate E2F-1 and p53 through a caspase-independent mechanism.29
The antiapoptotic action of TUDCA is relatively nonspecific. In addition to slowing retinal degeneration in the Pro23His rat,11 TUDCA has been reported to slow cataract formation in the mouse lens, ostensibly through a crystallin chaperone action.30 Crystallins are expressed in the retina, so this activity may also be related to the retinal protection. Systemic TUDCA is effective as a treatment in models of neurodegeneration such as Huntington disease,9 and high levels of TUDCA have been found in the brain (a surrogate for retina) when it is given subcutaneously in mice.4 TUDCA has also improves outcomes in cerebral ischemic stroke in animal models.31
TUDCA and UDCA are safe in humans; UDCA is FDA approved. TUDCA has been used in clinical trials in premature infants without significant side effects32 and is used in Europe to treat gastrointestinal disorders, albeit at lower doses than the equivalent human doses used in the animal studies. UDCA has been shown to treat liver disorders and prevent gallstone formation after bariatric surgery.33
A potential side effect in mice and humans is weight loss, and we found that the rd1 mice receiving daily injections from P6 did not gain weight as their untreated littermates did. Even mice that tolerated the protocols showed a trend toward slightly lower body weights than vehicle-treated controls. TUDCA had a greater effect on body weight in Bbs1 mice than in heterozygous or WT mice. Ironically, this could be an important part of treatment for patients with BBS, in whom debilitating obesity accompanies their RP. Leptin resistance in patients with BBS has been shown to be the cause of their obesity.18 Parents of children with BBS are often distressed by their inability to curb their children's weight, and when children are young, the massive obesity may be a more serious problem than vision loss. UDCA has been touted as a weight loss aid; however, in a study using UDCA to treat obese children who also had elevated liver enzymes, the weight loss they attained was attributed to diet alone.34 TUDCA and 4-phenyl butyric acid (PBA) have been reported to reduce obesity in a mouse model of leptin resistance due to mediation of the unfolded protein response and diminution of endoplasmic reticulum stress which both of these agents provide.25 TUDCA was noted to be more effective than PBA for treatment of obesity in this study. In a recent clinical trial in 20 obese adults, 1750 mg/d of TUDCA taken for 4 weeks led to an increase in hepatic and muscle insulin sensitivity by approximately 30% (P < 0.05) compared to no change in the placebo group.35 Both TUDCA and UDCA increase the amount of lipid in rat hepatic microsomes.36
Careful follow-up of patients would be needed to monitor for side effects, given the high doses apparently necessary to treat retinal disorders. High doses of bile acids can cause hepatotoxicity, although one study in mice found that UDCA was the least toxic of the bile acids tested (TUDCA was not tested).37 Since UDCA is FDA approved, it would be the easier agent with which to start a clinical trial in the United States. It is reportedly less well tolerated than TUDCA when taken by mouth, however, and it is possible that the taurine moiety is part of what conveys the retinal protection. Taurine is protective against the retinal toxicity of vigabatrin,38 and taurine deficiency causes retinal degeneration in cats.39 There is some evidence that the addition of taurine to UDCA is key in delivery to the brain26 and therefore to the retina. The relative efficacy of TUDCA compared with UDCA is a topic for further study.
The ciliopathies are complex disorders that affect many organ systems. Whereas TUDCA cannot correct the underlying structural and functional abnormalities in the cilia in photoreceptor cells, it appears to disrupt the pathway to apoptosis in the retina. Concomitant with the structural protection of the retina was the relative preservation of ERG, which may offer patients a longer window of useful vision. Given the favorable safety profile, availability, and demonstration of efficacy in several animal models, TUDCA appears to be an excellent candidate for a human retinal degeneration clinical trial in the near future. We propose that one of the first disorders for which this treatment should be offered in BBS, a currently untreatable cause of blindness in which TUDCA could potentially benefit not only the visual outcome, but through serendipity, may allow us to treat their leptin-resistant obesity at the same time if a safe and effective dose can be established.
Footnotes
Supported in part by a Marjorie Carr Adams Women's Career Development Award from the Foundation Fighting Blindness (AVD), the Howard Hughes Medical Institute (EMS, VCS), National Institutes of Health Grants RO1 EY-17168 and R01 EY-110298 (VCS), and Hope for Vision (AVD). AVD holds the Ronald V. Keech Professorship for Pediatric Genetic Eye Disease Research.
