The accommodative lag of young infants and children was determined prior to any optical correction or treatment. Young infants and children with hyperopia of less than 4 D were found to have equal performance, while those above 4 D or with amblyopia or strabismus demonstrated a range of lags.
Abstract
Purpose.
To determine the accommodative accuracy of infants and young children before they had had any form of clinical intervention or treatment, in an attempt to determine the difference between ‘normal’ and ‘abnormal’ visual experience for these individuals.
Methods.
Nott retinoscopy was performed on 111 subjects in binocular viewing conditions at a viewing distance of 50 cm. The target was a naturalistic cartoon image with a broadband spatial frequency amplitude spectrum.
Results.
Accommodative accuracy was not related to age (4–90 months). In the group found to have no apparent clinical abnormality (n = 71), the mean lag in the more hyperopic meridian of the least ametropic eye was 0.34 diopters (D). When considering the group as a whole, those with less than approximately 4 D of hyperopia demonstrated similar lags, while those with higher hyperopia, amblyopia, or strabismus had more variable lags. An ROC analysis designed to detect hyperopia >5 D in any meridian, amblyopia and/or strabismus had an area under the curve of 0.90 (95% confidence interval [CI], 0.82 to 0.95), and for a lag criterion of 1.3 D had a sensitivity of 83.3% and a specificity of 96.5%.
Conclusions.
These data from a relatively small but broad sampling of age and clinical status suggest that clinically normal young infants and children with low amounts of hyperopia have similar lags of accommodation from the first few months after birth. Subjects with greater than 4 D of hyperopia, or amblyopia or strabismus, have more variable lags and therefore evidence of abnormal visual experience.
Postnatal control of eye growth and the development of the neural visual system are both influenced by visual experience (e.g., 1–6). Studies of animal models have provided definitive examples of abnormal visual experience leading to abnormal visual development and parallels have been found in human clinical populations (e.g.,7,8).
Vision screening of young children has been largely aimed at finding individuals with the apparent consequences of abnormal experience, in particular amblyopia and refractive or accommodative strabismus. Treatment for these conditions can then be provided. It would be more effective to identify individuals who are at risk and to prevent these conditions if possible, especially as current treatment protocols do not routinely achieve full recovery.9,10 This is a difficult task however; as the natural history of some forms of amblyopia is poorly understood and children with matched refractive errors do not all follow the same clinical path. For example, one child with 5 D of hyperopia in both eyes may develop strabismus and amblyopia, while another might develop bilateral isoametropic amblyopia, and yet another may not develop amblyopia or strabismus. The management of the young hyperopic patient is therefore still debated, and current clinical guidelines for prescribing for asymptomatic refractive error are based solely on clinical consensus, rather than evidence-based prediction.11–13
In relatively severe cases of visual deprivation, such as ptosis or cataract, the nature of a patient's abnormal visual experience can be systematically defined in that spatial information is consistently removed from the retinal image. The nature of any abnormal visual experience in the case of hyperopia is more ambiguous. Retinal image quality is largely dependent on how well individuals accommodate to objects they are fixating, which cannot be judged by the casual observer.14 A hyperope may or may not be experiencing a focused retinal image at any moment in time, and it is feasible that unobserved individual differences in accommodative behavior may be related to an individual's clinical outcome.
The average data from samples of typically developing infants suggest that at younger than 2 to 3 months infants tend to overaccommodate to distant targets and exhibit a reduced gain of accommodation centered around a 33 to 50 cm near response. After that age the gain of the responses increases rapidly to become accurate over a wider range of distances.15–18 Ingram, Gill, and Goldacre19 conducted a longitudinal study of 1119 infants, demonstrating that the individuals who did not emmetropize and went on to develop strabismus also tended to accommodate poorly as infants. Mutti et al.20 have also recently shown that poor accommodation to a target at 57 cm during infancy is associated with poor emmetropization, and the recent data of Horwood and Riddell21 are in agreement with these previous studies in that they compared relatively accurate accommodation at near in 10- to 26-week-old emmetropizing infants to relatively inaccurate accommodation at near in infants who did not go on to emmetropize and in older hyperopic children who had previously reported to a clinic with decreased distance visual acuity.
We have made similar anecdotal observations in our laboratory (e.g., the data from the typically developing 12-week-old in Figure 2 of Tondel and Candy,22 who appears to demonstrate more accurate accommodation for a near target than at distance), and the goal of the present study was, therefore, to measure the accommodative lag of young human hyperopes, across a wider age range, to identify individuals who demonstrate inaccurate accommodation for near and who therefore may be at risk for abnormal visual development. The accommodative accuracy of infants and young children was measured before they had any form of intervention or treatment, in an attempt to understand the difference between ‘normal’ and ‘abnormal’ habitual retinal visual experience.
