Cytotoxic strains of Pseudomonas aeruginosa express a type III secreted toxin ExoU and are often isolated from cases of P. aeruginosa keratitis. This study showed that the phospholipase domain of ExoU is necessary for cytotoxic strains to traverse multilayered human corneal epithelial cells in vitro.
Abstract
Purpose.
Pseudomonas aeruginosa isolates from microbial keratitis are invasive or cytotoxic toward mammalian cells, depending on their type III secreted toxins. Cytotoxic strains express ExoU, a phospholipase that contributes to corneal virulence. This study determined whether the ExoU phospholipase domain is required for P. aeruginosa traversal of the human corneal epithelium.
Methods.
P. aeruginosa traversal of airlifted, multilayered, human corneal epithelial cells was quantified in vitro up to 8 hours after apical inoculation with ∼106 cfu of strain PA14, or an isogenic exoU mutant (PA14ΔexoU). In addition, PA14ΔexoU or its triple effector mutant PA14ΔexoUΔexoTΔexoY, were complemented with exoU (pUCPexoU), phospholipase-inactive exoU (pUCPexoUD344A), or control plasmid (pUCP18). Transepithelial resistance (TER) was measured (by epithelial volt ohmmeter), and cytotoxicity was determined by trypan blue staining.
Results.
PA14 traversed more efficiently than its exoU mutant at 4, 6, and 8 hours after inoculation (100-, 20-, and 8-fold, respectively; P < 0.05), but not at 2 hours. Cells exposed to PA14 lost TER to baseline (P < 0.05). Controls confirmed PA14 cytotoxicity toward these corneal epithelial cells that was absent with exoU mutants. Epithelial traversal, cytotoxicity, and lost TER were restored for PA14ΔexoU, or PA14ΔexoUΔexoTΔexoY, by complementation with pUCPexoU, but not by complementation with pUCPexoUD344A.
Conclusions.
Traversal of multilayered corneal epithelia in vitro by cytotoxic P. aeruginosa requires ExoU with an active phospholipase domain. Correlative loss of TER with traversal by wild-type, or exoU-complemented, bacteria suggests involvement of epithelial cell death and/or lost tight junction integrity. However, traversal by exoU mutants without reduced TER suggests that additional mechanisms are also operative.
The pathogen Pseudomonas aeruginosa is a leading cause of microbial keratitis, a sight-threatening infection associated with contact lens wear.1–3 Clinical isolates of P. aeruginosa can be divided into two major phenotypes on the basis of their interactions with corneal epithelial cells: invasive or cytotoxic.4 Cytotoxic strains differ from invasive isolates by encoding a toxin called ExoU,5 which allows bacteria to be acutely cytotoxic to epithelial and other mammalian cell types6 via potent phospholipase activity.7,8 ExoU and its phospholipase activity are important for P. aeruginosa virulence in the cornea9,10 and the respiratory tract11 and promote tissue colonization, although the mechanisms involved remain to be fully determined.
An important first step in the pathogenesis of infection of the cornea and other tissues is the ability of P. aeruginosa to traverse single- or multilayered epithelia. We have shown that traversal of multilayered corneal epithelia in vitro by an invasive strain of P. aeruginosa requires pilus-mediated twitching motility12 and that P. aeruginosa proteases play a role in overcoming basement membrane/extracellular matrix–mediated resistance to bacterial traversal.13 Others have used invasive strains or purified toxins13 to show roles for P. aeruginosa elastase and exotoxin A,14,15 the type III secretion system,16 and multidrug efflux pumps17 in P. aeruginosa traversal of respiratory or MDCK monolayers. We have shown that cytotoxic strains can traverse multilayered corneal epithelial cells in vitro18 and that the phospholipase activity of ExoU contributes to P. aeruginosa corneal virulence when the epithelium is breached by scarification.10 However, the role of ExoU and its phospholipase activity in corneal epithelial traversal in the absence of prior injury are unknown. In this study, we tested the hypothesis that traversal of intact multilayered corneal epithelia by a cytotoxic strain of P. aeruginosa in vitro would require ExoU with an active phospholipase domain.
