This study further characterizes the treatment course, visual outcomes, and response to adjunctive corticosteroid therapy of Pseudomonas aeruginosa keratitis.
Abstract
Purpose.
To compare the clinical course and effect of adjunctive corticosteroid therapy in Pseudomonas aeruginosa with those of all other strains of bacterial keratitis.
Methods.
Subanalyses were performed on data collected in the Steroids for Corneal Ulcers Trial (SCUT), a large randomized controlled trial in which patients were treated with moxifloxacin and were randomly assigned to 1 of 2 adjunctive treatment arms: corticosteroid or placebo (4 times a day with subsequent reduction). Multivariate analysis was used to determine the effect of predictors, organism, and treatment on outcomes, 3-month best-spectacle-corrected visual acuity (BSCVA), and infiltrate/scar size. The incidence of adverse events over a 3-month follow-up period was compared using Fisher's exact test.
Results.
SCUT enrolled 500 patients. One hundred ten patients had P. aeruginosa ulcers; 99 of 110 (90%) enrolled patients returned for follow-up at 3 months. Patients with P. aeruginosa ulcers had significantly worse visual acuities than patients with other bacterial ulcers (P = 0.001) but showed significantly more improvement in 3-month BSCVA than those with other bacterial ulcers, adjusting for baseline characteristics (−0.14 logMAR; 95% confidence interval, −0.23 to −0.04; P = 0.004). There was no significant difference in adverse events between P. aeruginosa and other bacterial ulcers. There were no significant differences in BSCVA (P = 0.69), infiltrate/scar size (P = 0.17), and incidence of adverse events between patients with P. aeruginosa ulcers treated with adjunctive corticosteroids and patients given placebo.
Conclusions.
Although P. aeruginosa corneal ulcers have a more severe presentation, they appear to respond better to treatment than other bacterial ulcers. The authors did not find a significant benefit with corticosteroid treatment, but they also did not find any increase in adverse events. (ClinicalTrials.gov number, NCT00324168.)
Among the causative organisms of bacterial keratitis, Pseudomonas aeruginosa is of particular concern for several reasons. P. aeruginosa, which causes a significant proportion of bacterial keratitis, is responsible for 6% to 39% of cases in the United States and 8% to 21% in South India.1–7 Additionally, P. aeruginosa corneal ulcers have been described as more severe at presentation than other bacterial corneal ulcers.1,8–10 P. aeruginosa is also known to be highly virulent, and P. aeruginosa ulcers are generally thought to be more difficult to treat and to result in worse visual outcomes than other bacterial corneal ulcers.1,8,9 Lastly, there is particular concern regarding the use of corticosteroids in the treatment of P. aeruginosa corneal ulcers. Animal studies have described increased rates of recurrence in P. aeruginosa ulcers treated with corticosteroids compared with other bacterial ulcers treated with corticosteroids.11,12 In a recent pair of editorials, one author advised readers to be “especially cautious” when considering corticosteroids for P. aeruginosa ulcers.13 A second stated the “prevailing bias against using topical corticosteroids in P. aeruginosa keratitis” was based on “inconclusive” evidence.14 Previous clinical trials investigating corticosteroid use in bacterial keratitis have not had enough power to detect a significant effect of corticosteroids and have only had a few cases of P. aeruginosa ulcers.15–17 More recently, the Steroids for Corneal Ulcers Trial (SCUT), a large randomized controlled trial, demonstrated no overall difference in visual outcomes with adjunctive corticosteroid use in bacterial corneal ulcers.18 In this study, we perform subgroup analyses of SCUT to better characterize the clinical course of P. aeruginosa corneal ulcers and to determine the effect of topical corticosteroid therapy on their outcomes.
Methods
SCUT was a National Institutes of Health–funded randomized, double-masked, placebo-controlled, multicenter clinical trial with two parallel arms comparing adjunctive topical prednisolone phosphate 1% (Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL) with topical placebo (NaCl 0.9% and preservative, prepared by Leiter's Pharmacy, San Jose, CA); all patients received topical moxifloxacin 0.5% (Viagmox, Alcon, Fort Worth, TX). Specific methods of the trial have been described elsewhere.18,19 The trial enrolled 500 patients from September 1, 2006, to February 22, 2010. Patients were enrolled at the Aravind Eye Care System (Madurai, Tirunelveli, and Coimbatore), the Dartmouth-Hitchcock Medical Center, and the Francis I. Proctor Foundation at the University of California, San Francisco. Patients were eligible for the trial if they had a culture-positive bacterial corneal ulcer. Corneal scrapings were smeared for Gram's stain and KOH wet mount and were cultured on sheep's blood agar, chocolate agar, and potato dextrose agar or Sabouraud's agar for bacterial and fungal cultures. Growth of organism on one solid medium at the site of inoculation was considered a positive bacterial culture except for coagulase-negative Staphylococcus and diphtheroid bacteria, in which moderate growth on at least two solid media or on one solid medium with appropriate morphology and staining characteristics on Gram's stain was needed. Exclusion criteria included evidence of fungus, acanthamoeba or herpes virus infection, any use of topical corticosteroids before presentation, bilateral ulcers, best spectacle-corrected visual acuity (BSCVA) worse than 6/60 in the fellow eye, no light perception in the affected eye, age younger than 16 years, and impending perforation of the corneal ulcer (defined as presence of descemetocele).