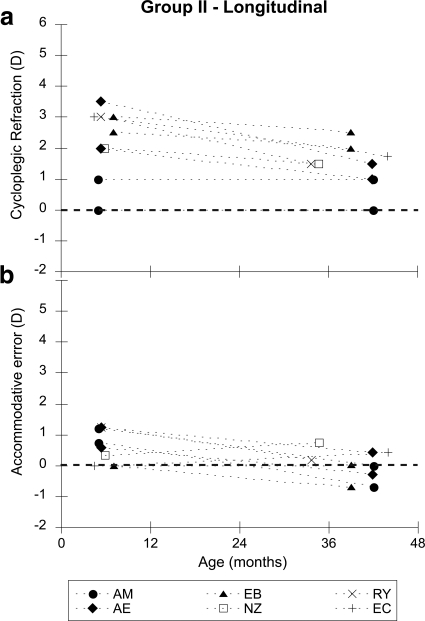
Figure 2.
Data from group II, who were tested at both 6 months and 2 or 3 years of age. Each symbol represents a different subject and is labeled with their initials. The filled symbols represent subjects with astigmatism (two principal meridia). (a) The refractive error of the principal meridia of their least hyperopic eye is plotted as a function of age. (b) The accommodative error of those meridia is plotted as a function of age.
Methods
Subjects
A total of 111 subjects were recruited consecutively either through local birth records or during a routine eye examination at the Indiana University School of Optometry's Pediatric Clinic. The subjects' ages ranged from 3.7 months to 90 months (mean age 37.7 months; 54 male, 57 female). They were all born within 2 weeks of their due date and had no medical concerns as reported by their parents. At the eye examination, a history of no prior treatment or spectacle correction was reported, and the examinations revealed no evidence of abnormal ocular health beyond strabismus, refractive error, and any associated amblyopia. Written informed consent was obtained from parents after the study had been fully explained to them. The study followed the tenets of the Declaration of Helsinki and had been approved by the local Indiana University Institutional Review Board. Of the 111 subjects, eight were studied longitudinally, with their first visit at age 6 months, and their second visit at age 2 or 3 years. At the time of their second visit, these subjects had had no spectacle correction or visual intervention.
Data Collection
Data collection consisted of a routine eye examination and an assessment of accommodative accuracy. They were both performed at the same visit and three pediatric residency-trained optometrists collected all data.
Eye Examination.
Each examination included an age-appropriate measure of visual acuity, assessment of ocular alignment, and then a cycloplegic retinoscopy and ocular health evaluation. The visual acuity tests were selected from the following tests based on age, and were tested at the appropriate distance for each method: Teller Cards, Cardiff Cards, Lea Symbols, HOTV, or Snellen letters. Binocular visual acuity was obtained before monocular visual acuity testing and an adhesive patch was used for occlusion. Prism neutralized distance and near cover test or Hirschberg assessment, if necessary, were used to assess ocular alignment. Children younger than the age of 6 months were then given one drop of 0.5% cyclopentolate in each eye, while older children received one drop of 1.0% cyclopentolate in each eye. Cycloplegic retinoscopy, using loose lenses or retinoscopy bars, was used to determine the sphero-cylindrical refractive error for each eye, and a fundus examination was then performed.
Accommodative Performance.
Accommodative accuracy was assessed using Nott retinoscopy.23–28 It was performed before instillation of the cycloplegic drops, and therefore without knowledge of the subject's refractive error. Any bias in the cycloplegic refraction data resulting from having performed the Nott retinoscopy measurements was minimal, as the retinoscopist only converted the neutralization distances for the Nott retinoscopy into diopters after the visit.
To perform the Nott technique, the retinoscopist provided the subject with a target at a defined viewing distance and then changed the distance of the retinoscope to find the neutral point and therefore the eye's focus. The advantages of this technique are that it is remote and objective, and that it minimizes potential distractions for the subject (for instance, the Monocular Estimation Method [MEM] involves holding potentially distracting lenses in front of the subject's eye26). Mean accommodative errors recorded using Nott retinoscopy have been found to be approximately 0.5 D for a target at 25 cm for children aged 3 to 10 years.25,29
Accommodative accuracy was measured in binocular viewing conditions with the subject wearing no optical correction, and therefore experiencing their habitual retinal image quality. A meter rule was held with the zero mark in alignment with the subject's corneal apex. The target, a high contrast cartoon picture with a broad spatial frequency amplitude spectrum, was placed at a 50 cm viewing distance where it subtended an angle of 8° vertically and horizontally. The cartoon target was chosen to have information at all spatial scales to imitate targets that children are typically exposed to in their daily visual environment.
The retinoscopist was aligned with the edge of the cartoon while the retinoscope was held as close to the meter rule as possible, between zero and 8° from the subject's axis of fixation on the cartoon depending on where on the picture they were fixating. The child was verbally encouraged to continue viewing the cartoon, which was the only object illuminated in an otherwise dim room. The retinoscopist assessed the reflex in the principal meridian closest to vertical in the right eye at the 50 cm target distance. If “with” movement was noted, indicating a relative lag of accommodation, the retinoscopist moved further away from the child to reach neutral. If “against” movement was noted, indicating a lead of accommodation, the retinoscopist moved closer to the child to reach neutral. The person holding the ruler then noted the working distance where the neutral reflex was observed, and the reflex closest to the horizontal meridian in the right eye was neutralized in the same manner. This procedure was then repeated for the left eye. At least two estimates were made for each measurement and they were then averaged.
