ZEB1-mediated alterations in COL4A3 expression are most likely associated with the pathogenesis of posterior polymorphous corneal dystrophy type 3 (PPCD3), although the demonstration of COL4A3 expression in healthy adult human corneal endothelial cells (HCEnCs) indicates that PPCD3 is not caused simply by the ectopic expression of COL4A3.
Abstract
Purpose.
To determine how nonsense mutations in the transcription factor ZEB1 lead to the development of posterior polymorphous corneal dystrophy type 3 (PPCD3).
Methods.
Whole-cell extracts were obtained from cultured human corneal epithelial cells (HCEpCs) as a source of ZEB1 protein. DNA-binding assays were performed using the whole-cell extract and oligonucleotide probes consisting of the two conserved E2-box motifs and surrounding nucleotides upstream of COL4A3. ZEB1 and COL4A3 mRNA expression in primary human corneal endothelial cells (HCEnCs) was assayed in both PPCD3 and control corneas by RT-PCR. Immunohistochemistry was used to localize ZEB1 and COL4A3 expression in normal human cornea.
Results.
Electromobility shift assays (EMSAs) and competition EMSAs demonstrated binding of protein(s) in the cultured HCEpCs to the E2-box motifs in the probes. The supershift EMSA confirmed that ZEB1, demonstrated to be present in the whole-cell extracts, binds to both the proximal and distal E2-box motifs in the COL4A3 promoter region. Both COL4A3 and ZEB1 are expressed in normal HCEnCs, although in PPCD3, ZEB1 expression is decreased and COL4A3 expression is increased compared with levels of both genes in healthy control corneas.
Conclusions.
Inversely related HCEnC expression levels of ZEB1 and COL4A3 in PPCD3 indicate that ZEB1-mediated alterations in COL4A3 expression are most likely associated with the pathogenesis of this corneal endothelial dystrophy. However, the demonstration of COL4A3 expression in healthy adult primary HCEnCs suggests that PPCD3 is more likely to involve an alteration in the timing and/or degree of COL4A3 expression than to result from the dichotomous change implied by the previously proposed ectopic expression model.
Posterior polymorphous corneal dystrophy (PPCD: MIM 122000; Mendelian Inheritance in Man; National Center for Biotechnology Information, Bethesda, MD) is a dominantly inherited corneal endothelial dystrophy that is associated with a varied phenotype, ranging from asymptomatic corneal endothelial changes to visually disabling corneal edema and glaucoma. Locus heterogeneity has been demonstrated for PPCD, with two dozen nonsense mutations identified in the zinc finger E-box binding homeobox 1 gene (ZEB1: MIM 189909) on chromosome 10 (the PPCD3 locus) and linkage to a locus on chromosome 20 (the PPCD1 locus) by our group and others.1–8 As coding region mutations in ZEB1, which is also known as transcription factor 8 (TCF8), AREB6, BZP, NIL2A, ZFHEP, ZFHX1A, and DELTAEF1, have been identified in 24 of the 73 PPCD probands screened to date, approximately one-third of PPCD is associated with mutations in ZEB1. Each identified ZEB1 mutation is a truncating mutation that is thought to result in haploinsufficiency, leading to the ectopic expression of collagen, type IV, alpha 3 (COL4A3; MIM 120070) in the corneal endothelium.2 COL4A3 is not typically expressed in the adult human corneal endothelium, although published studies are inconsistent as to whether it is expressed to any significant degree in the wild-type mouse corneal endothelium.2,9,10 However, in the presence of a ZEB1 truncating mutation, ectopic COL4A3 expression has been demonstrated in the corneal endothelium of an individual affected with PPCD3.2 Likewise, Col4a3 mRNA expression is significantly increased in the corneal endothelium of Zeb1-null mice and in embryonic fibroblasts derived from Zeb1-heterozygous and -null mice.10 Given these findings, as well as the presence of two conserved E2-boxes (to which ZEB1 is predicted to bind) upstream of the transcription initiation site of COL4A3, it appears that ZEB1 participates in the negative regulation of COL4A3 transcription. Therefore, loss of ZEB1 function is thought to lead to the corneal endothelial expression of COL4A3, resulting in the abnormal endothelial proliferation, corneal thickening, and iridocorneal adhesions observed in individuals affected with PPCD3 and in Zeb1-heterozygous and -null mice.10
We sought to elucidate the mechanisms through which ZEB1 mutations lead to PPCD3 through studying the effects of ZEB1 mutations on the binding of ZEB1 to the E2-box domains in the COL4A3 promoter region. We are the first to demonstrate the expression of both ZEB1 and COL4A3 in the normal human corneal endothelium and altered expression of both in the corneal endothelium in PPCD3. In addition, we provide initial evidence that ZEB1 interacts with both the proximal and distal E2-box motifs in the COL4A3 promoter region, leading to the conclusion that truncating mutations in ZEB1 result in alteration in E2-box binding affinity and loss of transcriptional repression of COL4A3.