Disclosure: A.V. Drack, None; A.V. Dumitrescu, None; S. Bhattarai, None; D. Gratie, None; E.M. Stone, None; R. Mullins, None; V.C. Sheffield, None
References
- 1. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 3. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boatright JH, Moring AG, McElroy C, et al. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. 2006;12:1706–1714 [PubMed] [Google Scholar]
- 5. Boatright JH, Nickerson JM, Moring AG, Pardue MT. Bile acids in treatment of ocular disease. J Ocul Biol Dis Infor. 2009;2:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillips MJ, Walker TA, Choi HY, et al. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Invest Ophthalmol Vis Sci. 2008;49:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramalho RM, Viana RJ, Low WC, Steer CJ, Rodrigues CM. Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer's disease. Trends Mol Med. 2008;14:54–62 [DOI] [PubMed] [Google Scholar]
- 8. Ramalho RM, Borralho PM, Castro RE, Sola S, Steer CJ, Rodrigues CM. Tauroursodeoxycholic acid modulates p53-mediated apoptosis in Alzheimer's disease mutant neuroblastoma cells. J Neurochem. 2006;98:1610–1618 [DOI] [PubMed] [Google Scholar]
- 9. Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington's disease. Proc Natl Acad Sci U S A. 2002;99:10671–10676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keene CD, Rodrigues CM, Eich T, et al. A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington's disease. Exp Neurol. 2001;171:351–360 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Sanchez L, Lax P, Pinilla I, Martin-Nieto J, Cuenca N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest Ophthalmol Vis Sci. 2011;52:4998–5008 [DOI] [PubMed] [Google Scholar]
- 12. Davis RE, Swiderski RE, Rahmouni K, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci USA. 2007;104:19422–19427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bardet G. On congenital obesity syndrome with polydactyly and retinitis-pigmentosa (a contribution to the study of clinical forms of hypophyseal obesity). (Reprinted from a Thesis, Faculte De Medecine De Paris. 1920). Obes Res. 1995;3:387–399 [DOI] [PubMed] [Google Scholar]
- 14. Biedl A. A pair of siblings with adiposo-genital dystrophy (reprinted 1922). Obes Res. 1995;3:404–404 [DOI] [PubMed] [Google Scholar]
- 15. Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213 [DOI] [PubMed] [Google Scholar]
- 16. Loktev AV, Zhang Q, Beck JS, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15:854–865 [DOI] [PubMed] [Google Scholar]
- 17. Seo S, Baye LM, Schulz NP, et al. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci U S A. 2010;107:1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sheffield VC. The blind leading the obese: the molecular pathophysiology of a human obesity syndrome. Trans Am Clin Climatol Assoc. 121:172–181; discussion 181 [PMC free article] [PubMed] [Google Scholar]
- 19. Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang B, Hawes NL, Pardue MT, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47:624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang B, Khanna H, Hawes N, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson LV, Blanks JC. Application of acrylamide as an embedding medium in studies of lectin and antibody binding in the vertebrate retina. Curr Eye Res. 1984;3:969–974 [DOI] [PubMed] [Google Scholar]
- 23. Sola S, Castro RE, Laires PA, Steer CJ, Rodrigues CM. Tauroursodeoxycholic acid prevents amyloid-beta peptide-induced neuronal death via a phosphatidylinositol 3-kinase-dependent signaling pathway. Mol Med. 2003;9:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramalho RM, Ribeiro PS, Sola S, Castro RE, Steer CJ, Rodrigues CM. Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J Neurochem. 2004;90:567–575 [DOI] [PubMed] [Google Scholar]
- 25. Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 26. Sola S, Aranha MM, Steer CJ, Rodrigues CM. Game and players: mitochondrial apoptosis and the therapeutic potential of ursodeoxycholic acid. Curr Issues Mol Biol. 2007;9:123–138 [PubMed] [Google Scholar]
- 27. Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beuers U, Kullak-Ublick GA, Pusl T, Rauws ER, Rust C. Medical treatment of primary sclerosing cholangitis: a role for novel bile acids and other (post-)transcriptional modulators? Clin Rev Allergy Immunol. 2009;36:52–61 [DOI] [PubMed] [Google Scholar]
- 29. Sola S, Ma X, Castro RE, Kren BT, Steer CJ, Rodrigues CM. Ursodeoxycholic acid modulates E2F-1 and p53 expression through a caspase-independent mechanism in transforming growth factor beta1-induced apoptosis of rat hepatocytes. J Biol Chem. 2003;278:48831–48838 [DOI] [PubMed] [Google Scholar]
- 30. Song S, Liang JJ, Mulhern ML, Madson CJ, Shinohara T. Cholesterol-derived bile acids enhance the chaperone activity of α-crystallins. Cell Stress Chaperones. 2011;16:475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodrigues CM, Spellman SR, Sola S, et al. Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab. 2002;22:463–471 [DOI] [PubMed] [Google Scholar]
- 32. Heubi JE, Wiechmann DA, Creutzinger V, et al. Tauroursodeoxycholic acid (TUDCA) in the prevention of total parenteral nutrition-associated liver disease. J Pediatr. 2002;141:237–242 [DOI] [PubMed] [Google Scholar]
- 33. Uy MC, Talingdan-Te MC, Espinosa WZ, Daez ML, Ong JP. Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: a meta-analysis. Obes Surg. 2008;18:1532–1538 [DOI] [PubMed] [Google Scholar]
- 34. Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–743 [PubMed] [Google Scholar]
- 35. Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 201059:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bellentani S, Chao YC, Ferretti I, Panini R, Tiribelli C. Chronic administration of ursodeoxycholic and tauroursodeoxycholic acid changes microsomal membrane lipid content and fatty acid compositions in rats. Biochem Biophys Res Commun. 1996;220:479–483 [DOI] [PubMed] [Google Scholar]
- 37. Song P, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile acid concentrations and hepatotoxicity in mice. Toxicol Sci. 2011;123:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jammoul F, Wang Q, Nabbout R, et al. Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Ann Neurol. 2009;65:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leon A, Levick WR, Sarossy MG. Lesion topography and new histological features in feline taurine deficiency retinopathy. Exp Eye Res. 1995;61:731–741 [DOI] [PubMed] [Google Scholar]