For some subjects with large lags of accommodation, a neutral reflex was not observed even when the retinoscopist reached a 1 m distance. In those cases a positive power trial lens was held in front of the eye briefly while the retinoscopist explored closer distances to find neutral. The lenses were held in place as briefly as possible, to minimize any accommodative response to the lens and to minimize potential distraction to the subject. The calculated accommodative lag was then adjusted appropriately for the effect of the lens.
Results
Of the 119 subject visits, Nott retinoscopy could not be performed at five visits (one was a repeat visit for longitudinal data) and cycloplegic retinoscopy could not be performed at one visit due to poor cooperation from the children. In seven cases the apparent axis of astigmatism changed by approximately 90° between the Nott retinoscopy and the cycloplegic refraction (one was a repeat visit for longitudinal data). These subjects were also excluded because the accommodative response presumably changed during the Nott retinoscopy, or the retinoscopist was unable to align reliably on the visual axis.
The subjects were then split into three groups: (I) The subjects with no clinical abnormality beyond bilateral spherical refractive error (inclusion criteria were a spherical equivalent anisometropia of <1 D, astigmatism ≤1 D, no evidence of strabismus, and normal acuity for age and test used (n = 71). (II) The subjects who provided usable data at both 6 months and 2 or 3 years of age (n = 6) (they were also included as 2- or 3 year-olds in group I if they met the inclusion criteria, and as 6-month-olds in group III if they met the inclusion criteria). (III) The subjects with a clinical abnormality as defined by the exclusion criteria for group I (n = 36). With regard to the acuity data, the acuity measurements were taken during the eye examination before the cycloplegic refraction. Thus they were not best-corrected measures of acuity. Most of the subjects were hyperopes, however, and so the acuity measurements were typically within the normal range. Any child was classified as an amblyope if their precycloplegic acuity testing revealed an acuity difference equivalent to two or more lines between the eyes in the presence of an amblyogenic factor: >1.0 D of anisometropia, or strabismus, or both, and they were then confirmed to be an amblyope in their follow-up care.
Group I—No Clinical Abnormality
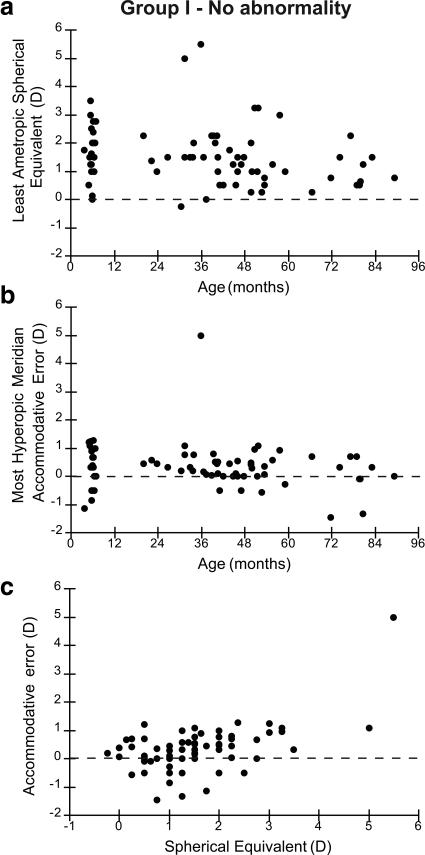
The spherical equivalent cycloplegic refraction of the least ametropic eye is plotted as a function of age for these cross-sectional data in Figure 1a (57 right eyes and 14 left eyes—the right eye was used if there was no anisometropia, n = 43). The spherical equivalent refraction of the other eye was no more than 1 D more, as a result of the anisometropia criterion for inclusion. In reality, the more ametropic eye was always the one with the greater hyperopia as none of the subjects had significant myopia. This figure is consistent with the general trend of emmetropization noted previously in the literature,30,31 with some individuals with greater hyperopia who did not appear to be emmetropizing.
Figure 1.
Data from group I, who had no clinical abnormality as defined by the inclusion criteria described in the text. (a) Spherical equivalent cycloplegic refractive error of the least ametropic eye is plotted as a function of age. (b) The accommodative error of the most hyperopic meridian of the least ametropic eye is plotted as a function of age. (c) Accommodative error of most hyperopic meridian is plotted as a function of spherical equivalent refractive error of the least ametropic eye.