Materials and Methods
Study approval was obtained from the Institutional Review Board at the University of California, Los Angeles (UCLA IRB 10-001932 and 11-000020).
Cellular Localization of ZEB1 and COL4A3 in the Normal Cornea by Immunohistochemistry
Donor corneal tissue obtained from commercial eye banks was embedded in OCT compound (Tissue-Tek; Sakura, Torrance, CA) and 5-μm frozen sections were cut onto slides (Superfrost Plus; Fisher Scientific, Tustin, CA). The sections were fixed with cold 4% paraformaldehyde solution in phosphate-buffered saline (PBS; Affymetrix Inc., Cleveland, OH) for 10 minutes. The fixative was removed, and the sections were briefly rinsed with PBS+0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO), followed by a rinse with PBS. The sections were then blocked with a solution of PBS containing 1% bovine serum albumin (BSA; U.S. Biological, Swampscott, MA), 20% serum from the species of the secondary antibody being used, and 0.05% sodium azide (Sigma-Aldrich). Primary antibodies to ZEB1 raised in rabbit (ab87280; Abcam, Inc., Cambridge, MA) or COL4A3 raised in goat (sc-10572; Santa Cruz Biotechnology, Santa Cruz, CA) were diluted in blocking solution, applied to the sections after removal of the primary blocking solution, and incubated overnight. Species-specific normal IgG (Jackson ImmunoResearch, West Grove, PA) was used as the negative control at the same concentration as the primary antibodies. The slides were then washed in PBS plus 1% BSA three times before the application of the secondary antibodies (donkey anti-rabbit Alexa Fluor 488 and donkey anti-goat Alexa Fluor 546; Life Technologies, Carlsbad, CA) and diluted in blocking solution for 2 hours in a dark box. The sections were washed again with PBS plus 1% BSA three times and incubated in PBS containing Hoechst dye (Pierce, Rockford, IL) at a concentration of 4 μg/mL for 15 minutes in a dark box. After three washes with PBS, the slides were mounted (Fluoromount; Sigma-Aldrich) and examined with a fluorescence microscope.
Relative Expression of COL4A3 and ZEB1
RNA and cDNA Preparation.
Five adult human corneas from three donors were obtained from commercial eye banks with the following characteristics: mean donor age, 55.3 years (range, 32–73); mean death to preservation time, 9.2 hours (range, 4.54–14.57); mean death to harvesting of Descemet's membrane and endothelium time, 4 days (range, 2–7); and mean central corneal endothelial cell density, 2810 cells/mm2 (range, 2092–3436). Descemet's membrane and the corneal endothelium were stripped from the donor corneas by using a published technique11 and placed into transcription reagent (TRIzol; Life Technologies). Corneal endothelium from a PPCD3 patient with a known ZEB1 mutation was obtained when the patient underwent Descemet's stripping endothelial keratoplasty (DSEK). The central 6 to 8 mm of Descemet's membrane and endothelium were removed during surgery, transferred to a piece of filter paper, and placed into the reagent (TRIzol; Life Technologies). All six samples were then disrupted by using a stainless steel probe (Omni International, Kennesaw, GA) and processed according to the manufacturer's instructions, to obtain total RNA. First-strand cDNA synthesis was performed using oligo (dT) primers and a reverse transcriptase kit (SuperScript III; Life Technologies), according to the manufacturer's instructions.
Quantitative PCR.
Semiquantitative PCR (qPCR) was performed using master mix (ABsolute Blue; ABgene, Thermo Fisher Scientific, Rockford, IL) to determine the expression of COL4A3 mRNA and ZEB1 mRNA in normal and PPCD3 corneas using GAPDH mRNA levels as the reference. Intron-spanning primers were designed to amplify mRNA, but not genomic DNA, using the mRNA sequence and the Primer3 web tool (http://frodo.wi.mit.edu/primer3; Massachusetts Institute of Technology, Boston, MA): GAPDH F: CTGGCCAAGGTCATCCATG and R: GCCATGCCAGTGAGCTTCC; ZEB1 F: GACAGTGTTACCAGGGAGGAGCA and R: TTCAGGTGCCTCAGGAAAAATGA; and COL4A3 F: CCCACAAGGTCCCAGTGGTC and R:GATCTCCAGGTGGACCCTTGC.