Moxifloxacin, administered to both treatment arms, was administered as 1 drop every hour while awake for the first 48 hours, then 1 drop every 2 hours until reepithelialization, and then 4 times a day until 3 weeks from enrollment. Topical corticosteroid or placebo was started 48 hours after moxifloxacin therapy and was administered 1 drop 4 times a day for the first week after enrollment, then twice daily for 1 week, and then once a day for 1 week. The primary aim of the trial was to determine whether adjunctive topical corticosteroid therapy improves visual outcomes of bacterial corneal ulcers as measured by BSCVA 3 months after enrollment. Prespecified secondary outcomes included infiltrate/scar size at 3 months, time to reepithelialization, and adverse events. Additional prespecified aims of SCUT included subgroup analyses comparing the overall efficacy and safety of corticosteroid therapy in ulcers caused by P. aeruginosa versus other bacteria and any difference in efficacy based on enrollment characteristics.
Patients were evaluated at baseline, every 3 days (±1 day) until reepithelialization (defined as epithelial defect ≤0.5 mm), at 3 weeks, and at 3 months by certified, masked refractionists and ophthalmologists who conducted visual acuity examinations and slit lamp examinations. LogMAR visual acuity was measured by tumbling “E” charts at 4 meters (Charts 2305 and 2305A; Precision Vision, La Salle, IL). Using a slit lamp, infiltrate/scar size was measured as the longest dimension and the longest perpendicular to this dimension in millimeters.
Informed consent was obtained for all subjects enrolled in SCUT. Institutional Review Board approval was granted by the Aravind Eye Care System Institutional Review Board, the University of California, San Francisco Committee on Human Research, and the Dartmouth-Hitchcock Medical Center Committee for Protection of Human Subjects. This study adhered to the tenets of the Declaration of Helsinki.
Statistical Methods
This study performed subanalyses on all patients in SCUT except mixed bacterial infections (two or more distinct bacterial organisms on culture at enrollment). Baseline demographic features and risk factors for P. aeruginosa ulcers were compared with those of all other bacterial ulcers and were analyzed by treatment group using the Fisher's exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. BSCVA (logMAR) and infiltrate/scar size (mm) of P. aeruginosa versus all other bacteria ulcers were compared at baseline and at 3 months using linear regression analysis. To assess the response to treatment of P. aeruginosa versus other bacterial organisms of similar presentation, a prespecified multiple linear regression model including a dichotomous term of P. aeruginosa versus all other bacteria (the predictor of interest), treatment arm, and enrollment characteristics (BSCVA or infiltrate/scar size) as covariates was used to predict both BSCVA (logMAR) and infiltrate/scar size (geometric mean of the two measurements in mm) at enrollment and 3 months. To test for a possible differential effect of corticosteroid treatment on P. aeruginosa ulcers, an interaction term (organism × treatment) was added to our model. Sensitivity analysis including treatment arm and enrollment BSCVA, scar size, ulcer depth, and ulcer location as covariates was used to assess for confounders. Sensitivity analyses were also performed to test for effects of study site, examiner, mixed infections, and nonlinear distribution of baseline visual acuity. Comparison of adverse events including perforation, elevated intraocular pressure (IOP), and recurrence of epithelial defect (defined as resolution of the epithelial defect ≤0.5 mm followed by new epithelial defect ≥1 mm) between P. aeruginosa and all other bacteria was performed using the Fisher's exact test. Within the P. aeruginosa subgroup, time to reepithelialization analyses comparing corticosteroid and placebo arms were conducted using Cox proportional hazards regression. The incidence of adverse events in each of the treatment arms of the P. aeruginosa subgroup was compared using the Fisher's exact test. Multivariate regression models were used to test for differing effects of corticosteroids on outcomes of BSCVA and infiltrate/scar size at 3 months by subgroups of baseline visual acuity, infiltrate size, ulcer depth, and location. All analyses were performed using statistical analysis software (Stata 10; StatCorp, College Station, TX). Negative logMAR values indicate improvement in visual acuity, and negative infiltrate/scar size values indicate decreases in infiltrate/scar size. P values reported are nominal values and have not been adjusted for multiple comparisons.
