ADAMTSL4 mutations result in recessively inherited isolated ectopia lentis, a dysgenesis of the fibrillin-1–rich zonule of Zinn. This research shows that ADAMTSL4 binds fibrillin-1 microfibrils and accelerates their biogenesis, thus providing a potential underlying mechanism for this disorder.
Abstract
Purpose.
ADAMTSL4 mutations cause autosomal recessive isolated ectopia lentis (IEL) and ectopia lentis et pupillae. Dominant FBN1 mutations cause IEL or syndromic ectopia lentis (Marfan syndrome and Weill-Marchesani syndrome). The authors sought to characterize recombinant ADAMTSL4 and the ocular distribution of ADAMTSL4 and to investigate whether ADAMTSL4 influences the biogenesis of fibrillin-1 microfibrils, which compose the zonule.
Methods.
ADAMTSL4 was expressed by the transfection of HEK293F cells. Protein extracts and paraffin sections from human eyes were analyzed by Western blot analysis and by immunoperoxidase staining, respectively. Immunofluorescence was used to evaluate fibrillin-1 deposition in the ECM of fetal bovine nuchal ligament cells after culture in ADAMTSL4-conditioned medium or control medium. Confocal microscopy was performed to investigate ADAMTSL4 and fibrillin-1 colocalization in these cultures.
Results.
Western blot analysis identified ADAMTSL4 as a glycoprotein in HEK293F cells and as a major band of 150 kDa in ocular tissues including ciliary body, sclera, cornea, and retina. Immunoperoxidase staining showed a broad ocular distribution of ADAMTSL4, associated with both cells and fibrillar ECM. When cultured in ADAMTSL4-containing medium, fetal bovine nuchal ligament cells showed accelerated fibrillin-1 deposition in ECM. ADAMTSL4 colocalized with fibrillin-1 microfibrils in the ECM of these cells.
Conclusions.
ADAMTSL4 is a secreted glycoprotein that is widely distributed in the human eye. Enhanced fibrillin-1 deposition in the presence of ADAMTSL4 and colocalization of ADAMTSL4 with fibrillin-1 in the ECM of cultured fibroblasts suggest a potential role for ADAMTSL4 in the formation or maintenance of the zonule.
The suspensory ligament of the lens (zonule of Zinn) centers the ocular lens in the path of light and transmits ciliary muscle forces involved in accommodation. The zonule is a circular cell-free structure that is composed of radially oriented cablelike microfibrils. It extends from the ciliary body to the equatorial region of the lens capsule. Zonule microfibrils are composed primarily of the matrix glycoprotein fibrillin-1.1–4 Fibrillin-1 is also a major component of tissue microfibrils, which are especially abundant in association with elastic fibers in skin, arteries, and lung.3,5 Ectopia lentis, which refers to subluxation of the lens from its centered position, may result from trauma to the zonule or may be caused by dysgenesis or fragility of the zonule as a consequence of inherited disorders. For example, dominantly inherited FBN1 mutations cause Marfan syndrome (MFS), in which ectopia lentis is a major clinical manifestation.6 Less commonly, dominantly inherited FBN1 mutations cause dominantly inherited isolated ectopia lentis (MIM129600), i.e., without other clinical connective tissue manifestations of MFS.7,8 Recently, ADAMTSL4 mutations were identified in both recessively inherited isolated ectopia lentis9–12 and ectopia lentis et pupillae.13 Ectopia lentis has also been described in Weill-Marchesani syndrome (MIM277600; MIM608328),14 which is caused by either ADAMTS1015 or FBN1 mutations,16 inherited in a recessive or dominant fashion, respectively. ADAMTS17 mutations were identified in a WMS-like syndrome in which ectopia lentis was also present.17 These genetic findings suggest that at least three members of the ADAMTS superfamily of secreted proteins are involved in the normal formation, maintenance, or both of the zonule and the abnormalities therein in case of defects in these proteins. Recently, ADAMTSL6, which of all family members is the most similar to ADAMTSL4, was shown to bind directly to fibrillin-1 and to enhance its assembly both in tissue culture and in transgenic mice overexpressing it.18 Similarly, binding of ADAMTS10 to fibrillin-1 and to fibrillin microfibrils was identified recently, and ADAMTS10 was shown to enhance microfibril biogenesis in vitro.19 Collectively, these findings led to the hypothesis that ADAMTSL4 functions to promote fibrillin-1 assembly in the genesis, maintenance, or both of the zonular apparatus.
The ADAMTS superfamily comprises 19 ADAMTS proteases and seven ADAMTS-like proteins.20 ADAMTS proteases consist of two distinct domains, namely, a zinc-binding metalloprotease domain and an ancillary domain containing one or more thrombospondin type 1 repeats. In contrast, ADAMTS-like proteins have a modular structure related to the ADAMTS ancillary domain and lack the protease domain.20 Hence, they are not enzymes but are secreted glycoproteins deposited into the ECM. Because little is known about ADAMTSL4, we determined ADAMTSL4 distribution in the eye and investigated its potential function vis-à-vis fibrillin-1. Our results suggest that ADAMTSL4 is a widely distributed glycoprotein that associates with fibrillin-1 and may accelerate fibrillin-1 assembly.