Materials and Methods
Bacteria
The wild-type P. aeruginosa strain PA14 was used. This strain is known to encode exoU19 and is cytotoxic toward mammalian cells.20 An exoU mutant (PA14ΔexoU) and triple effector mutant (PA14ΔexoUΔexoTΔexoY) were also used.21 Mutants were complemented with plasmid pUCP18 containing either the exoU gene (pUCPexoU), which fully restores cytotoxic activity toward eukaryotic cells22; or the exoU gene, in which the aspartate catalytic site of the N-terminal phospholipase domain is mutated (pUCPexoUD344A) and thus lacks phospholipase and in vitro cytotoxic activity7; or an empty vector control (pUCP18). Plasmid-complemented mutants were grown on trypticase soy agar (TSA) supplemented with carbenicillin 300 μg/mL overnight (∼18 hours) at 37°C. For use in experiments, bacteria were resuspended in serum-free tissue culture medium without antibiotics, to an optical density (at 650 nm) of ∼0.1 corresponding to a viable count of ∼1 × 108 cfu/mL. This suspension was diluted to achieve the desired inocula. Plasmid-complemented strains grow equally well in vitro with or without exposure to mammalian cells.
Cell Culture
Human corneal epithelial cells (SV-40 immortalized) were used.23 The cells were air-lifted on permeable tissue culture inserts (3-μm pores; Transwell; Corning Costar, Corning, NY) to induce a confluent, polarized, multilayered epithelium, as previously described.23,24
Epithelial Traversal Assay with TER Measurements
Experiments were performed as described elsewhere.24 Bacterial inocula (∼106 cfu in 1 mL of media) were carefully added to the apical compartment of human corneal epithelial cells (multiplicity of infection ∼1), grown on permeable tissue culture inserts (Transwell; Corning Costar), and incubated (5% CO2 at 37°C) for up to 8 hours. Apical and basal compartments were sampled at 2, 4, 6, and 8 hours after inoculation to determine the number of viable bacteria. Uninfected epithelial cells were sham inoculated as controls. All samples were performed in triplicate, and experiments were repeated at least three times. TER was measured before and after each experiment with an epithelial volt ohmmeter (EVOM; World Precision Instruments, Sarasota, FL). Controls included sham-inoculated cells (normal tight junctions) and filters without cells (baseline). To measure bacterial traversal, the bacteria were added only to the apical compartment, and bacteria traversing to the basal compartment were enumerated at various times after inoculation.24
Cytotoxicity
Trypan blue exclusion assays were used to measure P. aeruginosa cytotoxic activity, as previously described.4,5 Trypan blue staining was used to indicate dead/dying human corneal epithelial cells 3 hours after exposure to bacterial inocula (∼106 cfu/mL). At least three wells of cells were used for each sample. All experiments were repeated once.
Statistical Analysis
Data are expressed as the mean ± SD. Student's t-test was used to assess the statistical significance of differences between means. P < 0.05 denoted significance.
Results
ExoU Is Essential for Cytotoxic P. aeruginosa to Traverse Multilayered Corneal Epithelia
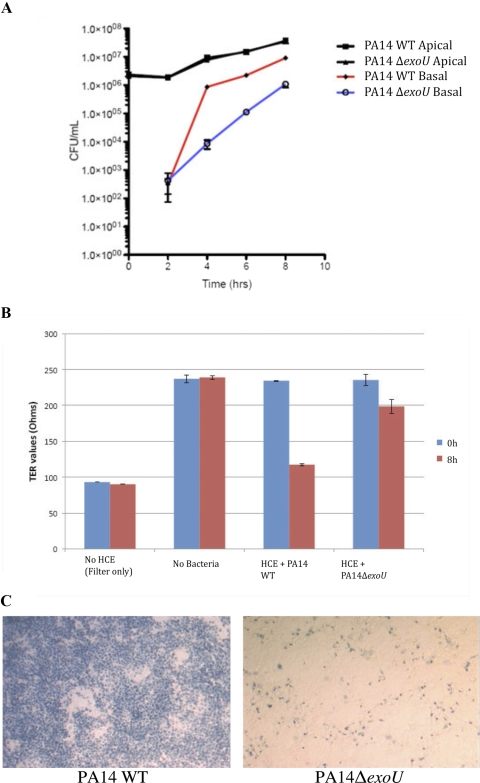