Results
Among the 500 patients enrolled in SCUT, the most common organisms isolated were Streptococcus pneumoniae, P. aeruginosa, and Nocardia spp. Six patients were excluded from analysis because they had mixed bacterial infections; one of those was a mixed P. aeruginosa and S. pneumoniae infection. This left 494 patients for analysis comparing P. aeruginosa (110 patients) with all other bacteria (384 patients) (Table 1). Of 110 P. aeruginosa patients, 105 were from India and 5 were from the United States. Among patients with P. aeruginosa ulcers, 10% were lost to follow-up at 3 months in both the corticosteroid (53/59) and the placebo (46/51) treatment arms (P > 0.99). Within the P. aeruginosa subgroup, the median enrollment BSCVA in the corticosteroid group was 1.50 logMAR (approximate Snellen equivalent 20/640); the median enrollment BSCVA in the placebo group was 1.12 logMAR (Snellen equivalent 20/250, P = 0.10). There were no significant differences in baseline demographic features and clinical examination findings between the two treatment arms of the P. aeruginosa subgroup (Table 2).
Table 1.
Baseline Characteristics of Patients in SCUT: Comparison between P. aeruginosa and All Other Bacterial Ulcers
| P. aeruginosa (n = 110) | All Other Bacteria* (n = 384) | P | |
|---|---|---|---|
| Age in years, median (IQR) | 43 (30–54) | 55 (42.5–63) | <0.001† |
| Sex, n (%) | 0.051‡ | ||
| Male | 69 (63) | 199 (52) | |
| Female | 41 (37) | 185 (48) | |
| Duration of symptoms, n (%) | 0.008‡ | ||
| <4 days | 55 (50) | 144 (38) | |
| 4–7 days | 42 (38) | 151 (39) | |
| 8–14 days | 10 (9) | 44 (11) | |
| >14 days | 3 (3) | 45 (12) | |
| Contact lens use, n (%) | 7 (6) | 1 (0.2) | <0.001‡ |
| Systemic disease,§n (%) | 6 (5) | 21 (5) | >0.99‡ |
| Ulcer location, n (%) | 0.007‡ | ||
| Entirely in periphery | 9 (8) | 56 (15) | |
| Partially covering 4 mm | 65 (59) | 253 (66) | |
| Completely fills 4 mm | 36 (33) | 73 (19) | |
| Visual acuity, logMAR, median (IQR) | 1.26 (0.46–1.7) | 0.74 (0.35–1.6) | 0.002† |
| Infiltrate/scar size in mm, median (IQR) | 3.8 (2.5–5.6) | 2.5 (1.8–3.7) | <0.001† |
| Treatment arm, n (%) | 0.45‡ | ||
| Corticosteroid | 59 (54) | 188 (49) | |
| Placebo | 51 (46) | 196 (51) |
Other bacteria include Streptococcus pneumoniae (n = 250), Nocardia spp. (n = 56), coagulase-negative Staphylococcus (n = 22), Staphylococcus aureus (n = 16), Moraxella spp. (n = 15), Viridans group Streptococcus (n = 11), Corynebacterium spp. (n = 6), Bacillus spp. (n = 1), Mycobacteria spp. (n = 1), Klebsiella spp. (n = 3), Enterobacter spp. (n = 2), Haemophilus influenzae (n = 1), other Pseudomonas spp. (non-Aeruginosa, n = 3), other Gram-positive (n = 3), and Gram-negative bacteria (n =5).
Rank sum test.
Fisher's exact test.
Systemic diseases included diabetes, asthma, Hansen's disease, eczema, psoriasis, HIV, ichthyosis, and malnutrition.
Table 2.
Baseline Characteristics of Patients from SCUT with P. aeruginosa Corneal Ulcers: Comparison between Corticosteroid and Placebo Treatment Arms
| Placebo (n = 51) | Corticosteroid (n = 59) | P | |
|---|---|---|---|
| Age in years, median (IQR) | 41 (30–50) | 45 (30–56) | 0.39* |
| Sex, n (%) | |||
| Male | 34 (67) | 35 (59) | 0.44† |
| Female | 17 (33) | 24 (41) | |
| Duration of symptoms, n (%) | |||
| <4 days | 27 (53) | 28 (47) | 0.71† |
| 4–7 days | 20 (39) | 22 (37) | |
| 8–14 days | 3 (6) | 7 (12) | |
| >14 days | 1 (2) | 2 (3) | |
| Contact lens use, n (%) | 4 (8) | 4 (7) | >0.99† |
| Systemic disease, n (%)‡ | 4 (8) | 2 (3) | 0.41† |
| Ulcer location, n (%) | |||
| Entirely in periphery | 4 (8) | 5 (8) | 0.15† |
| Partially covering 4 mm | 35 (69) | 30 (51) | |
| Completely fills 4 mm | 12 (24) | 24 (41) | |
| Visual acuity, logMAR, median (IQR) | 1.12 (0.46–1.7) | 1.50 (0.46–1.8) | 0.10* |
| Infiltrate/scar size in mm, median (IQR) | 3.75 (2.4–5.5) | 3.75 (2.7–5.5) | 0.29* |
Rank sum test.