The qPCR reactions were performed on a thermocycler (LC 480 LightCycler; Roche Applied Science, Indianapolis, IN) using the following program: 95°C for 15 minutes and then 40 cycles at 95°C for 20 seconds, 62°C for 20 seconds, and 72°C for 30 seconds.
Gel Mobility-Shift Assays
Gel Electrophoresis and Immunoblot Analysis.
Using a kit (NE-PER; Pierce), we prepared whole-cell extracts from a human corneal epithelial cell line (CRL-11515; ATCC, Manassas, VA). Western blot analysis was performed to confirm the presence of ZEB1 protein in the whole-cell extracts. Briefly, 15 μg of total protein from the human corneal epithelial cell (HCEpC) extracts was denatured at 70°C in reducing agent (NuPAGE; Life Technologies) and sample buffer, loaded onto 4% to 12% polyacrylamide gradient gels (NuPage Bis-Tris gels; Life Technologies), and electrophoresed at 200 V for 45 minutes. The gels were then transferred to PVDF membranes at 100 V for 1 hour at 4°C. The membranes were probed (SNAP i.d. Protein Detection System; Millipore, Billerica, MA) with 0.125 μg/mL of rabbit anti-ZEB1 antibody (ab64098; Abcam) as the primary antibody, and goat anti-rabbit antibody conjugated to horse radish peroxidase secondary antibody at a dilution of 1:30000 (sc-2004; Santa Cruz Biotechnology, Inc.). All blocking and incubation steps were performed in blocking buffer (Superblock with 0.1% SufactAmps-20; Pierce). Chemiluminescence was used to detect bound antibody using an extended-duration substrate (Super Signal West Dura; Pierce) and autoradiograph film (CL-Xposure; Pierce).
Electromobility Shift Assay.
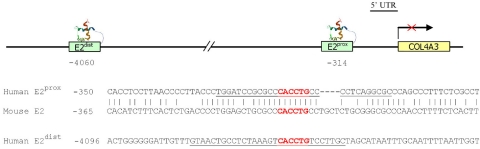
Each of the gel shift assays was performed according to previously published techniques.12 The EMSAs were performed using whole-cell extracts prepared from cultured HCEpCs as the source of ZEB1 protein. The probes consisted of 30-base 32P-labeled synthesized oligonucleotides (Sigma-Aldrich) containing the E2-box in the center with the same 12 bases on each side that flank both the proximal and distal E2-box motifs upstream of COL4A3 (Fig. 1). The E2-box motifs were identified by searching the 5000 bases upstream of the COL4A3 transcription initiation site using the DNA Pattern Tool.13 The oligonucleotide sequences used were (sense strand shown only): COL4A3 proximal probe: TGGATCCGCGCCCACCTGCCC-CTCAGGCGC; and COL4A3 distal probe: GTAACTGCCTCTAAAGTCA-CCTGTCCTTGCT. The COL4A3 distal probe identifies the E2-box predicted by Krafchak et al.2 Approximately 5 to 7.5 μg of protein from the whole-cell extract and approximately 50 femtomoles of the selected oligonucleotide (double-stranded) were used in a typical assay in the presence of 20 mM HEPES, 100 mM KCl, 0.1 mM DTT, 17% glycerol, 1.0 μg poly(dI-dC), and 0.6 μg bovine serum albumin (pH 7.9). The reactions were assembled on ice, incubated at 30°C for 15 minutes, and electrophoresed at 4°C on 8% acrylamide gels in a buffer containing 50 mM Tris, 40 mM glycine, and 1 mM EDTA (pH 7.9). Gels were run at 150 V for ∼150 minutes and dried, and complexes were detected by exposing the gels to x-ray film for 12 to 16 hours.
Figure 1.
The COL4A3 promoter region. The location of the proximal (E2prox) and distal (E2dist) E2-boxes in the human COL4A3 promoter region are shown, as is the sequence homology between the human E2prox box and the mouse E2-box. The sequence corresponding to the probe sequence is underscored.
Competition EMSAs with Unlabeled and Mutant Probes.
Competition EMSAs were performed to confirm that protein(s) in the cultured HCEpCs interact with the E2 binding motif in the COL4A3 promoter region. The previously described EMSA experiments were repeated with the addition of synthesized probes (Sigma-Aldrich) that were identical in sequence but were unlabeled and in 50- and 100-fold excess compared with the labeled probes. In addition, we repeated the competition EMSA using mutant probes with single-nucleotide alterations in the E2-box sequence of the COL4A3 proximal and distal probes that were in 100-fold excess compared with the labeled probes containing the conserved E2-box motif. The oligonucleotide sequences of the mutant probes were as follows (sense strand shown only; E2-box motif in italic; altered nucleotides indicated in lowercase): COL4A3 mutant proximal probe M5: GTAACTGCCTCTAAAGTCACtTGTCCTTGCT; COL4A3 mutant proximal probe M6: GTAACTGCCTCTAAAGTCAtCTGTCCTTGCT; COL4A3 mutant distal probe M5: TGGATCCGCGCCCACtTGCCCCTCAGGCGC; and COL4A3 mutant distal probe M6: TGGATCCGCGCCCAtCTGCCCCTCAG GCGC.
