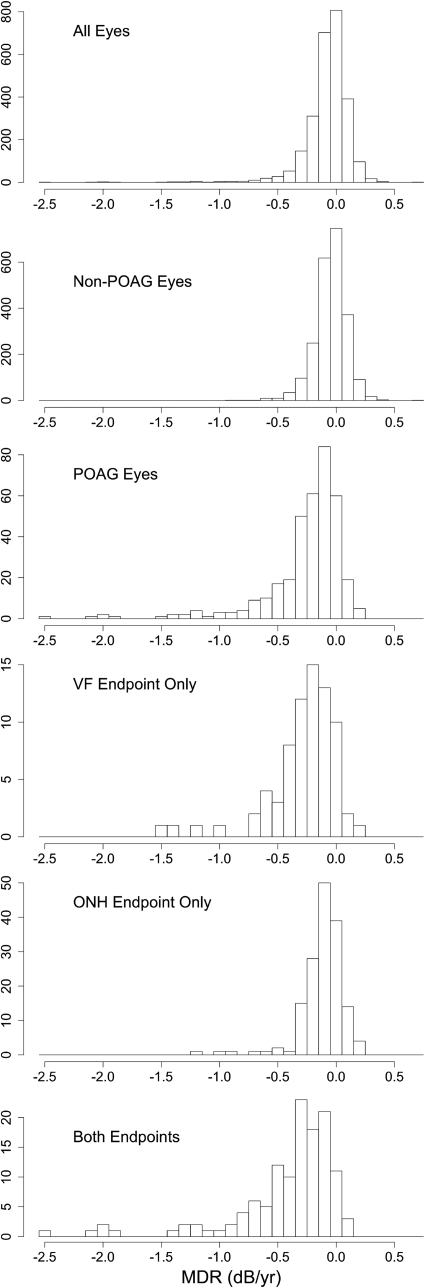
The rate of visual field change was more rapid in OHTS eyes that reached a POAG endpoint, even if it was an optic disc endpoint. Eyes that reached visual field and optic disc endpoints had the most rapid rates of visual field change.
Abstract
Purpose.
To assess the rate of change of visual field (VF) mean deviation (MD) in the Ocular Hypertension Treatment Study (OHTS).
Methods.
OHTS data were filtered to exclude eyes that had fewer than 10 reliable VFs or less than 5 years of follow-up or that reached a nonglaucomatous endpoint. The rate of change of MD (MDR) was calculated for each eye. Differences were sought between groups of eyes differing in primary open angle glaucoma (POAG) outcome, how POAG was determined, and original randomization.
Results.
In total, 2609 eyes (1379 participants) met the selection criteria. The mean MDR was −0.08 ± 0.20 dB/y (±SD). POAG eyes (n = 359) had significantly worse MDRs (−0.26 ± 0.36 dB/y) than non-POAG eyes (n = 2250; −0.05 ± 0.14 dB/y; P < 0.001). Eyes that reached POAG endpoints based on only VF change (n = 74; −0.29 ± 0.31 dB/y) or only optic disc change (n = 158; −0.12 ± 0.19 dB/y) had significantly worse MDRs than non-POAG eyes (both P < 0.001). Eyes that reached POAG endpoints for both VF and optic disc change (n = 127) deteriorated more rapidly (−0.42 ± 0.46 dB/y) than eyes showing only VF change (P = 0.017) or only optic disc change (P < 0.001). There was not a significant association between MDR and original OHTS randomization (observe vs. treat, P = 0.168).
Conclusions.
Eyes that develop POAG have significantly worse MDRs than eyes that do not. Eyes that reached endpoints due to both VF and optic disc change had worse MDRs than eyes displaying change in only one of these. MDR was not significantly associated with randomization, suggesting that MDR may not be the best measure of VF change in early-stage POAG. (ClinicalTrials.gov number, NCT00000125.)