Confluent multilayered human corneal epithelial cells on permeable tissue culture filters (Transwell; Corning Costar) were exposed to wild-type cytotoxic P. aeruginosa PA14 or an isogenic exoU mutant (PA14ΔexoU) on their apical surface, and traversal of viable bacteria to the basal compartment was quantified over an 8-hour period (Fig. 1A). Interestingly, both wild-type and exoU mutant bacteria traversed in equal numbers after 2 hours, albeit at levels >3 log lower than the number in the apical compartment. Over the next 6 hours, however, significantly greater traversal of the corneal epithelia was observed with wild-type than with exoU mutants (∼100-fold at 4 hours, ∼20-fold at 6 hours, and 8-fold at 8 hours; P < 0.05, t-test in each instance). Both wild-type and exoU mutants grew at identical rates in the apical compartment. Exposure of corneal epithelial cells to wild-type PA14 reduced TER nearly to baseline after 8 hours, whereas TER of cells exposed to the exoU mutant retained most of their TER throughout the assay, although a small, but significant (P < 0.05, t-test) TER decline was observed after 8 hours (Fig. 1B). Control experiments (Fig. 1C) using trypan blue staining to mark dead/dying cells confirmed, as expected, that P. aeruginosa PA14 was highly cytotoxic to these corneal epithelial cells under these experimental conditions and that this cytotoxicity was lost with the exoU mutant. Although we had previously shown that cytotoxic P. aeruginosa can traverse a multilayered corneal epithelium in vitro,18 these new data showed that the exoU gene plays a significant part in that epithelial traversal. The data also showed, however, that even without exoU, P. aeruginosa could still traverse the corneal epithelia, albeit at lower levels than wild-type.
Figure 1.
(A) Cytotoxic P. aeruginosa strain PA14 traversed multilayered human corneal epithelial cells in vitro with significantly greater efficiency than its isogenic exoU mutant (PA14ΔexoU) at 4, 6, and 8 hours after inoculation. At 2 hours, however, there was no difference in traversal. Bacteria grew at similar rates in the apical compartment. (B) PA14 caused a significant loss in TER of human corneal epithelial cells relative to its exoU mutant after 8 hours, and (C) PA14 was cytotoxic to human corneal epithelial cell monolayers in vitro after 3 hours, as shown by trypan blue staining (left), whereas PA14ΔexoU caused minimal cell death (right).
ExoU-Mediated Epithelial Traversal Requires Its Phospholipase Domain
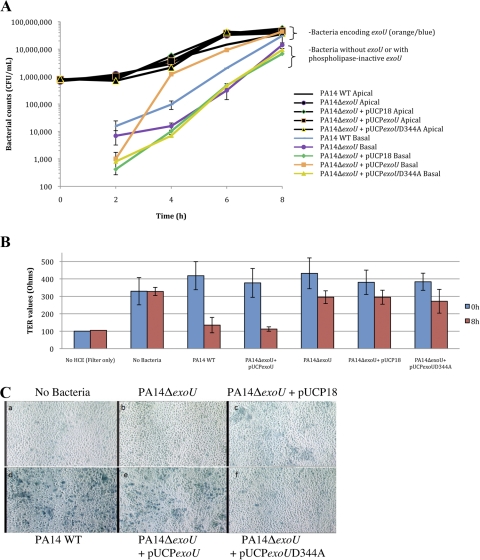
ExoU is known to possess a phospholipase domain with potent activity.7,8 The requirement of the phospholipase domain for ExoU-mediated corneal epithelial traversal was tested by comparing traversal of the exoU mutant of P. aeruginosa PA14 when complemented with active exoU (pUCPexoU) or with phospholipase-inactive exoU (pUCPexoUD344A). Complementation with exoU fully restored (or exceeded) epithelial traversal shown by wild-type bacteria, whereas the phospholipase-inactive form of exoU did not affect traversal of the exoU mutant and was not different from plasmid vector control (Fig. 2A). At 2 hours, traversal by wild-type PA14 and the exoU mutant did not differ from one another, nor did traversal by the plasmid-complemented bacteria, with or without exoU. As seen previously (Fig. 1), decreased epithelial TER coincided with traversal by bacteria expressing the phospholipase-active form of exoU (Fig. 2B). Control experiments showed that complementation of PA14ΔexoU with pUCPexoU restored bacterial cytotoxicity to human corneal epithelial cells, whereas complementation with the phospholipase mutant pUCPexoUD344A did not (Fig. 2C). These data show that the phospholipase domain of exoU is necessary for its role in epithelial traversal.