Fisher's exact test.
Systemic diseases included diabetes, asthma, Hansen's disease, eczema, psoriasis, HIV, ichthyosis, and malnutrition.
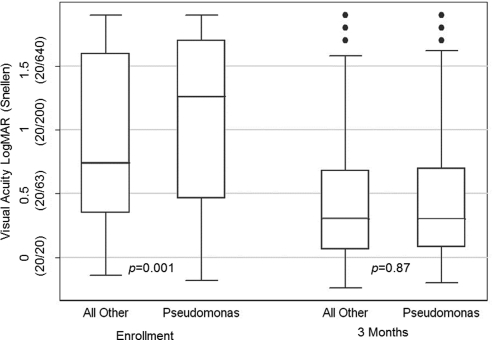
Patients with P. aeruginosa ulcers presented with significantly worse visual acuities than did patients with other bacterial ulcers (0.24 logMAR [∼2.5 lines difference]; 95% confidence interval [CI], 0.10–0.38; P = 0.001; Fig. 1). However, multiple linear regression analysis adjusting for enrollment characteristics showed P. aeruginosa ulcers to show significantly greater improvement in visual acuity at 3 months than other bacterial ulcers of similar presentation severity (−0.14 logMAR [∼1.5 lines]; 95% CI, −0.23 to −0.04; P = 0.004; Table 3). As a result, there was no significant difference in 3-month BSCVA between P. aeruginosa ulcers and all other bacterial ulcers (0.01 logMAR [∼0.1 line difference]; 95% CI, −0.12 to 0.14; P = 0.87; Fig. 1). Sensitivity analyses supported this finding and demonstrated that infiltrate/scar size, depth, and location were not confounders. Sensitivity analysis also demonstrated that study site, examiner, inclusion of mixed infections, and nonlinear distribution of baseline visual acuity did not affect outcomes. Multiple linear regression including enrollment characteristics did not find a significant difference in the geometric mean of infiltrate/scar size between P. aeruginosa ulcers and other bacterial ulcers at 3 months (−0.07 mm; 95% CI, −0.23 to 0.09; P = 0.40; Table 3). There was no significant difference in rates of adverse events between P. aeruginosa and all other bacteria (regardless of treatment arm), including corneal perforation (P = 0.54) and recurrence of epithelial defect (P = 0.69; Table 4).
Figure 1.
Visual acuity comparison of P. aeruginosa and all other bacterial ulcers at enrollment and 3 months. The line in the middle of each box represents the median, and the lower and upper bounds of the box represents the 25th and 75th percentiles, the span of which is the interquartile range (IQR). Whiskers extend to the lowest data point within 1.5× IQR below the 25th percentile and to the highest data point within 1.5× IQR above the 75th percentile. Individually plotted points represent outliers or values falling outside the whiskers. P values were obtained by rank sum test.
Table 3.
Comparison of Visual Acuity and Infiltrate/Scar Size Outcomes between P. aeruginosa and All Other Bacterial Ulcers
| Covariate | Coefficient (95% CI) | P |
|---|---|---|
| Linear regression predicting 3-month logMAR BSCVA (n = 438) | ||
| Enrollment BSCVA | 0.64 (0.58 to 0.70) | <0.001 |
| Corticosteroid treatment | −0.01 (−0.09 to 0.07) | 0.79 |
| Bacterial type (P. aeruginosa vs. other) | −0.14 (−0.23 to −0.04) | 0.004 |
| Constant | −0.07 (−0.15 to 0.01) | 0.07 |
| Linear regression predicting 3-month infiltrate/scar size in mm (n = 436) | ||
| Enrollment infiltrate/scar size | 0.92 (0.88 to 0.96) | <0.001 |
| Corticosteroid treatment | 0.05 (−0.07 to 0.17) | 0.41 |
| Bacterial type (P. aeruginosa vs. other) | −0.07 (−0.23 to 0.09) | 0.40 |
| Constant | 0.17 (0.03 to 0.31) | 0.02 |
Table 4.