The accommodative error of the most hyperopic meridian of the least ametropic eye is plotted as a function of age in Figure 1b. These data demonstrate that by 6 months of age typical performance has matured to an accuracy of within approximately 1 D of the target in this population with small amounts of anisometropia and astigmatism. There are a small number of outliers with larger errors, in particular a 3-year-old with approximately 5 D of lag, but no statistically significant simple effect of age (r = −0.13; P = 0.27). Some of the subjects demonstrate leads of accommodation, which may indicate that they are accommodating more accurately with their other more hyperopic eye, or that their fixation was attracted to the retinoscopist who had to move closer than the cartoon to estimate a lead of accommodation.
The same accommodative accuracy data, from the most hyperopic meridian of the least ametropic eye, are plotted as a function of the spherical equivalent refractive error of the least ametropic eye in Figure 1c. There is no clear relationship with refractive error, although, of note, the 3-year-old with the large lag is the most hyperopic subject.
Group II—Longitudinal Data
Six of the 2- and 3-year-olds in group I had also been examined at 6 months of age (the 6-month-old data were not used in the analyses above to keep a fully between subject analysis). Figure 2a shows the change in refractive error of the principal meridia of the least hyperopic eye as a function of age, and Figure 2b shows the accommodative accuracy of those meridia as a function of age. Although this small group of subjects demonstrates a range of hyperopic refractive error of approximately 4 D at their first visit, the range of accommodative error was only approximately 0 to 1 D of lag at the same visit. The refraction data exhibit the typical characteristics of emmetropization, with a reduction in both the amount and variability of hyperopia, while the mean accommodative performance demonstrates a small shift toward a lead (mean first visit = 0.57 D, SD ± 0.53 D; mean second visit = 0.13 D, SD ± 0.48 D; paired t-test: t = 2.10, df = 11; P = 0.06).
Group III—Subjects with Clinical Abnormalities
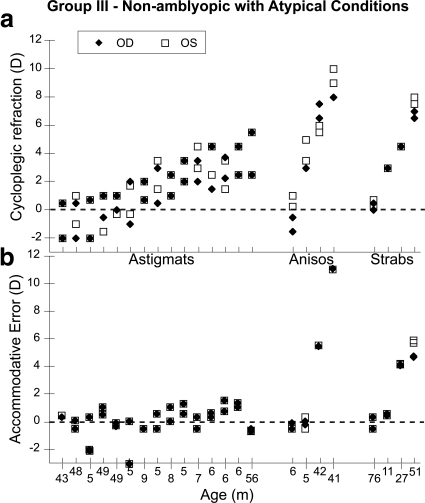
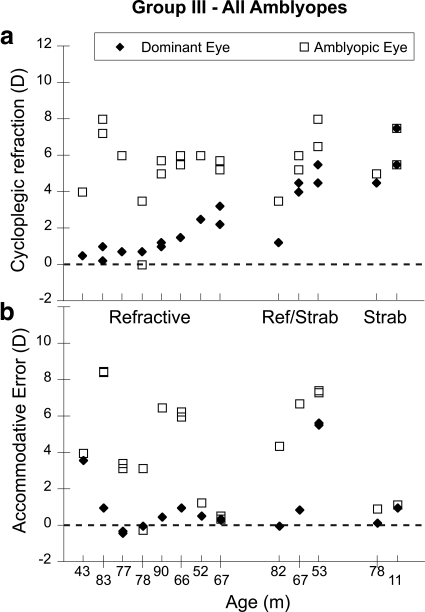
The data from the subjects who did not meet the inclusion criteria for group I are shown in Figures 3 and 4. The group is split into subjects with and without amblyopia (with amblyopia defined as an uncorrected acuity difference equivalent to two or more lines between the eyes in the presence of an amblyogenic factor: >1.0 D of anisometropia, or strabismus, or both). This classification was confirmed for each child in follow-up care. The group without amblyopia consists of subjects who did not meet the amblyopia acuity criterion, but who had astigmatism ≥1 D, anisometropia ≥1 D, or strabismus. The amblyopic group includes patients with refractive, strabismic, or mixed amblyopia. The data collected from all four principal meridia (both principal meridia of both eyes) of each subject are shown, with each subject's data aligned vertically in the upper and lower panels. Figures 3a and 4a show the cycloplegic refraction for each meridian for each subject, and Figures 3b and 4b show the corresponding accommodative error or defocus for each meridian. While the subjects with relatively small refractive errors are able to maintain consistently small accommodative errors, the subjects with the larger amounts of hyperopia exhibit larger accommodative errors when viewing the cartoon, in some cases more than 5 D of error. The amblyopes with the larger amounts of anisometropia in Figure 4 (those with refractive amblyopia) also have large defocus errors in their more hyperopic eyes, as a result of the relatively accurate performance of their dominant eyes.
Figure 3.
Data from the nonamblyopic subjects in group III, who all had astigmatism, anisometropia, or strabismus. The subjects are ordered based on increasing refractive error, with the subject group labeled on the abscissa of (a) and age labeled on the abscissa of (b). (a) Cycloplegic refractive error for all meridia plotted for each subject. (b) Accommodative error for all meridia plotted for the vertically corresponding subject in each group.