Figure 2.
(A) Complementation of PA14ΔexoU with pUCPexoU returned bacterial traversal of human corneal epithelial cells to wild-type PA14 levels. Complementation of PA14ΔexoU with pUCPexoUD344A, which lacks phospholipase activity, did not promote traversal and was similar to the empty vector (pUCP18) control, except at 2 hours. All strains grew similarly in the apical compartment. (B) Complementation of PA14ΔexoU with pUCPexoU caused TER disruption similar to wild-type. Complementation with phospholipase inactive exoU (pUCPexoUD344A) did not affect TER. (C) Complementation of PA14ΔexoU with pUCPexoU also restored cytotoxicity toward multilayered human corneal epithelial cells after 3 hours, as shown by trypan blue staining. Top (left to right): no bacteria control, PA14ΔexoU, and PA14ΔexoU complemented with empty vector pUCP18. Bottom (left to right): PA14 wild-type, PA14ΔexoU complemented with pUCPexoU and PA14ΔexoU complemented with pUCPexoUD344A (phospholipase domain mutant).
ExoU Encoding Phospholipase Activity Is Sufficient to Promote Epithelial Traversal by Cytotoxic P. aeruginosa in the Absence of Other Known Type III Secreted Effectors
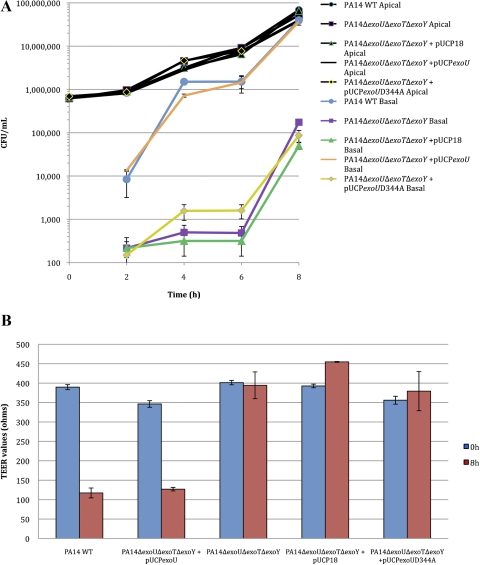
In previous experiments we showed that exoU complementation of an exoU mutant of PA14 restored the ability to traverse corneal epithelia to levels similar to, or greater than, wild-type bacteria. However, wild-type PA14 also encode genes for other type III secreted effectors (i.e., exoT and exoY), although it is not known under which conditions they are all actually expressed.19 To determine whether exoU alone was sufficient for epithelial traversal in vitro, a noncytotoxic triple effector gene knockout of PA14 was complemented with pUCPexoU and compared with the same bacteria complemented with phospholipase-inactive exoU (pUCPexoUD344A) or a vector control (Fig. 3). Complementation of the triple effector mutant with exoU alone was sufficient to restore bacterial traversal to that of wild-type bacteria, whereas complementation with phospholipase-inactive exoU produced significantly lower bacterial traversal levels similar to the uncomplemented triple effector mutant, with and without the plasmid vector control. However, a small, but statistically significant, increase in epithelial traversal was also noted at 4 and 6 hours for the triple effector mutant complemented with the phospholipase-inactive exoU versus the pUCP18 control. Of note, in contrast to experiments using a single effector (ΔexoU) mutant (Figs. 1, 2), there was a difference in traversal at 2 hours between wild-type PA14 and the triple effector (ΔexoUΔexoTΔexoY) mutant and between the pUCPexoU and pUCPexoUD344A-complemented triple effector mutants. TER changes after 8 hours showed that epithelial traversal by wild-type bacteria or the triple effector mutant complemented with exoU correlated with a large decrease in TER that was not seen with the other mutant or complemented strains (Fig. 3B).