Supershift Assay
A supershift assay was performed to confirm that ZEB1 was the (one of the) protein(s) in the cultured HCEpCs that interacted with the proximal and distal E2-box motifs in the COL4A3 promoter region. A goat antibody to human, mouse, and rat ZEB1 that has been used by other investigators for supershift assays (E-20X; Santa Cruz Biotechnology) was selected.14 Heat shock factor 1 (HSF1) was used as a negative control for the supershift assay, with a rabbit antibody to human HSF1 (Sigma Genosys, St. Loius, MO). Supershift assays were performed as described above with the addition of 4 μg antibody to the whole-cell extracts for 60 minutes at room temperature before addition of the probe.
Results
HCEpCs Express ZEB1
Whole-cell extracts from HCEpCs demonstrated the presence of ZEB1 protein by Western blot analysis (Fig. 2).
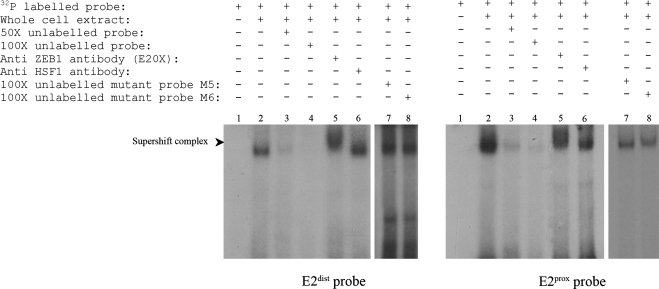
Figure 2.
ZEB1 protein from cultured human corneal epithelial cell (HCEpC) extracts binds to the proximal and distal E2-boxes in the COL4A3 promoter region. EMSA was performed using cultured HCEpC extracts as a source of ZEB1 protein and oligonucleotide probes containing both the proximal (E2prox) and distal (E2dist) E2-box motifs upstream of COL4A3. Lane 1: no complex was formed with the addition of 32P-labeled E2dist and E2prox probe only. Lane 2: protein(s) in the HCEpC extracts interacted with 32P-labeled E2dist and E2prox probes, forming complexes of the same location and similar intensity. Lanes 3 and 4: the addition of unlabeled E2prox and E2dist probes in (lane 3) 50- and (lane 4) 100-fold excess compared with the labeled probe resulted in the disappearance of the complex observed in lane 2. Lane 5: the addition of ZEB1 antibody E20X to the HCEpC extracts and 32P-labeled E2dist and E2prox probes resulted in replacement of the band seen in lane 2 by a slower migrating band. Lane 6: addition of an antibody to heat shock factor I (HSF1) to the HCEpC extracts and 32P-labeled E2dist and E2prox probes did not alter the migration of the complex seen in lane 2. Lanes 7 and 8: the addition of mutant probes M5 (lane 7) and M6 (lane 8) in 100-fold excess compared with the 32P-labeled E2dist and E2prox probes did not result in an alteration of the complex observed in lane 2.
Proteins in HCEpC Interact with the E2-Box Motifs in the COL4A3 Promoter Region
Electromobility Shift Assay.
EMSAs were performed on whole-cell extracts from cultured HCEpCs with radiolabeled oligonucleotide probes consisting of the conserved E2-box motif and surrounding nucleotides upstream of COL4A3. A single nucleic acid–protein complex that was of the same location, and similar intensity was formed with both the distal and proximal human E2-box-containing probes (Fig. 2).
Competition EMSA with Unlabeled and Mutant Probes.
The addition of an unlabeled probe in 50- and 100-fold excess compared to the labeled probe resulted in the disappearance of the complex that was observed with the addition of only the labeled probe to the cultured HCEpCs (both distal and proximal; Fig. 2). To confirm the specificity of binding of the protein(s) from the HCEpCs to the E2-box motif in both of the unlabeled probes, we repeated the competition EMSA using mutant probes with an alteration in the E2-box sequence. When each of these unlabeled mutant probes was added to the whole-cell extracts in 100-fold excess compared with the corresponding labeled probe containing the conserved E2-box motif, a complex of similar intensity and location to that seen with the addition of only the wild-type labeled probe was observed (Fig. 2).