The Ocular Hypertension Treatment Study (OHTS) was a randomized clinical trial that demonstrated a benefit of lowering intraocular pressure (IOP) in patients with ocular hypertension. The OHTS found that the proportion of participants with ocular hypertension converting to primary open angle glaucoma (POAG) was approximately 50% lower in those randomly assigned to treatment than in those randomly assigned to observation.1 Along with this primary result, the OHTS improved our understanding of the associations between certain clinical findings and the risk for conversion to POAG.2–6
Detection of VF change over time is challenging largely because of the numerous factors that can influence VF outcomes.7–12 Major clinical trials in glaucoma have formulated different criteria for detecting VF change over time.13–16 In the OHTS, for instance, participants were required to have qualifying standard automated perimetry (SAP) VFs that were within normal limits.13 A change in VFs from within normal limits to outside normal limits (Glaucoma Hemifield Test (GHT) outside normal limits or Corrected Pattern Standard Deviation (CPSD)/Pattern Standard Deviation (PSD) greater than the normal 5% level) on originally two, but later three, consecutive reliable examinations was considered confirmation of VF change.17 This is an event-based approach because progression is deemed to have occurred when a particular event takes place, regardless of how soon after baseline it happens.
One disadvantage of event-based methods is that many intervening VF tests are not used when determining whether change has taken place and no estimate of the rate of change is produced. Other methods for quantifying VF change that use the entire test sequence have been developed. These methods have become known as trend-based techniques because they generate a continuous, numeric trend value (dB/y). Trend-based analyses can be performed using global indices, such as mean deviation (MD) or the newer visual field index (VFI)18 or using threshold values from individual test locations, such as pointwise linear regression (PLR).19–21
The present study had two main purposes, to examine the rate of VF change in OHTS participants as quantified by the rate of MD change over time (MDR) and to test the hypothesis that the MDR is significantly associated with endpoint status (POAG vs. no POAG), with how the POAG endpoint was established (VF change, optic disc change, or change in both), and with the initial randomization (observed vs. treated).
Methods
The design of the OHTS adhered to the Declaration of Helsinki. All participants signed statements of informed consent before study entry, after having the risks and benefits of participation explained to them. Institutional review boards at each participating clinical site approved their respective informed consent statements and procedures. A list of all participating OHTS sites and personnel can be found at https://vrcc.wustl.edu/clinics.html. The analysis data set for this study contained all VF data and endpoint determinations as of March 9, 2009. Several inclusion and exclusion criteria were then applied, as described.
The OHTS recruited and randomly assigned 1636 subjects. Inclusion and exclusion criteria along with qualifying requirements have been previously reported.13 To qualify, both optic nerve heads of participants had to be within normal limits as judged by readers at the Optic Disc Reading Center. Potential participants also had to have two qualifying SAP VFs that were reliable and within normal limits as judged by readers at the Visual Field Reading Center.13 If results of either of the first two VF tests were unreliable or questionable, a third qualifying test was allowed. During follow-up, VFs were scheduled to be measured every 6 months. If there was suspicion of VF deterioration during follow-up (originally only once but later twice), then an off-schedule confirmatory VF was requested. For eyes that reached a POAG endpoint, VF testing continued every 6 months, and all VFs before and after endpoint were included in this analysis.
Because we were interested in glaucomatous VF change, we excluded all eyes that reached a study endpoint not attributed to POAG (261 eyes of 202 participants). We then selected the last qualifying VF for each eye and only those follow-up VFs that were considered reliable (false positives, false negatives, and fixation loss all <33% if full threshold; false positives <15%, false negatives and fixation loss <33% if SITA). We selected the last qualifying VF for each eye because there was evidence of significant learning between the first and second qualifying tests. It would not be appropriate to use the mean of the qualifying VFs to represent the initial time point because this would reduce the variance at this time point and violate the constant variance assumption of linear regression. We excluded all eyes that had fewer than 10 VFs (qualifying + nine follow-up) or whose VF sequence spanned less than 5 years so that all VF sequences were long enough to allow precise estimates of the MDR to be made. Exclusion criteria resulted in the analysis of 2609 eyes of 1379 participants. We then compiled endpoint information for these 2609 eyes and recorded whether the POAG endpoint was attributed to change in the VF, optic disc appearance, or both. An eye was considered to have both POAG endpoints if change occurred in both the VF and the optic disc at any time during follow-up.