Figure 4.
Data from the amblyopic subjects in group III. The subjects are ordered based on increasing refractive error, with the subject group labeled on the abscissa of (a) and age labeled on the abscissa of (b). (a) Cycloplegic refractive error for all meridia plotted for each subject. (b) Accommodative error for all meridia plotted for the vertically corresponding subject in each group. The fact that the data were collected in binocular viewing means that the more hyperopic eyes of the anisometropics are more defocused merely as a result of the accurate accommodation in the dominant eye.
Combined Analysis
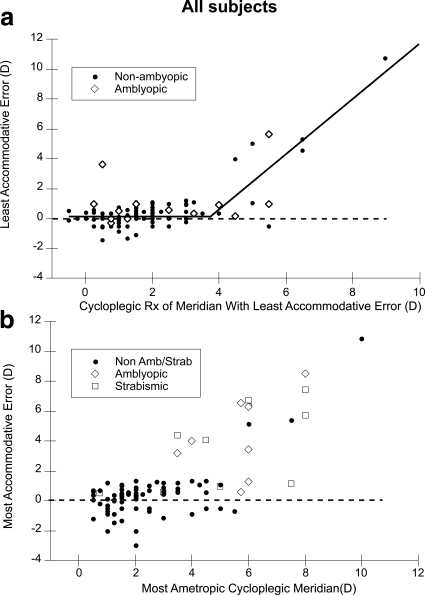
Figure 5 presents two analyses of the accommodative accuracy of the whole group of subjects. In Figure 5a, the accommodative error of the meridian focused closest to the target in binocular viewing conditions is plotted as a function of the cycloplegic refractive error in that meridian. The data are split into two groups, amblyopic and nonamblyopic subjects, to reveal any effect of amblyopic spatial vision on performance when viewing with both eyes. The data from the amblyopic group are taken from the dominant eye (the best-focused meridian in the dominant, nonamblyopic, eye). The data from the nonamblyopic subjects are from the subject's best-focused meridian. This graph demonstrates the most accurate response that subjects achieved across all meridia. It reveals that most subjects with a refractive error of 4 D of hyperopia or less in the most accurately focused meridian exhibit an accommodative error of <2 D as measured with this Nott retinoscopy technique. At higher refractive errors, some subjects start to exhibit much greater lags even for this short duration task. A bilinear fit to the nonamblyopic data, that forced the slope of one line fit to the smaller refractive error data to be zero while allowing the slope of the second line fit to the higher refractive error data and the intersection of the two lines to vary, resulted in an adjusted R2 of 0.74, a y-axis intercept of 0.16 D (95% confidence interval [CI], −0.12 to 0.32 D), a slope of the higher refractive error line of 1.84 (95% CI, 1.48 to 2.22), and an intersection of the lines at 3.77 D (95% CI, 3.27 to 4.26). This fit, shown in Figure 5a, suggests that accommodative error starts to become abnormal in at least some subjects at a cycloplegic refractive error of approximately 4 D. While the subject with the highest refractive error largely drives the slope of the second line, the intersection of the lines at around 4 D does divide the data into consistently accurate accommodation at lower refractive errors and more variable accommodative performance at higher refractive errors. Further analyses of the different types of refractive error is limited as the opportunistic recruitment did not provide any astigmatic subjects with refractive errors above this value, and therefore the interaction between amount of hyperopia and astigmatism could not be assessed for example.
Figure 5.
Two analyses of accommodative error of the whole group. (a) Accommodative error for the meridian focused closest to the target in binocular viewing conditions plotted as a function of refractive error in that meridian. The full group of subjects is split into two groups, amblyopes and nonamblyopic subjects. The lines fit to the data are described in the text. (b) Least accurate accommodative error of any meridian plotted as a function of cycloplegic refractive error in the most ametropic meridian from either eye of nonstrabismic amblyopes, strabismics, and the other more typically-developing children with no amblyopia or strabismus.
This study was cross-sectional in design and therefore it was not possible to predict who would develop strabismus or amblyopia from these data. The subjects were not recruited as infants and then examined periodically until they either developed apparently activity-dependent abnormalities or not.19 In fact, a number of the subjects exhibiting the large lags turned out to be strabismic or amblyopic at the time of testing, and therefore their large lag could be related to either the cause or effect of the condition. In this broader context, in Figure 5b, the subject's least accurate meridian in the accommodative task is plotted as a function of the cycloplegic refractive error of their most ametropic meridian from either eye. The data are split into three groups: nonstrabismic amblyopes, strabismics, and more typically developing children with no amblyopia or strabismus. The goal of Figure 5 was to examine the potential for accommodative performance, measured using the current technique, to be used as a screening tool or an indicator of clinical abnormality. An ROC analysis was conducted on these data by classifying individuals with strabismus, amblyopia, or a most ametropic meridian of above 5 D of hyperopia (given that accommodative performance appeared to be affected above the intersection point of approximately 4 D in Fig. 5a) as the target of a vision screening. With these criteria, the area under the ROC curve was 0.90 (95% CI, 0.82 to 0.95) and using a cut point of 1.3 D of lag for example resulted in a sensitivity of 83.3% with a specificity of 96.5% (correct classification rate of 94.9%).