ZEB1 Interacts with the E2-Box Motifs in the COL4A3 Promoter Region: Supershift Assay
The addition of the E20X antibody to the mixture containing whole-cell extracts from cultured HCEpCs and either the proximal or distal human E2-box-containing probes resulted in loss of the band seen in the EMSA experiment and the appearance of a slower migrating band (Fig. 2). This supershift was not seen with the addition of an antibody specific for HSF1, confirming that the ZEB1 protein is present and binds to both the proximal and distal E2-box motifs in the COL4A3 promoter region.
ZEB1 and COL4A3 in Normal Human Corneal Endothelial Cells
Immunohistochemical Detection.
To determine whether COL4A3 is expressed in the normal adult human corneal endothelium, we stained sections of eye bank corneas with a goat anti-COL4A3 antibody. Staining of the endothelial cell cytoplasm was observed, whereas no significant staining was seen using the negative control IgG (data not shown; Fig. 3). Staining eye bank cornea sections with a rabbit anti-ZEB1 antibody demonstrated staining of the endothelial cell nuclei, while no significant staining was observed using the negative control IgG (data not shown) (Fig. 3).
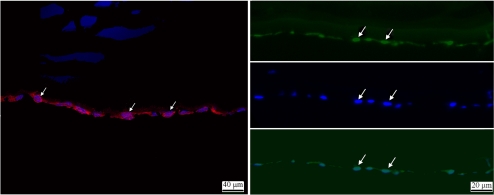
Figure 3.
ZEB1 and COL4A3 proteins are expressed in normal primary human corneal endothelial cells. Left: COL4A3 protein expression is seen as intense red fluorescence (Alexa Fluor 546) in the cytoplasm of the endothelial cells (arrows). The nuclei of the endothelial cells are stained blue with the Hoechst nuclear stain. Right: ZEB1 protein expression is noted as green fluorescence (Alexa Fluor 488) in the endothelial cell nuclei (arrows, top), the location of which are confirmed using the Hoechst nuclear stain (arrows, middle). Staining for both ZEB1 protein expression and the endothelial cell nuclei confirms the nuclear localization of ZEB1 protein expression (arrows, bottom).
RT-PCR Detection of ZEB1 and COL4A3 mRNA.
ZEB1 mRNA was demonstrated in normal adult human primary corneal endothelium obtained from eye bank corneas (Fig. 4). COL4A3 mRNA was also identified in the normal adult human primary corneal endothelium, although at a significantly lower level when compared with ZEB1 mRNA.
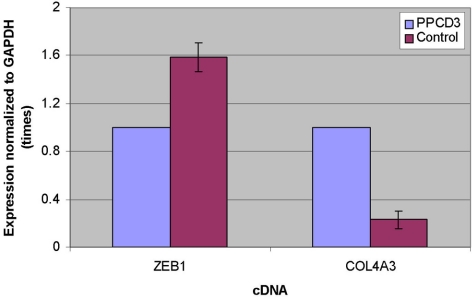
Figure 4.
PPCD3 is associated with altered expression of ZEB1 and COL4A3 mRNA. Expression of ZEB1 and COL4A3 mRNA in primary corneal endothelial cells is shown relative to mRNA expression of the housekeeping gene GAPDH. Corneal endothelial expression of ZEB1 mRNA was decreased and COL4A3 mRNA was increased in PPCD3 relative to expression levels in control corneas. Values shown for control represent average expression from five adult human corneas from three donors.
PPCD3 Is Associated with Decreased ZEB1 mRNA Expression and Increased COL4A3 mRNA Expression in the Corneal Endothelium: RT-PCR Detection
To determine whether a ZEB1 mutation associated with PPCD3 results in decreased ZEB1 mRNA expression in primary HCEnC, we repeated the RT-PCR protocol described above, this time amplifying ZEB1 cDNA derived from the corneal endothelium of a patient with PPCD3 obtained at the time of corneal endothelial transplantation. ZEB1 sequencing in this individual revealed a His230fsX6 mutation that was also identified in an affected sibling. Semiquantitative RT-PCR demonstrated significantly reduced ZEB1 mRNA expression in the HCEnCs compared with the expression level in healthy adult HCEnCs (Fig. 4). In contrast, the level of COL4A3 mRNA expression in the PPCD3 HCEnCs was found to be four- to fivefold higher than in the healthy adult HCEnC.