To estimate the MDR, we performed linear regression of MD over time for each of the selected eyes. Mean, 95% confidence intervals (CIs) about the mean, median, and 2.5th and 97.5th percentile MDRs for different groupings of eyes were calculated. The MDRs of eyes in different groups were then compared to see whether significant differences existed between them.
Analysis was performed using the R language and environment for statistical computing (version 2.11.1; http://cran.stat.ucla.edu; accessed April 22, 2010).22 All comparisons included data from both eyes of a participant when available but took account of the correlation between the data from both eyes (generalized estimating equation [GEE])23 as implemented in the geepack library.24 The P-value for significance was set at 0.05 for all comparisons between groups.
Results
For the selected cohort, the mean duration of follow-up was 12.2 years (SD, 2.0; median, 12.9; interquartile range [IQR], 12.1–13.5; range, 5.0–14.6) and the mean number of VF tests, including the qualifying test, was 23.7 (SD, 4.9; median, 25; IQR, 21–27; range, 10–35). Table 1 shows MDR values for eyes within different categories. The values given are the mean and 95% CI about the mean in the third column and the median, 2.5th percentile, and 97.5th percentile values for each category in the fourth column. The mean MDR for each of the groups of eyes shown in Table 1 were highly significantly different from zero (P < 0.001 all groups). Figure 1 depicts the information contained for some of the groups in Table 1 but better displays the distributions of the data.
Table 1.
MDRs in dB/y for Different Categories of Eyes
| OHTS Classification | Eyes (n) | Mean MDR (95% CI) | Median (2.5%, 97.5%) MDR for Group |
|---|---|---|---|
| All eyes | 2609 | −0.08 (−0.08 to −0.07) | −0.05 (−0.52, 0.19) |
| No POAG end point | 2250 | −0.05 (−0.05 to −0.04) | −0.04 (−0.35, 0.20) |
| All POAG (optic disc and/or VF change) | 359 | −0.26 (−0.30 to −0.22) | −0.17 (−1.31, 0.12) |
| POAG due to VF change only | 74 | −0.29 (−0.36 to −0.22) | −0.22 (−1.21, 0.07) |
| POAG due to optic disc change only | 158 | −0.12 (−0.15 to −0.09) | −0.09 (−0.60, 0.13) |
| POAG due to either VF or optic disc change | 232 | −0.17 (−0.20 to −0.14) | −0.12 (−0.90, 0.13) |
| POAG due to both VF and optic disc change | 127 | −0.42 (−0.50 to −0.34) | −0.27 (−1.98, 0.05) |
| Randomly assigned to observation in OHTS 1 | 1302 | −0.08 (−0.10 to −0.07) | −0.05 (−0.55, 0.19) |
| Randomly assigned to treatment in OHTS 1 | 1307 | −0.07 (−0.08 to −0.06) | −0.05 (−0.46, 0.19) |
Figure 1.
Histograms of MDRs for different classes of eyes. The number of eyes in each class can be found in Table 1.
Not surprisingly, the MDR was more negative (more rapid deterioration) in eyes that reached a POAG endpoint compared with eyes that did not (P < 0.001). Of note, the MDR was significantly more negative in eyes whose determination of POAG was based on optic disc change only versus non-POAG eyes (P < 0.001). Furthermore, the MDR was significantly more negative in eyes that displayed change in both VF and optic disc compared with eyes that only displayed change in a single modality (P < 0.001). In other words, deterioration in optic disc appearance during follow-up was associated with significantly more rapid VF deterioration, even if the OHTS criteria for VF change was not met.