Discussion
The data collected across a range of ages and clinical status in this study are consistent with the results of the previous studies of young infants19–21 in that individuals with higher hyperopia or abnormal visual development demonstrate greater accommodative lags. For example, Ingram et al.19 provide apparent accommodative deficit data for infants with cycloplegic refractions of 5 D of hyperopia in their Figure 1. The data are provided as a function of age and were collected before a number of these individuals developed squint or microtropia. The individuals who went on to develop strabismus tended to exhibit larger accommodative deficits consistently across age. Although these data were collected using a different nonstandard retinoscopy technique, the absolute values of the errors are also comparable to the data collected in the present study.
It is feasible that individuals with untreated amblyopia or uncorrected astigmatism will demonstrate a larger lag as a result of their increased depth of field, but as shown in Figures 3 and 4, these lags also increase with increasing hyperopic refractive error.
These qualitative effects did not appear to vary as a function of age and even the youngest typically developing infants, at around 6 months of age (Fig. 1b, Fig. 2), were routinely able to accommodate to within a diopter of the target, as would be predicted from the previous literature.15–18 The data collected here, together with the results of previous studies of typically developing children older than 3 years,25,29,32 suggest that young children with low amounts of hyperopia typically exhibit accommodative errors measured with dynamic retinoscopy of less than 1 D across typical near working distances (for example, Rouse et al.32 suggest that a lag greater than 0.75 D should be considered abnormal for MEM retinoscopy with printed words on a card). We would therefore define this level of performance, of the typically developing low hyperopes, as a clinically normal visual experience in a developmental sense, and Figure 5 demonstrates that subjects of all ages with low hyperopia were able to achieve this performance.
Impact of Visual Experience
Retinal image quality has been shown to have a role in both the control of eye growth and synaptic refinement in visual cortex. How might the range of accommodative performance seen here affect an individual's visual development? Studies of animal models have not addressed this question directly because they have typically employed anisometropic defocus, which cannot be compensated for with accommodation.2,33–36 The three prospective trials of preventative optical correction for moderate to high hyperopia that have been conducted to date,37–39 did not track the retinal image quality of the subjects in relation to the clinical outcome. They all suggested that the prevalence of moderate to severe amblyopia is likely to be reduced with preventative spectacle correction, but the reasons for both this and the incidence of strabismus and amblyopia even in their optically corrected hyperopes are yet to be determined. The data collected here do suggest however that there is a wide range of accommodative performance in at risk patients during early childhood.
Clinical Implications
These data have implications for abnormal visual development in two clinical contexts. First, if the infants who exhibit the larger accommodative lags are the ones who are least likely to emmetropize, these data suggest that the risk of failure to emmetropize increases most dramatically above 4 D of hyperopia in nonamblyopic and nonstrabismic individuals. This is consistent with the data of Mutti et al.20 from infants showing that lag for a target at 57 cm started to increase, and emmetropization became less likely, for cycloplegic refractive errors above 3 to 4 D of hyperopia at younger than 1 year of age. Second, with regard to vision screening designed to identify individuals who need treatment or monitoring, the analysis based on Figure 5b suggests accommodative lag at this target distance can be used to detect higher hyperopia, amblyopia, and strabismus, with sensitivity, specificity, and targeted conditions all comparable with those of the Vision in Preschoolers Study40 ‘very important’ to detect conditions for this relatively small sample. The protocol used here only required the participant to accommodate and attend to the target for a relatively short period, and so other subjects could have exhibited increased lags if given a more sustained task. The question of sustained performance is still poorly understood.
A number of approaches could be used to measure defocus for near targets in the clinic: retinoscopy, autorefractors, or photorefractors for example. While the Nott dynamic retinoscopy technique revealed significant lags here, it is clear from the differences in astigmatism and anisometropia in sequential measures plotted in Figures 3 and 4 that an alternative technique that records focus in all principal meridia simultaneously is likely to give more repeatable data if it is available in a clinical setting.
Underlying Reason for Increased Accommodative Lags
Patients with amblyopia may exhibit an increased accommodative lag as a result of an increased depth of field resulting from their limited spatial vision,41 but why might infants and children with higher hyperopia and/or small to moderate amounts of astigmatism have increased lags, that apparently fall beyond their depth of focus? Either they are incapable of accommodating accurately, or they adopt a strategy of not doing so. Ingram et al.19 speculated that the infants who neither accommodate nor emmetropize have a defect in blur sensitivity and that they are at risk for strabismus and amblyopia. They suggest, therefore, that these individuals are not capable of accommodating accurately. Alternatively, Al-Bagdady et al.42 and Nandakumar and Leat43 have observed some patients with Down syndrome who have apparently learned to produce more accurate accommodative responses after exhibiting significant lags, and Hunter44 notes anecdotally that some patients may exhibit bursts of accommodation that are not sustained. Presumably poorly sustained accommodation may be more apparent with the use of a target with a naturalistic spatial frequency amplitude spectrum, or use of a retinoscope light source as in Ingram et al.,19 than with a detailed target that motivates maximal accommodative effort. This distinction is relevant to the selection of a clinical approach.