Figure 3.
(A) Complementation of the triple effector mutant PA14Δ exoUΔexoTΔexoY with pUCPexoU also fully restored P. aeruginosa traversal of multilayered human corneal epithelial cells in vitro (similar to wild-type PA14). Complementation with pUCP exoUD344A (phospholipase inactive exoU) did not restore traversal and was similar to vector pUCP18 control. All bacteria grew similarly in apical compartments. (B) TER readings of multilayered corneal epithelia after 8 hours' exposure to plasmid complemented triple effector mutants showed a pattern similar to that in previous experiments. Complementation of PA14ΔexoUΔexoTΔ exoY with pUCPexoU diminished TER similarly to wild-type. Complementation with pUCPexoUD344A or the pUCP18 control plasmid did not reduce TER.
Discussion
The data presented show that the type III secreted effector gene exoU is sufficient to enable a cytotoxic strain of P. aeruginosa to traverse multilayered corneal epithelial cells in vitro. Moreover, the data show that exoU-mediated epithelial traversal requires the phospholipase domain of the gene. Epithelial traversal by cytotoxic P. aeruginosa in vitro correlated with the loss of TER and acute cytotoxic effects of exoU-bearing bacteria on corneal epithelial cells. Together, these data suggest that ExoU-mediated epithelial traversal requires the breakdown of epithelial tight junctions and/or epithelial injury and cell death.
It is perhaps not surprising that ExoU can mediate epithelial traversal by P. aeruginosa, at least in vitro. The ability of this toxin to cause mammalian cell death and the requirement of its phospholipase domain are already well documented6,7,22 and were confirmed in the present study for the P. aeruginosa strain (PA14) and cells (human corneal epithelia) that were used. Moreover, traversal coincided with a significant decline in TER, which would be consistent with the acute death of multiple epithelial cells. Thus, one likely scenario to explain these results is that cytotoxic P. aeruginosa simply physically breaks through the multilayered epithelium by killing one epithelial cell after another until the basal compartment is reached. In this scenario, exposure of basolateral epithelial cell surfaces as bacteria traverse could also enhance traversal, as these surfaces are more vulnerable to P. aeruginosa cytotoxicity.25 Alternatively, bacteria may first directly or indirectly target epithelial tight junctions (also decreases TER) to expose basolateral cell surfaces,26 followed by traversal via epithelial cell death. In other host cell types, ExoU induces the release of proinflammatory mediators including IL-6, IL-8, and eicosanoids,27–29 one or more of which could modulate epithelial barrier function of the cornea (including effects on tight junctions) before ExoU-mediated cell death.29,30 Thus, we cannot exclude the possibility that additional, or alternative, mechanisms participate in ExoU-mediated epithelial traversal by P. aeruginosa through other effects of ExoU intoxication of mammalian cells before cell death or of factors released from cells killed by ExoU, both of which are yet to be fully defined.
Our data show that factors other than ExoU also participate in epithelial traversal by cytotoxic P. aeruginosa. At 2 hours and later time points, mutants lacking exoU or complemented with phospholipase-inactive exoU also traversed the epithelia, albeit at reduced rates compared to exoU-bearing bacteria. ExoU-independent traversal could reflect activities of multidrug efflux pumps (MexAB/OprM),17 exotoxin A,15 and proteases,13,15 which have been shown to be involved in P. aeruginosa traversal of epithelia and which are expressed by most P. aeruginosa strains. Clearly, only ExoS-mediated epithelial traversal16 can be excluded, since it is not encoded by PA14 or other cytotoxic strains. ExoU-independent traversal did not result in significant changes in TER (compared with ExoU-dependent traversal), suggesting that differences in traversal mechanisms/pathways may exist. One of these differences may involve ExoT, another type III secreted effector protein with both GAP (GTPase activating protein) and ADPr (ADP ribosylation) activity (see review31), or ExoY an adenylate cyclase.32 There was little difference in traversal between wild-type P. aeruginosa and its isogenic (ΔexoU) mutant at 2 hours in any given experiment (i.e., within Fig. 1, 2), whereas a significant difference in traversal was observed at the same time point in experiments in which wild-type bacteria were compared with a triple (ΔexoUΔexoTΔexoY) mutant (Fig. 3). These data suggest that ExoT and/or ExoY also influence the traversal process. However, since ExoY is encoded by PA14, but not necessarily expressed,19 ExoT may especially warrant further investigation in the context of cytotoxic strain traversal. ExoT has numerous effects on host cells through effects on the cytoskeleton31 and focal adhesion signaling33 that could influence bacterial traversal. These include epithelial cell rounding,22 inhibition of P. aeruginosa internalization,34,35 and alteration of tight junction proteins.16 Nevertheless, further studies are needed to determine the relationship between this and other ExoU-independent traversal mechanisms and ExoU-mediated traversal.