Comparison of Adverse Events in P. aeruginosa Ulcers versus All Other Bacterial Ulcers
| P. aeruginosa (n = 110)*n (%) | All Other Bacteria (n = 384)*n (%) | Total n (%) | P† | |
|---|---|---|---|---|
| Change in antibiotic/addition of other antibiotic | 15 (14) | 53 (14) | 68 (14) | >0.99 |
| Corneal perforation | 2 (2) | 13 (3) | 15 (3) | 0.54 |
| Death | 2 (2) | 10 (3) | 12 (2) | >0.99 |
| IOP elevated >25 but ≤35 mm Hg with medications within 1 week of therapy‡ | 4 (4) | 8 (2) | 12 (2) | 0.31 |
| Increase in infiltrate size (>50%) and >1 mm in maximum diameter | 1 (1) | 12 (3) | 13 (3) | 0.32 |
| No resolution of epithelial defect at 21 days or later | 19 (17) | 52 (14) | 71 (14) | 0.36 |
| Progressive corneal thinning ≥50% of enrollment thickness | 1 (1) | 1 (0.3) | 2 (0.4) | 0.40 |
| Recurrence of epithelial defect | 1 (1) | 7 (2) | 8 (2) | 0.69 |
| Total subjects with any adverse events | 27 (25) | 76 (20) | 103 (21) | 0.29 |
Adverse events were recorded from enrollment until the 3-month follow-up. These values (n) reflect the number of cases at enrollment.
Fisher's exact test.
IOP elevation >35 was considered a serious adverse event. There were no cases of IOP elevation >35 in SCUT.
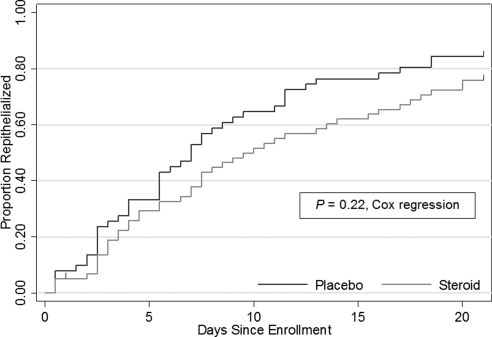
Corticosteroid effects on visual outcomes were compared between patients with and without P. aeruginosa infection, with a −0.04 logMAR (95% CI, −0.21 to 0.13) change at 3 months for those with P. aeruginosa ulcers and a −0.003 logMAR (95% CI, −0.09 to 0.08) change at 3 months for those with all other bacterial ulcers. The difference in response to corticosteroid treatment between P. aeruginosa and all other bacterial ulcers was not significant (−0.04 logMAR [∼0.5 line]; 95% CI, −0.22 to 0.15; P = 0.69). For infiltrate/scar size at 3 months, corticosteroids contributed to a −0.11 mm (95% CI, −0.38 to 0.15) change in P. aeruginosa ulcers and a 0.10 mm (95% CI, −0.04 to 0.26) change in all other bacterial ulcers; the difference in response to corticosteroid treatment between P. aeruginosa and all other bacterial ulcers was not significant (−0.21 mm; 95% CI, −0.51 to 0.09; P = 0.17). Within the P. aeruginosa subgroup, there were more changes or additions of antibiotics in the placebo group than in the corticosteroid group (Table 5). There was no evidence of any other difference in incidence of adverse events over a 3-month follow-up period between the corticosteroid and the placebo groups of the P. aeruginosa subgroup, including corneal perforations, increased IOP, recurrence of epithelial defect, and delay in epithelial defect resolution (Table 5). The median time to reepithelialization was 9.5 days (interquartile range [IQR]: 4, 20) in the corticosteroid group and 7.0 days (IQR: 3, 13) in the placebo group. Kaplan-Meier curves showed that the corticosteroid group had slower reepithelialization than the placebo group (Fig. 2), but the difference in time to reepithelialization was not statistically significant (P = 0.22). Prespecified subgroup analyses based on enrollment characteristics (including enrollment visual acuity, infiltrate/scar size, ulcer location, and depth of defect) did not find any significant effect of corticosteroid treatment on primary or secondary outcomes.
Table 5.