Discussion
The role of ZEB1 in modulating epithelial-to-mesenchymal transition (EMT) during development by downregulation of E-cadherin and other genes encoding cell adhesion and basement membrane proteins is well characterized.15,16 Although ZEB1-mediated cell differentiation plays an essential role during the development of a variety of tissues, such as the delamination of the neural crest from the neural tube,17 overexpression of ZEB1 results in uncontrolled EMT.18,19 ZEB1 overexpression leads to the transformation of epithelial cells into invasive and/or metastatic mesenchymal cells in a variety of pathologic conditions, including breast cancer and interstitial lung diseases.20–22 However, the role of ZEB1 in PPCD, in which proliferation of corneal endothelial cells demonstrating immunohistochemical and histopathologic features of epithelial cells is observed in affected individuals23,24 and ZEB1 mutant mice,10 consistent with a mesenchymal (neural crest of the surface ectoderm) to epithelial transition (MET) remains to be elucidated. It is possible that decreased ZEB1 expression leads to the MET observed in the corneal endothelium in PPCD, based on the observation that when ZEB1 expression is decreased by overt expression of the miR-200 family and miR-205, which are repressors of ZEB1 and its counterpart ZEB2, MET occurs.18
To clarify the role of ZEB1 in the pathogenesis of PPCD3, we tested the previous hypothesis that ZEB1 mutations lead to PPCD3 via the ectopic expression of COL4A3 in the human corneal endothelium.2 Our demonstration of COL4A3 expression in the normal adult human corneal endothelium challenges this hypothesis. In addition, the inversely related expression levels of ZEB1 and COL4A3 mRNA that we demonstrated in normal and PPCD3 human corneal endothelium indicates that it is the relative expression, not the simple presence or absence of COL4A3 expression, that differentiates normal from affected corneal endothelium. Support for this contention is provided by the identification in Descemet's membrane of COL4A3, which is produced by the corneal endothelial cells, in normal and keratoconic human corneas and in the mouse cornea.9,25–27 As this is the first report of the differential expression of COL4A3 mRNA between normal and PPCD3 corneal endothelium and we measured expression in only a single affected individual, corneal endothelial expression levels of COL4A3 mRNA must be determined in other individuals with PPCD3, to confirm that the COL4A3 expression level is approximately four to fivefold higher than in normal corneal endothelium. However, in this study, we have confirmed that the measurement of COL4A3 mRNA is reflective of the levels of COL4A3 protein in the corneal endothelium (posttranslational regulation does not result in endogenous protein degradation, as is presumed to occur for collagen I produced in rabbit corneal endothelial cells28) via the demonstration of the presence of endogenous COL4A3 protein by immunostaining.
Although altered levels and/or timing of COL4A3 expression in the corneal endothelium are likely to play a role in the pathogenesis of PPCD3, it is possible that alterations in expression of other collagen subtypes during development play a role as well. Even though we have demonstrated COL4A3 protein in the adult human cornea, we have not had the opportunity to demonstrate expression in the developing cornea, when the anomalies that cause PPCD are thought to occur.29 However, type IV collagen has been demonstrated in a subendothelial layer of the developing avian cornea, lending support to the hypothesis that COL4A3 is expressed in the developing human corneal endothelium and, to a lesser degree, in the adult human corneal endothelium.30 Other genetic factors that may play a role in the pathogenesis of PPCD include the genes that encode other collagens and basal membrane proteins identified in the posterior collagenous layer (PCL) that is found in corneas of individuals with PPCD and other endothelial disorders, including Fuchs endothelial corneal dystrophy (FECD; MIM 610158) and iridocorneal endothelial syndrome, which share many clinical and histopathologic features with PPCD.31–33 The identification of collagens I, III to VI, and VIII; laminin; tenascin; and fibronectin in the PCL,32,34 and recently reported alterations in the staining intensity and pattern of collagens IV and VIII in PPCD corneas when compared to normal corneas,26 indicate that each of these proteins may play a role in the pathogenesis of PPCD. In addition, it should be kept in mind that in the original publication identifying ZEB1 mutations as the cause of PPCD3, Krafchak et al.2 screened for ZEB1-binding sites only in the promoter regions of the α-type 4 collagen genes (COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, and COL4A6), which had been implicated in Alport syndrome (previously associated with PPCD), and COL8A2, which had been linked to both FECD and PPCD. E2-box domains were identified in the promoter region of each of these genes, except for COL4A4, although they evaluated the expression of only three (COL4A1, COL4A3, and COL4A5) in HCEnCs from an individual with PPCD3 and in a control individual. Therefore, further studies are needed to measure the differential level of expression in PPCD3 and healthy corneal endothelium of each of the collagen genes and basal membrane proteins that contain an E2-box domain in the promoter region and to investigate ZEB1-mediated regulation of expression of these genes to more fully understand the mechanisms through which ZEB1 mutations lead to PPCD3.26,31,32
The significance of the identification of the genetic basis of PPCD1 and characterization of ZEB1-mediated transcriptional control of COL4A3 in the pathogenesis of PPCD3 extends well beyond an increased understanding of the molecular mechanisms of PPCD. Based on clinical and histopathologic similarities between FECD and PPCD, vision scientists have long suspected a shared genetic basis for the two endothelial dystrophies. Last year, researchers reported that missense mutations in ZEB1 cause FECD, leading to the investigators' conclusion that PPCD and FECD are allelic variants, with the phenotype dependent on the interaction between the genes associated with these corneal dystrophies.35 Even more recently, two independent groups of investigators reported that genetic variation in the transcription factor 4 gene (TCF4; MIM 602272) located on chromosome 18 contributes to the development of FECD, possibly by altering the expression of ZEB1.36,37 In the United States, FECD affects as much as 5% of the population over 40 years of age and visually significant corneal edema secondary to FECD is the most common indication for corneal transplantation, accounting for 51% of endothelial keratoplasty procedures and 28% of all corneal transplant procedures performed in 2010.36,38 Therefore, the identification and characterization of the molecular pathways by which ZEB1 mutations result in corneal endothelial cell transformation and dysfunction will provide important insights into the genetic control of corneal clarity and targets for molecular therapeutic strategies that may benefit thousands of individuals in this country alone each year.