Cytotoxic P. aeruginosa strains appear more commonly (∼50%) among corneal isolates of P. aeruginosa compared to isolates from other infections (∼28%).36–38 Indeed, exoU is one of several genes that appear to be selected in microbial keratitis,37 and its expression correlates with resistance to contact lens disinfection systems.39 From this study, we conclude that ExoU (phospholipase)-mediated epithelial traversal has the potential to offer a pathogenic advantage for cytotoxic P. aeruginosa when the bacteria encounter the intact multilayered corneal epithelium in vivo. Indeed, these findings build on our previous work showing that cytotoxic P. aeruginosa can damage intact corneal epithelia ex vivo40 that ExoU contributes to P. aeruginosa corneal virulence if the epithelium is injured before bacterial exposure9,41 and that ExoU-mediated ocular colonization and corneal disease severity require its phospholipase activity.10 However, we have also shown that the multilayered corneal epithelium is a formidable barrier to P. aeruginosa traversal in vivo and ex vivo24,42,43 and that even cytotoxic P. aeruginosa do not infect the intact mouse cornea in vivo.44 Clearly, the clinical prevalence of cytotoxic P. aeruginosa suggests that in vivo circumstances occur that allow ExoU-mediated virulence. However, further studies are needed to elucidate the relationship between ExoU-mediated penetration of multilayered corneal epithelia in vitro, ExoU-mediated virulence in vivo, and their combined significance in the pathogenesis of microbial keratitis, especially in the context of contact lens wear for which mechanisms of P. aeruginosa epithelial traversal to cause infection are unknown.
Acknowledgments
The authors thank Dara Frank (Medical College of Wisconsin) and Fred Ausubel (Harvard Medical School) for providing the P. aeruginosa mutants and exoU constructs.
Footnotes
Supported by National Institutes of Health Grant EY011221 (SMJF).
Disclosure: J.C. Ramirez, Alcon (F); S.M.J. Fleiszig, Alcon (F), P; A.B. Sullivan, Alcon (F); C. Tam, Alcon (F); R. Borazjani, Alcon (E, F); D.J. Evans, Alcon (F), P
References
- 1. Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–1324 [DOI] [PubMed] [Google Scholar]
- 2. Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27(1):33–39 [DOI] [PubMed] [Google Scholar]
- 3. Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95(6):762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleiszig SM, Zaidi TS, Preston MJ, et al. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64(6):2288–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fleiszig SM, Wiener-Kronish JP, Miyazaki H, et al. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65(2):579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finck-Barbancon V, Goranson J, Zhu L, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25(3):547–557 [DOI] [PubMed] [Google Scholar]
- 7. Sato H, Frank DW, Hillard CJ, et al. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin. ExoU. EMBO J. 2003;22(12):2959–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato H, Feix JB, Hillard CJ, Frank DW. Characterization of phospholipase activity of the Pseudomonas aeruginosa type III cytotoxin. ExoU. J Bacteriol. 2005;187(3):1192–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun. 2006;74(7):3880–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tam C, Lewis SE, Li WY, et al. Mutation of the phospholipase catalytic domain of the Pseudomonas aeruginosa cytotoxin ExoU abolishes colonization promoting activity and reduces corneal disease severity. Exp Eye Res. 2007;85(6):799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72(12):6969–6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alarcon I, Evans DJ, Fleiszig SM. The role of twitching motility in Pseudomonas aeruginosa exit from and translocation of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(5):2237–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alarcon I, Kwan L, Yu C, et al. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect Immun. 2009;77(8):3264–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azghani AO, Gray LD, Johnson AR. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect Immun. 1993;61(6):2681–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azghani AO. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am J Respir Cell Mol Biol. 1996;15(1):132–140 [DOI] [PubMed] [Google Scholar]