Comparison of Adverse Events in Placebo versus Corticosteroid Treatment Arms within the P. aeruginosa Subgroup
| Placebo (n = 51)*n (%) | Corticosteroid (n = 59)*n (%) | P† | |
|---|---|---|---|
| Change in antibiotic/addition of other antibiotic‡ | 11 (22) | 4 (7) | 0.03 |
| Corneal perforation | 1 (2) | 1 (2) | >0.99 |
| Death | 1 (2) | 1 (2) | >0.99 |
| IOP elevated >25 but ≤35 mm Hg with medications within 1 week of therapy§ | 3 (6) | 1 (2) | 0.34 |
| Increase in infiltrate size (>50%) and >1 mm in maximum diameter | 1 (2) | 0 (0) | 0.46 |
| No resolution of epithelial defect at 21 days or later | 6 (12) | 13 (22) | 0.21 |
| Progressive corneal thinning ≥50% of enrollment thickness | 1 (2) | 0 (0) | 0.46 |
| Recurrence of epithelial defect | 0 (0) | 1 (2) | >0.99 |
| Total subjects with any adverse events | 12 (24) | 15 (25) | >0.99 |
Adverse events were recorded from enrollment until the 3-month follow-up. These values (n) reflect the number of cases at enrollment.
Fisher's exact test.
Other antibiotics included gentamicin, ceftazidime, amikacin, doxycycline, tobramycin, and ciprofloxacin.
IOP elevation >35 was considered a serious adverse event. There were no cases of IOP elevation >35 in SCUT.
Figure 2.
Kaplan-Meier curves comparing time to reepithelialization in corticosteroid versus placebo treatment arms within the P. aeruginosa subgroup (n = 110). Curves are right-censored at 21 days; tick marks represent censoring. One patient in the corticosteroid arm was censored at day 1; 8 patients in the placebo arm and 14 patients in the corticosteroid arm were censored at day 21.
Discussion
P. aeruginosa ulcers are often thought to respond poorly to treatment and have worse clinical outcomes than other bacterial ulcers.1,9,10 However, in this subanalysis of a large clinical trial, we found that, when compared with other bacterial ulcers of the same presentation severity, P. aeruginosa corneal ulcers responded significantly better to treatment, with approximately 1.5 lines greater improvement in visual acuity from presentation to 3-month follow-up that was not attributable to ulcer enrollment characteristics (BSCVA, infiltrate/scar size, depth, and location). In fact, even though they have significantly worse clinical presentations, P. aeruginosa ulcers do not have significantly worse overall visual outcomes at 3 months when compared with all other bacterial ulcers. There was also no evidence of a significant difference in adverse events between P. aeruginosa ulcers and other bacterial ulcers, including perforations. These findings suggest that P. aeruginosa ulcers may not be as difficult to treat or may not result in worse clinical outcomes, as had been previously thought.
The use of adjunctive corticosteroid therapy is particularly controversial for P. aeruginosa corneal ulcers. Corticosteroids have been shown to reduce the host inflammatory response, which may be damaging to the cornea.14,20,21 However, corticosteroids may also significantly slow the process of corneal wound healing, prolong infection, and predispose to stromal thinning and perforation, especially in P. aeruginosa infections, which are known to be more invasive and to cause rapid systemic tissue destruction.1,9,10,13,22 Patients receiving corticosteroid treatment before presentation for corneal infection have been reported to be at triple the risk for complications, including treatment failure.22 Additionally, animal studies have found corticosteroid treatment in the absence of antibiotic treatment to be associated with increased bacterial growth and recurrence of P. aeruginosa keratitis in rabbit corneas.11,12 However, other animal studies have demonstrated that corticosteroid treatment given concurrently with appropriate antibiotic therapy reduces inflammation but does not interfere with the bactericidal action of antibiotics and does not lead to increased bacterial growth.23,24
The results of this study indicate there is no significant difference, either benefit or harm, in 3-month BSCVA or infiltrate/scar size among P. aeruginosa ulcers treated with adjunctive corticosteroids compared with placebo. Although multivariate analysis suggested a slight benefit with corticosteroid treatment over placebo for both 3-month BSCVA and infiltrate/scar size, these findings were not statistically significant. The large number of P. aeruginosa ulcers from SCUT allowed for the detection of small changes in visual acuity: a priori power calculations estimated >80% power to detect approximately a 2-line difference in visual acuity; post hoc, the 95% CI of the interaction term was approximately ±2 lines of visual acuity, suggesting that a large true effect would not be missed. Subgroup analysis did not find any differential effect for corticosteroid efficacy dependent on baseline clinical characteristics.
This study also found no evidence of an increase in adverse events with corticosteroid treatment. Corneal perforation, increased intraocular pressure, and delayed corneal healing are recognized potential adverse events associated with corticosteroid therapy, particularly in P. aeruginosa infections.1,9–12,22 One previous prospective study was discontinued after a patient with a P. aeruginosa ulcer worsened following the addition of corticosteroid treatment and required a penetrating keratoplasty for a large central scar.13 However, we found no difference in the number of perforations between corticosteroid and placebo-treated P. aeruginosa ulcers with one patient in each treatment arm. Although corticosteroids may increase IOP, patients with P. aeruginosa ulcers receiving corticosteroid treatment in SCUT were not more likely to have elevated IOP than those receiving placebo; in fact, there were more patients with elevated IOP in the placebo arm than in the corticosteroid arm.