Acknowledgments
The authors thank Josh Z. Lee for assistance with the design and performance of the initial EMSA experiments.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2011.
Supported by an unrestricted grant from Research to Prevent Blindness and an Administrative Supplement to National Institutes of Health K08 EY016079 (AJA).
Disclosure: V.S. Yellore, None; S.A. Rayner, None; C.K. Nguyen, None; R.K. Gangalum, None; Z. Jing, None; S.P. Bhat, None; A.J. Aldave, None
References
- 1. Aldave AJ, Yellore VS, Yu F, et al. Posterior polymorphous corneal dystrophy is associated with TCF8 gene mutations and abdominal hernia. Am J Med Genet A. 2007;143A:2549–2556 [DOI] [PubMed] [Google Scholar]
- 2. Krafchak CM, Pawar H, Moroi SE, et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005;77:694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liskova P, Tuft SJ, Gwilliam R, et al. Novel mutations in the ZEB1 gene identified in Czech and British patients with posterior polymorphous corneal dystrophy. Hum Mutat. 2007;28:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen DQ, Hosseini M, Billingsley G, Heon E, Churchill AJ. Clinical phenotype of posterior polymorphous corneal dystrophy in a family with a novel ZEB1 mutation. Acta Ophthalmol. 2009 [DOI] [PubMed] [Google Scholar]
- 5. Gwilliam R, Liskova P, Filipec M, et al. Posterior polymorphous corneal dystrophy in Czech families maps to chromosome 20 and excludes the VSX1 gene. Invest Ophthalmol Vis Sci. 2005;46:4480–4484 [DOI] [PubMed] [Google Scholar]
- 6. Heon E, Mathers WD, Alward WL, et al. Linkage of posterior polymorphous corneal dystrophy to 20q11. Hum Mol Genet. 1995;4:485–488 [DOI] [PubMed] [Google Scholar]
- 7. Yellore VS, Papp JC, Sobel E, et al. Replication and refinement of linkage of posterior polymorphous corneal dystrophy to the posterior polymorphous corneal dystrophy 1 locus on chromosome 20. Genet Med. 2007;9:228–234 [DOI] [PubMed] [Google Scholar]
- 8. Vincent AL, Niederer RL, Richards A, Karolyi B, Patel DV, McGhee CN. Phenotypic characterisation and ZEB1 mutational analysis in posterior polymorphous corneal dystrophy in a New Zealand population. Mol Vis. 2009;15:2544–2553 [PMC free article] [PubMed] [Google Scholar]
- 9. Saito K, Yonezawa T, Minaguchi J, et al. Distribution of alpha(IV) collagen chains in the ocular anterior segments of adult mice. Connect Tissue Res. 2011;52:147–156 [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Peng X, Tan J, Darling DS, Kaplan HJ, Dean DC. Zeb1 mutant mice as a model of posterior corneal dystrophy. Invest Ophthalmol Vis Sci. 2008;49:1843–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price MO, Giebel AW, Fairchild KM, Price FW., Jr Descemet's membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368 [DOI] [PubMed] [Google Scholar]
- 12. Somasundaram T, Bhat SP. Canonical heat shock element in the alpha B-crystallin gene shows tissue-specific and developmentally controlled interactions with heat shock factor. J Biol Chem. 2000;275:17154–17159 [DOI] [PubMed] [Google Scholar]
- 13. Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques. 2000;28:1102, 1104 [DOI] [PubMed] [Google Scholar]
- 14. Manavella PA, Roqueiro G, Darling DS, Cabanillas AM. The ZFHX1A gene is differentially autoregulated by its isoforms. Biochem Biophys Res Commun. 2007;360:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aigner K, Dampier B, Descovich L, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385 [DOI] [PubMed] [Google Scholar]
- 17. Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–192 [DOI] [PubMed] [Google Scholar]
- 18. Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601 [DOI] [PubMed] [Google Scholar]