Conclusion
These data collected from a relatively broad sampling of age and clinical status suggest that clinically normal young infants and children with low amounts of hyperopia maintain an accommodative response within approximately 1 D of a 50 cm target in their least ametropic eye, from the first few months after birth. Subjects who do not appear to be emmetropizing, with >4–5 D of hyperopia, have more variable lags and therefore evidence of abnormal visual experience for some individuals. These preliminary data from a relatively small group of subjects suggest that these highly detectable lags may be used as evidence of abnormal retinal visual experience for consideration in prescribing optical correction and also as an approach to screening for abnormal development. This assessment of accommodative error and criterion could be applied broadly irrespective of age.
Acknowledgments
The authors thank Diane Goss for help with subject recruitment and data collection, the infants and children and their parents for their participation, and Maureen Maguire for helpful discussion of statistics.
Footnotes
Supported by National Eye Institute Grant R01 EY014460 (TRC) and the National Institutes of Health Loan Repayment Program (KHG, DWL).
Disclosure: T.R. Candy, None; K.H. Gray, None; C.C. Hohenbary, None; D.W. Lyon, None
References
- 1. Mitchell DE, Timney B. Postnatal development of function in the mammalian visual system. In: Kandel ER, ed. Handbook of Physiology. The Nervous System III. Vol. 3 Washington DC: American Physiological Society; 1984:507–555 [Google Scholar]
- 2. Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. J Neurosci. 1987;7:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998;18:6411–6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Troilo D. Neonatal eye growth and emmetropisation–a literature review. Eye. 1992;6:154–160 [DOI] [PubMed] [Google Scholar]
- 5. Smith EI. Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, eds. Myopia and Nearwork. Oxford: Butterworth-Heinemann; 1998:57–90 [Google Scholar]
- 6. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468 [DOI] [PubMed] [Google Scholar]
- 7. Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981;20:561–564 [PubMed] [Google Scholar]
- 8. Birch EE, Stager DR. Monocular acuity and stereopsis in infantile esotropia. Invest Ophthalmol Vis Sci. 1985;26:1624–1630 [PubMed] [Google Scholar]
- 9. Shotton K, Powell C, Voros G, Hatt SR. Interventions for unilateral refractive amblyopia. Cochrane Database Syst Rev. 2008;CD005137. [DOI] [PubMed] [Google Scholar]
- 10. Donahue SP, Ruben JB, American Academy of Ophthalmology, et al. US Preventive Services Task Force vision screening recommendations. Pediatrics. 2011;127:569–570 [DOI] [PubMed] [Google Scholar]
- 11. Lyons SA, Jones LA, Walline JJ, et al. A survey of clinical prescribing philosophies for hyperopia. Optom Vis Sci. 2004;81:233–237 [DOI] [PubMed] [Google Scholar]
- 12. Miller JM, Harvey EM. Spectacle prescribing recommendations of AAPOS members. J Pediatr Ophthalmol Strabismus. 1998;35:51–52 [DOI] [PubMed] [Google Scholar]
- 13. Farbrother JE. Spectacle prescribing in childhood: a survey of hospital optometrists. Br J Ophthalmol. 2008;92:392–395 [DOI] [PubMed] [Google Scholar]
- 14. Candy TR, Wang J, Ravikumar S. Retinal image quality and postnatal visual experience during infancy. Optom Vis Sci. 2009;86:E556–E571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haynes H, White BL, Held R. Visual accommodation in human infants. Science. 1965;148:528–530 [DOI] [PubMed] [Google Scholar]
- 16. Banks MS. The development of visual accommodation during early infancy. Child Dev. 1980;51:646–666 [PubMed] [Google Scholar]
- 17. Braddick O, Atkinson J, French J, Howland HC. A photorefractive study of infant accommodation. Vision Res. 1979;19:1319–1330 [DOI] [PubMed] [Google Scholar]
- 18. Brookman KE. Ocular accommodation in human infants. Am J Optom Physiol Opt. 1983;60:91–99 [DOI] [PubMed] [Google Scholar]
- 19. Ingram RM, Gill LE, Goldacre MJ. Emmetropisation and accommodation in hypermetropic children before they show signs of squint–a preliminary analysis. Bull Soc Belge Ophtalmol. 1994;253:41–56 [PubMed] [Google Scholar]