Delayed corneal wound healing is also a recognized potential adverse effect of corticosteroid therapy.11,12,22 The pilot study for SCUT found adjunctive corticosteroid treatment to be associated with a significant delay in the reepithelialization of corneal ulcers secondary to all bacterial organisms.17 However, though there were more reported cases of epithelial defects at 21 days and beyond in the corticosteroid arm, time to reepithelialization was not significantly different between the two treatment arms in SCUT.18 In this substudy, within the P. aeruginosa subgroup, there were more patients who had an epithelial defect at 21 days or beyond in the corticosteroid arm than in the placebo arm, but this difference was not statistically significant. Cox regression analysis also demonstrated that time to reepithelialization was not significantly different between the two study arms. Recurrence of epithelial defect can be another measure of corticosteroid effect on corneal healing. Only one patient with a P. aeruginosa ulcer experienced a recurrence of epithelial defect. Although this patient was treated with corticosteroids, there was no significant difference between the two treatment arms. Therefore, our present study did not find a significant delay in the measures of healing time and recurrence of epithelial defect for P. aeruginosa ulcers treated with corticosteroids.
This study has a few potential limitations. Because most patients were enrolled at the Aravind Eye Care System in India, it is possible that differences in risk factors, bacteriology, and response to treatment in this population may make these results not generalizable to other populations. P. aeruginosa infections are most common among contact lens wearers in the United States.1 In SCUT, although most contact lens wearers had P. aeruginosa infection, a low proportion of the total P. aeruginosa cases occurred in contact lens wearers because contact lens use is less common in India.2,19 This difference in associated risk factors may reflect genotypic variation in P. aeruginosa strains between the two countries. Variations of pathogenicity between P. aeruginosa strains could contribute to differing outcomes and responses to adjunctive corticosteroid therapy. Sensitivity analysis demonstrated no effect of study site on outcome; nevertheless, results may be biased toward P. aeruginosa strains found in India. The SCUT criteria for a positive bacterial culture, defined as growth of the organism on one solid medium at the site of inoculation (with more stringent criteria for coagulase-negative Staphylococcus and diphtheroids), may differ from microbiologic criteria in other studies. The choice of treatment regimen in SCUT, both antibiotic and corticosteroid, might also have affected outcomes because choice of agent, number of agents used, concentration, and administration timing and route are all factors in treatment efficacy. Because of potential safety concerns, a more aggressive corticosteroid regimen was not chosen. The treating physician did have the option of adding or changing antibiotics during the trial if deemed necessary. Within the P. aeruginosa subgroup, the number of antibiotic changes was greater in the placebo treatment arm than in the corticosteroid treatment arm.
Although P. aeruginosa ulcers in SCUT had a more severe presentation than the other bacterial ulcers, this subanalysis suggests that P. aeruginosa ulcers respond better to treatment than previously thought. There appears to be no definite benefit for visual outcome and infiltrate/scar size with corticosteroid treatment in P. aeruginosa ulcers. However, the lack of any significant difference in adverse events with corticosteroid therapy suggests that adjunctive corticosteroid therapy may be used according to clinical judgment that takes into account the specific features of an individual case.
Acknowledgments
The authors thank the patients who enrolled in the Steroids for Corneal Ulcers Trial (SCUT), their families, and the research staffs at all the trial sites; and Marian Fisher (chair), Anthony Aldave, Donald Everett, Jacqueline Glover, K. Ananda Kannan, Steven Kymes, Gudlavalleti V. S. Murthy, and Ivan Schwab of the SCUT data and safety monitoring board for their invaluable guidance and advice.
Footnotes
Supported by National Eye Institute Grants U10 EY015114 (SCUT) and K23EY017897 (NRA) and Core Grant EY02162; a Research to Prevent Blindness Award (NRA); the Proctor Foundation (AS); a Dean's Research Fellowship from the UCSF School of Medicine (AS); a Pathways to Careers in Clinical and Translational Research Fellowship (NIH/NCRR/OD UCSF-CTSI Grant Number TL1 RR024129) (AS); an unrestricted grant from Research to Prevent Blindness; and That Man May See.