- 19. Paterson EL, Kolesnikoff N, Gregory PA, Bert AG, Khew-Goodall Y, Goodall GJ. The microRNA-200 family regulates epithelial to mesenchymal transition. ScientificWorldJournal. 2008;8:901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho JH, Gelinas R, Wang K, et al. Systems biology of interstitial lung diseases: integration of mRNA and microRNA expression changes. BMC Med Genomics. 2011;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 21. Singh M, Spoelstra NS, Jean A, et al. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol. 2008;21:912–923 [DOI] [PubMed] [Google Scholar]
- 22. Soini Y, Tuhkanen H, Sironen R, et al. Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer. 2011;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413–475 [PMC free article] [PubMed] [Google Scholar]
- 24. Rodrigues MM, Sun TT, Krachmer J, Newsome D. Epithelialization of the corneal endothelium in posterior polymorphous dystrophy. Invest Ophthalmol Vis Sci. 1980;19:832–835 [PubMed] [Google Scholar]
- 25. Kabosova A, Azar DT, Bannikov GA, et al. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci. 2007;48:4989–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merjava S, Liskova P, Sado Y, Davis PF, Greenhill NS, Jirsova K. Changes in the localization of collagens IV and VIII in corneas obtained from patients with posterior polymorphous corneal dystrophy. Exp Eye Res. 2009;88:945–952 [DOI] [PubMed] [Google Scholar]
- 27. Tuori AJ, Virtanen I, Aine E, Kalluri R, Miner JH, Uusitalo HM. The immunohistochemical composition of corneal basement membrane in keratoconus. Curr Eye Res. 1997;16:792–801 [DOI] [PubMed] [Google Scholar]
- 28. Kay EP, Gu X, Choi SH, Ninomiya Y. Posttranslational regulation of type I collagen in corneal endothelial cells. Invest Ophthalmol Vis Sci. 1996;37:11–19 [PubMed] [Google Scholar]
- 29. de Felice GP, Braidotti P, Viale G, Bergamini F, Vinciguerra P. Posterior polymorphous dystrophy of the cornea. An ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1985;223:265–271 [DOI] [PubMed] [Google Scholar]
- 30. Fitch JM, Birk DE, Linsenmayer C, Linsenmayer TF. The spatial organization of Descemet's membrane-associated type IV collagen in the avian cornea. J Cell Biol. 1990;110:1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gottsch JD, Zhang C, Sundin OH, Bell WR, Stark WJ, Green WR. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46:4504–4511 [DOI] [PubMed] [Google Scholar]
- 32. Levy SG, McCartney AC, Sawada H, Dopping-Hepenstal PJ, Alexander RA, Moss J. Descemet's membrane in the iridocorneal-endothelial syndrome: morphology and composition. Exp Eye Res. 1995;61:323–333 [DOI] [PubMed] [Google Scholar]
- 33. Waring GO., 3rd Posterior collagenous layer of the cornea. Ultrastructural classification of abnormal collagenous tissue posterior to Descemet's membrane in 30 cases. Arch Ophthalmol. 1982;100:122–134 [DOI] [PubMed] [Google Scholar]
- 34. Levy SG, Moss J, Noble BA, McCartney AC. Early-onset posterior polymorphous dystrophy. Arch Ophthalmol. 1996;114:1265–1268 [DOI] [PubMed] [Google Scholar]
- 35. Riazuddin SA, Zaghloul NA, Al-Saif A, et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010;86:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baratz KH, Tosakulwong N, Ryu E, et al. E2–2 protein and Fuchs's corneal dystrophy. N Engl J Med. 2010;363:1016–1024 [DOI] [PubMed] [Google Scholar]
- 37. Riazuddin SA, McGlumphy EJ, Yeo WS, Wang J, Katsanis N, Gottsch JD. Replication of the TCF4 Intronic Variant in Late-Onset Fuchs Corneal Dystrophy and Evidence of Independence from the FCD2 locus. Invest Ophthalmol Vis Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eye Bank Association of America 2010 eye banking statistical report. 2010 [Google Scholar]