- 16. Soong G, Parker D, Magargee M, Prince AS. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J Bacteriol. 2008;190(8):2814–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirakata Y, Srikumar R, Poole K, et al. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med. 2002;196(1):109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwong MS, Evans DJ, Ni M, et al. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect Immun. 2007;75(5):2325–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyata S, Casey M, Frank DW, et al. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect Immun. 2003;71(5):2404–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Alst NE, Wellington M, Clark VL, et al. Nitrite reductase NirS is required for type III secretion system expression and virulence in the human monocyte cell line THP-1 by Pseudomonas aeruginosa. Infect Immun. 2009;77(10):4446–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati NT, Urbach JM, Miyata S, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A. 2006;103(8):2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vallis AJ, Finck-Barbancon V, Yahr TL, Frank DW. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67(4):2040–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNamara NA, Van R, Tuchin OS, Fleiszig SM. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69(5):483–490 [DOI] [PubMed] [Google Scholar]
- 24. Augustin DK, Heimer SR, Tam C, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011;79(2):595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fleiszig SM, Evans DJ, Do N, et al. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65(7):2861–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee A, Chow D, Haus B, et al. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am J Physiol. 1999;277(1 Pt 1):L204–L217 [DOI] [PubMed] [Google Scholar]
- 27. Saliba AM, Nascimento DO, Silva MC, et al. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell Microbiol. 2005;7(12):1811–1822 [DOI] [PubMed] [Google Scholar]
- 28. Plotkowski MC, Brandao BA, de Assis MC, et al. Lipid body mobilization in the ExoU-induced release of inflammatory mediators by airway epithelial cells. Microb Pathog. 2008;45(1):30–37 [DOI] [PubMed] [Google Scholar]
- 29. Cuzick A, Stirling FR, Lindsay SL, Evans TJ. The type III pseudomonal exotoxin U activates the c-Jun NH2-terminal kinase pathway and increases human epithelial interleukin-8 production. Infect Immun. 2006;74(7):4104–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coyne CB, Vanhook MK, Gambling TM, et al. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13(9):3218–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barbieri JT, Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol. 2004;152:79–92 [DOI] [PubMed] [Google Scholar]
- 32. Yahr TL, Vallis AJ, Hancock MK, et al. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95(23):13899–13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng Q, Sun J, Barbieri JT. Uncoupling Crk signal transduction by Pseudomonas exoenzyme T. J Biol Chem. 2005;280(43):35953–35960 [DOI] [PubMed] [Google Scholar]
- 34. Cowell BA, Chen DY, Frank DW, et al. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun. 2000;68(1):403–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrity-Ryan L, Kazmierczak B, Kowal R, et al. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun. 2000;68(12):7100–7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lomholt JA, Poulsen K, Kilian M. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect Immun. 2001;69(10):6284–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stewart RM, Wiehlmann L, Ashelford KE, et al. Genetic characterization indicates that a specific subpopulation of Pseudomonas aeruginosa is associated with keratitis infections. J Clin Microbiol. 2011;49(3):993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feltman H, Schulert G, Khan S, et al. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147(Pt 10):2659–2669 [DOI] [PubMed] [Google Scholar]
- 39. Lakkis C, Fleiszig SM. Resistance of Pseudomonas aeruginosa isolates to hydrogel contact lens disinfection correlates with cytotoxic activity. J Clin Microbiol. 2001;39(4):1477–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fleiszig SM, Lee EJ, Wu C, et al. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. Clao J. 1998;24(1):41–47 [PubMed] [Google Scholar]
- 41. Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44(9):3892–3898 [DOI] [PubMed] [Google Scholar]
- 42. Alarcon I, Tam C, Mun JJ, et al. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci. 2011;52(3):1368–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tam C, Ledue J, Mun JJ, et al. 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One. 2011;6(8):e24008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mun JJ, Tam C, Kowbel D, et al. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect Immun. 2009;77(6):2392–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]