Interestingly, there was no significant difference between the MDR of eyes originally randomly assigned to observation and eyes originally randomly assigned to treatment (Table 1, compare rows 8 and 9; P = 0.168). To further explore this finding, we examined those eyes that reached an OHTS VF endpoint (n = 201) using PLR. In these eyes, a median of only two VF locations (median, 2 of 75 non–blind spot locations; 25th and 75th percentiles = 0 and 9 locations, respectively) were considered to be changing rapidly (slope ≤ −1.0 dB/y with P ≤ 0.01, a commonly applied PLR criterion). When a less stringent criterion was applied (slope ≤ −0.5 dB/y with P ≤ 0.05) a median of 14 locations (25th and 75th percentiles = 4 and 33) were considered to be changing rapidly.
Because the distribution of MDR values in all groups was skewed, we performed two additional analyses. First, we transformed MDR values using a power transformation until they were normally distributed. Second, we analyzed only one randomly chosen eye per participant and used the nonparametric Mann-Whitney rank sum test. These approaches produced findings almost identical to those reported.
To quantify the variance about the linear regression models for each eye, we calculated the SD of the residuals for each of the 2609 linear regressions performed. The mean ± SD was 0.96 ± 0.43 dB. The range was 0.25 to 6.71 dB, with 25th and 75th percentile values of 0.67 and 1.12 dB, respectively.
For participants who remained in the OHTS through its second phase, there was a transition from full threshold to the Swedish interactive thresholding algorithm (SITA standard25) testing. We sought an effect of this change in strategy on MD. We fit a linear model of MD against time for each eye while including “strategy” as a binary covariate. We then determined whether the mean coefficient for strategy was significantly different from zero; it was not (P = 0.752). We conclude that there was no significant effect of test strategy (full threshold vs. SITA standard) on MD. Similar findings have been reported in the literature.26–30
Discussion
Perhaps the most striking finding from this study was that the mean MDR in eyes with ocular hypertension, including those that converted to POAG, was slow (−0.08 dB/y). At this rate it would take approximately three decades for a visual field to progress from the normal mean (MD = 0 dB) to the 5th percentile of a healthy reference group (MD ≈ −2.2 dB).
When we applied a criterion of MDR ≤ −1 dB/y significant at the P < 0.01 level31 to all 2609 selected eyes, only 16 (∼0.6%) were considered to be changing rapidly. No eyes displayed positive trends at the same rate (i.e., MDR > +1 dB/y). When we applied a less stringent criterion (MDR ≤ −0.5 dB/y; P < 0.05), 67 eyes (2.6%) were considered to be changing rapidly. The overall proportion of eyes in the wider OHTS study (without the lower limits on follow-up duration and number of tests that we applied in the current analysis) considered to have developed POAG was 11.1% (362/3272 eyes); the rate was 6.2% (204/3272) if we consider only those eyes that reached an endpoint because of confirmed VF change.32 These values are substantially higher than the proportions reported for MDR and suggest that monitoring the rate of change of MD in ocular hypertensive eyes may underestimate the number of eyes displaying glaucomatous VF deterioration. It may also suggest that when the VFs of persons with ocular hypertension begin to change during the development of glaucoma, this change occurs at only a small number of VF locations. Local change is more likely to cause CPSD/PSD or GHT, the indices that were used to make a determination of VF change in the OHTS, to move outside their normal limits but will not cause much change in MD. The MD index is designed to “average ” data from all VF locations. However, if only a few VF locations change early in the disease, the average will not contain much “change signal ” and the application of pointwise methods may be preferable.33,34
Not surprisingly, eyes reaching a POAG endpoint based on confirmed VF change had MDRs that were significantly more negative (more rapid deterioration) than non-POAG eyes. However, eyes reaching a POAG endpoint based on change evident in both the VF and the optic disc displayed the most rapid rate of MD change of any subgroup. This suggests that an eye displaying glaucomatous change at the optic disc and within the VF may have a more rapidly progressing form of the disease.