- 20. Mutti DO, Mitchell GL, Jones LA, et al. Accommodation, acuity, and their relationship to emmetropization in infants. Optom Vis Sci. 2009;86:666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horwood AM, Riddell PM. Hypo-accommodation responses in hypermetropic infants and children. Br J Ophthalmol. 2011;95:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tondel GM, Candy TR. Human infants' accommodation responses to dynamic stimuli. Invest Ophthalmol Vis Sci. 2007;48:949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nott IS. Dynamic skiametry, accommodation and convergence. Am J Physiol Optics. 1925;6:490–503 [Google Scholar]
- 24. Locke LC, Somers W. A comparison study of dynamic retinoscopy techniques. Optom Vis Sci. 1989;66:540–544 [DOI] [PubMed] [Google Scholar]
- 25. Leat SJ, Gargon JL. Accommodative response in children and young adults using dynamic retinoscopy. Ophthalmic Physiol Opt. 1996;16:375–384 [PubMed] [Google Scholar]
- 26. Rosenfield M, Portello JK, Blustein GH, Jang C. Comparison of clinical techniques to assess the near accommodative response. Optom Vis Sci. 1996;73:382–388 [DOI] [PubMed] [Google Scholar]
- 27. Garcia A, Cacho P. MEM and Nott dynamic retinoscopy in patients with disorders of vergence and accommodation. Ophthalmic Physiol Opt. 2002;22:214–220 [DOI] [PubMed] [Google Scholar]
- 28. McClelland JF, Saunders KJ. The repeatability and validity of dynamic retinoscopy in assessing the accommodative response. Ophthalmic Physiol Opt. 2003;23:243–250 [DOI] [PubMed] [Google Scholar]
- 29. McClelland JF, Saunders KJ. Accommodative lag using dynamic retinoscopy: age norms for school-age children. Optom Vis Sci. 2004;81:929–933 [PubMed] [Google Scholar]
- 30. Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–1628 [DOI] [PubMed] [Google Scholar]
- 31. Mutti DO. To emmetropize or not to emmetropize? The question for hyperopic development. Optom Vis Sci. 2007;84:97–102 [DOI] [PubMed] [Google Scholar]
- 32. Rouse MW, Hutter RF, Shiftlett R. A normative study of the accommodative lag in elementary school children. Am J Optom Physiol Opt. 1984;61:693–697 [DOI] [PubMed] [Google Scholar]
- 33. Boothe RG, Kiorpes L, Hendrickson A. Anisometropic amblyopia in Macaca nemestrina monkeys produced by atropinization of one eye during development. Invest Ophthalmol Vis Sci. 1982;22:228–233 [PubMed] [Google Scholar]
- 34. Harwerth RS, Smith EL, 3rd, Boltz RL, Crawford ML, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: spatial modulation sensitivity. Vision Res. 1983;23:1501–1510 [DOI] [PubMed] [Google Scholar]
- 35. Kiorpes L, Boothe RG, Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS. Effects of early unilateral blur on the macaque's visual system. I. Behavioral observations. J Neurosci. 1987;7:1318–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS, Boothe RG, Kiorpes L. Effects of early unilateral blur on the macaque's visual system. II. Anatomical observations. J Neurosci. 1987;7:1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ingram RM, Arnold PE, Dally S, Lucas J. Results of a randomised trial of treating abnormal hypermetropia from the age of 6 months. Br J Ophthalmol. 1990;74:158–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atkinson J. Infant vision screening: prediction and prevention of strabismus and amblyopia from refractive screening in the Cambridge Photorefraction Program. In: Simons K, ed). Early Visual Development, Normal and Abnormal. New York: Oxford University Press; 1993:335–348 [Google Scholar]
- 39. Anker S, Atkinson J, Braddick O, Nardini M, Ehrlich D. Non-cycloplegic refractive screening can identify infants whose visual outcome at 4 years is improved by spectacle correction. Strabismus. 2004;12:227–245 [DOI] [PubMed] [Google Scholar]
- 40. Kulp MT, Vision in Preschoolers Study Group Findings from the Vision in Preschoolers (VIP) Study. Optom Vis Sci. 2009;86:619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ciuffreda KJ, Hokoda SC, Hung GK, Semmlow JL. Accommodative stimulus/response function in human amblyopia. Doc Ophthalmol. 1984;56:303–326 [DOI] [PubMed] [Google Scholar]
- 42. Al-Bagdady M, Stewart RE, Watts P, Murphy PJ, Woodhouse JM. Bifocals and Down's syndrome: correction or treatment? Ophthalmic Physiol Opt. 2009;29:416–421 [DOI] [PubMed] [Google Scholar]
- 43. Nandakumar K, Leat SJ. Bifocals in children with Down syndrome (BiDS) - visual acuity, accommodation and early literacy skills. Acta Ophthalmol. 2010;88:e196–e204 [DOI] [PubMed] [Google Scholar]
- 44. Hunter DG. Dynamic retinoscopy: the missing data. Surv Ophthalmol. 2001;46:269–274 [DOI] [PubMed] [Google Scholar]