Disclosure: A. Sy, None; M. Srinivasan, None; J. Mascarenhas, None; P. Lalitha, None; R. Rajaraman, None; M. Ravindran, None; C.E. Oldenburg, None; K.J. Ray, None; D. Glidden, None; M.E. Zegans, None; S.D. McLeod, None; T.M. Lietman, None; N.R. Acharya, None
References
- 1. American Academy of Ophthalmology Cornea/External Disease Panel Bacterial Keratitis: Preferred Practice Pattern. American Academy of Ophthalmology. 2000:1–22 [Google Scholar]
- 2. Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–69 [DOI] [PubMed] [Google Scholar]
- 3. Bharathi MJ, Ramakrishnan R, Shivakumar C, Meenakshi R, Lionalraj D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in South India. Indian J Ophthalmol. 2010;58:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharathi MJ, Ramakrishnan R, Vasu S, Palaniappan R. Aetiological diagnosis of microbial keratitis in South India—a study of 1618 cases. Indian J Med Microbiol. 2002;20:19–24 [PubMed] [Google Scholar]
- 5. Forster RK. Conrad Berens Lecture: the management of infectious keratitis as we approach the 21st century. CLAO J. 1998;24:175–180 [PubMed] [Google Scholar]
- 6. Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23:360–364 [DOI] [PubMed] [Google Scholar]
- 8. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27 [DOI] [PubMed] [Google Scholar]
- 9. Krachmer JH, Mannis MJ, Holland EJ. Cornea. 1st ed St. Louis: Mosby; 1997:1139–1189 [Google Scholar]
- 10. Pepose JS, Holland GN, Wilhelmus KR. Ocular Infection and Immunity. 1st ed St. Louis: Mosby; 1996:970–1031 [Google Scholar]
- 11. Badenoch PR, Hay GJ, McDonald PJ, Coster DJ. A rat model of bacterial keratitis: effect of antibiotics and corticosteroid. Arch Ophthalmol. 1985;103:718–722 [DOI] [PubMed] [Google Scholar]
- 12. Gritz DC, Kwitko S, Trousdale MD, Gonzalez VH, McDonnell PJ. Recurrence of microbial keratitis concomitant with antiinflammatory treatment in an animal model. Cornea. 1992;11:404–408 [DOI] [PubMed] [Google Scholar]
- 13. Cohen EJ. The case against the use of steroids in the treatment of bacterial keratitis. Arch Ophthalmol. 2009;127:103–104 [DOI] [PubMed] [Google Scholar]
- 14. Hindman HB, Patel SB, Jun AS. Rationale for adjunctive topical corticosteroids in bacterial keratitis. Arch Ophthalmol. 2009;127:97–102 [DOI] [PubMed] [Google Scholar]
- 15. Blair J, Hodge W, Al-Ghamdi S, et al. Comparison of antibiotic-only and antibiotic-steroid combination treatment in corneal ulcer patients: double-blinded randomized clinical trial. Can J Ophthalmol. 2011;46:40–45 [DOI] [PubMed] [Google Scholar]
- 16. Carmichael TR, Gelfand Y, Welsh NH. Topical steroids in the treatment of central and paracentral corneal ulcers. Br J Ophthalmol. 1990;74:528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srinivasan M, Lalitha P, Mahalakshmi R, et al. Corticosteroids for bacterial corneal ulcers. Br J Ophthalmol. 2009;93:198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: The Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2011. October 10 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The Steroids for Corneal Ulcers Trial: study design and baseline characteristics. Arch Ophthalmol. 2011. October 10 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leibowitz HM, Kupferman A. Anti-inflammatory effectiveness in the cornea of topically administered prednisolone. Invest Ophthalmol. 1974;13:757–763 [PubMed] [Google Scholar]
- 21. Kupferman A, Leibowitz HM. Anti-inflammatory effectiveness of topically administered corticosteroids in the cornea without epithelium. Invest Ophthalmol. 1975;14:252–255 [PubMed] [Google Scholar]
- 22. Wilhelmus KR. Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology. 2002;109:835–842, quiz 843 [DOI] [PubMed] [Google Scholar]
- 23. Hobden JA, Engel LS, Hill JM, Callegan MC, O'Callaghan RJ. Prednisolone acetate or prednisolone phosphate concurrently administered with ciprofloxacin for the therapy of experimental Pseudomonas aeruginosa keratitis. Curr Eye Res. 1993;12:469–473 [DOI] [PubMed] [Google Scholar]
- 24. Engel LS, Callegan MC, Hobden JA, Reidy JJ, Hill JM, O'Callaghan RJ. Effectiveness of specific antibiotic/steroid combinations for therapy of experimental Pseudomonas aeruginosa keratitis. Curr Eye Res. 1995;14:229–234 [DOI] [PubMed] [Google Scholar]