MDR in eyes originally randomly assigned to treatment in the OHTS was not significantly different from that found in eyes originally randomly assigned to observation. This finding initially seems difficult to explain given the significant difference in the number of eyes reaching a POAG endpoint in the two randomization groups.1 However, this finding can be somewhat explained if it is taken together with the number of VF locations that displayed rapid deterioration (median VF locations, 2) in eyes that displayed confirmed VF change. These findings support the notion that VF change in early glaucoma might be primarily localized. In addition, all participants were offered treatment at the commencement of phase 2 of the OHTS. Because we used the entire VF sequence from each eye, most of the eyes originally randomly assigned to observation were actually treated for a substantial portion of their follow-up. The current analysis was not optimally designed to examine the effect of treatment on MDR because that would require looking at the change in MDR at either side of a change in treatment. This question is the subject of another study.
One limitation of the method used to asses the rate of VF change in this study (linear regression) is the assumption of a constant rate of change (slope) in the calculation of the statistical significance of the slope estimate. It is not yet established that the rate of VF change in glaucoma is constant.
In conclusion, our analyses show that eyes meeting the OHTS definition of a POAG endpoint display significantly faster VF deterioration than eyes not meeting the endpoint definition, regardless of whether POAG was identified by change occurring in the VF or change in the appearance of the optic disc. Ocular hypertensive eyes that develop POAG and display both optic disc and VF change, not necessarily concurrently, had significantly more rapid VF deterioration than eyes developing POAG but only displaying change in their VF or in their optic disc.
Footnotes
Supported by the National Eye Institute, National Center on Minority Health and Health Disparities, National Institutes of Health Grants EY09341 and EY09307 and Vision Core Grant P30 EY02687, Horncrest Foundation, awards to the Department of Ophthalmology and Visual Sciences at Washington University, Merck Research Laboratories, Pfizer, Inc, unrestricted grants from Research to Prevent Blindness, Gildor Research Fund of the New York Glaucoma Research Institute, Glaucoma Research and Education Fund of Lenox Hill Hospital, and The Legacy Good Samaritan Foundation.
Disclosure: S. Demirel, None; C.G.V. De Moraes, None; S.K. Gardiner, None; J.M. Liebmann, None; G.A. Cioffi, None; R. Ritch, None; M.O. Gordon, None; M.A. Kass, None
References
- 1. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713, discussion 829–730 [DOI] [PubMed] [Google Scholar]
- 2. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720, discussion 829–730 [DOI] [PubMed] [Google Scholar]
- 3. Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122:813–820 [DOI] [PubMed] [Google Scholar]
- 4. Zangwill LM, Weinreb RN, Beiser JA, et al. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma: the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123:1188–1197 [DOI] [PubMed] [Google Scholar]
- 5. Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine RA, Demirel S, Fan J, Keltner JL, Johnson CA, Kass MA. Asymmetries and visual field summaries as predictors of glaucoma in the ocular hypertension treatment study. Invest Ophthalmol Vis Sci. 2006;47:3896–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klewin KM, Radius RL. Background illumination and automated perimetry. Arch Ophthalmol. 1986;104:395–397 [DOI] [PubMed] [Google Scholar]
- 8. Haas A, Flammer J, Schneider U. Influence of age on the visual fields of normal subjects. Am J Ophthalmol. 1986;101:199–203 [DOI] [PubMed] [Google Scholar]
- 9. Heuer DK, Anderson DR, Feuer WJ, Gressel MG. The influence of decreased retinal illumination on automated perimetric threshold measurements. Am J Ophthalmol. 1989;108:643–650 [DOI] [PubMed] [Google Scholar]
- 10. Lindenmuth KA, Skuta GL, Rabbani R, Musch DC. Effects of pupillary constriction on automated perimetry in normal eyes. Ophthalmology. 1989;96:1298–1301 [DOI] [PubMed] [Google Scholar]
- 11. Lindenmuth KA, Skuta GL, Rabbani R, Musch DC, Bergstrom TJ. Effects of pupillary dilation on automated perimetry in normal patients. Ophthalmology. 1990;97:367–370 [DOI] [PubMed] [Google Scholar]
- 12. Henson DB, Morris EJ. Effect of uncorrected refractive errors upon central visual field testing. Ophthalmic Physiol Opt. 1993;13:339–343 [DOI] [PubMed] [Google Scholar]
- 13. Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583 [DOI] [PubMed] [Google Scholar]
- 14. Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. The European Glaucoma Prevention Study design and baseline description of the participants. Ophthalmology. 2002;109:1612–1621 [DOI] [PubMed] [Google Scholar]
- 15. Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–662 [DOI] [PubMed] [Google Scholar]
- 16. The Advanced Glaucoma Intervention Study Group The Advanced Glaucoma Intervention Study (AGIS), 1: study design and methods and baseline characteristics of study patients. Controlled Clin Trials 1994;15:299–325 [DOI] [PubMed] [Google Scholar]
- 17. Keltner JL, Johnson CA, Quigg JM, Cello KE, Kass MA, Gordon MO. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118:1187–1194 [DOI] [PubMed] [Google Scholar]
- 18. Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353 [DOI] [PubMed] [Google Scholar]
- 19. Fitzke FW, Hitchings RA, Poinoosawmy D, McNaught AI, Crabb DP. Analysis of visual field progression in glaucoma. Br J Ophthalmol. 1996;80:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gardiner SK, Crabb DP. Examination of different pointwise linear regression methods for determining visual field progression. Invest Ophthalmol Vis Sci. 2002;43:1400–1407 [PubMed] [Google Scholar]
- 21. Manassakorn A, Nouri-Mahdavi K, Koucheki B, Law SK, Caprioli J. Pointwise linear regression analysis for detection of visual field progression with absolute versus corrected threshold sensitivities. Invest Ophthalmol Vis Sci. 2006;47:2896–2903 [DOI] [PubMed] [Google Scholar]
- 22. R Development Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010 [Google Scholar]
- 23. Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23:859–874 [DOI] [PubMed] [Google Scholar]
- 24. Yan J. geepack: Yet Another Package for Generalized Estimating Equations. R-News;2002:12–14 [Google Scholar]
- 25. Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol Scand. 1997;75:368–375 [DOI] [PubMed] [Google Scholar]
- 26. Bengtsson B, Heijl A, Olsson J. Evaluation of a new threshold visual field strategy, SITA, in normal subjects: Swedish Interactive Thresholding Algorithm. Acta Ophthalmol Scand. 1998;76:165–169 [DOI] [PubMed] [Google Scholar]
- 27. Bengtsson B, Heijl A. Evaluation of a new perimetric threshold strategy, SITA, in patients with manifest and suspect glaucoma. Acta Ophthalmol Scand. 1998;76:268–272 [DOI] [PubMed] [Google Scholar]
- 28. Bengtsson B, Heijl A. Comparing significance and magnitude of glaucomatous visual field defects using the SITA and Full Threshold strategies. Acta Ophthalmol Scand. 1999;77:143–146 [DOI] [PubMed] [Google Scholar]
- 29. Shirato S, Inoue R, Fukushima K, Suzuki Y. Clinical evaluation of SITA: a new family of perimetric testing strategies. Graefes Arch Clin Exp Ophthalmol. 1999;237:29–34 [DOI] [PubMed] [Google Scholar]
- 30. Musch DC, Gillespie BW, Motyka BM, Niziol LM, Mills RP, Lichter PR. Converting to SITA-standard from full-threshold visual field testing in the follow-up phase of a clinical trial. Invest Ophthalmol Vis Sci. 2005;46:2755–2759 [DOI] [PubMed] [Google Scholar]
- 31. Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wild JM, Hutchings N, Hussey MK, Flanagan JG, Trope GE. Pointwise univariate linear regression of perimetric sensitivity against follow-up time in glaucoma. Ophthalmology. 1997;104:808–815 [DOI] [PubMed] [Google Scholar]
- 34. Artes PH, O'Leary N, Hutchison DM, et al. Properties of the Statpac Visual Field Index. Invest Ophthalmol Vis Sci. 2011;52:4030–4038 [DOI] [PubMed] [Google Scholar]