Methods
Expression Plasmid, Cell Culture, and Transfection
A full-length human ADAMTSL4 cDNA including untranslated regions in the pCR4-TOPO vector (IMAGE clone 9021668, GenBank accession number BC140800) was purchased from Open Biosystems (Huntsville, AL). The stop codon was mutated by site-directed mutagenesis (QuikChange kit; Stratagene, Thousand Oaks, CA) and replaced with an Xho I site for cloning of the ADAMTSL4 protein coding region in-frame with a tandem myc+ His6 tag in pcDNA 3.1/myc-His A (+) (Invitrogen, Carlsbad, CA). The ADAMTSL4 expression plasmid was transfected into HEK293F cells or COS-1 cells (ATCC, Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin using transfection reagent (FuGENE 6; Roche Applied Science, Indianapolis, IN). Stably transfected HEK293F clones were selected using G418 (1 mg/mL).
Human Tissue
Frozen normal human cadaveric eye tissue was obtained from the Cleveland Eye Bank. Tissue procurement was adherent with the Declaration of Helsinki. The globe was initially cut sagittally and transversally in four equal parts. The different ocular tissues were dissected and weighed. Modified cell lysis reagent (T-Per Tissue Protein Extraction Reagent; Thermo Fisher Scientific, Waltham, MA) premixed with protease inhibitor cocktail tablet (complete, EDTA-free, Roche) was added (20 mL/g tissue) and homogenized (Ultra-Turrax T25; Janke & Kunkel IKA Labortechnik, Staufen, Germany). The homogenate was centrifuged at 3500 rpm for 10 minutes; 50 mL supernatant was used for immunoblotting. Archival formalin-fixed paraffin sections of cadaveric human eye (IRB exempt, with no patient identifiers) were used for immunohistochemistry.
Antibodies and Immunoblotting
A commercial rabbit polyclonal antibody generated to an ADAMTSL4 peptide (amino acids 601–706) and affinity-purified against the immobilized peptide was purchased from Sigma-Aldrich (St. Louis, MO; catalog no. HPA006279). Because of the length of the immunogenic peptide (105 residues), five overlapping 20- to 25-mer peptides (3–4 residue overlap) spanning this sequence were synthesized and dissolved in PBS (final concentration of each peptide was 400 mg/mL). Anti-ADAMTSL4 antibody (320 mg/mL) was incubated with peptide (ratio 1:2) in PBS at 37°C for 40 minutes before use in Western blot controls. For the immunostaining control, an antibody/peptide ratio of 1:5 was incubated at 37°C for 2.5 hours before use.
Monoclonal antibody to the myc tag (clone 9E10) was from the Lerner Research Institute Hybridoma Core. Monoclonal antibody to human fibrillin-1 (clone 11C1.3) was purchased from Millipore (Billerica, MA). Secondary antibodies generated in goat (AlexaFluor 488, Cy3, or peroxidase tagged) were purchased from Invitrogen. For protein electrophoresis, reducing 6% SDS-PAGE was used. Conditioned medium was mixed with 5× Laemmli sample buffer and reconstituted to 20% β-mercaptoethanol before boiling for 5 minutes. After electrophoresis, proteins were transferred to polyvinylidine difluoride (PVDF) membrane for Western blot analysis using ADAMTSL4 antibody at 1:2000 dilution (overnight at 4°C) and horseradish peroxidase-conjugated goat–anti-rabbit polyclonal antibody at 1:10,000 (room temperature for 1 hour). Antibody binding was detected using chemiluminescence (Hyglo; Denville Scientific Inc., South Plainfield, NJ). Conditioned medium from stably transfected cells was treated with peptide N-glycosidase F (PNGase F; New England Biolabs, Ipswich, MA), as previously described,21,22 before electrophoresis. Treatment of ADAMTSL4 with O-glycosidase (endo-α-N-acetlygalactosaminidase; New England BioLabs) was conducted in accordance with the manufacturer's instructions. Briefly, ADAMTSL4 conditioned medium was mixed with 10× glycoprotein denaturing buffer provided by the manufacturer and heated at 100°C for 10 minutes. Then 10× G7 reaction buffer, 10% NP40, and O-Glycosidase (provided by the manufacturer) were added to the denatured sample, which was incubated at 37°C for 3 hours. A control sample without addition of the O-glycosidase was processed in parallel. Samples were electrophoresed and immunoblotted as described.
Immunofluorescence and Immunohistochemistry
Immunofluorescence staining with anti-ADAMTSL4 was performed on live COS-1 cells (unfixed and nonpermeabilized) to exclusively detect extracellular signal, and after fixation and permeabilization, essentially as previously described.21 An indirect immunoperoxidase method was used for 5- to 10-μm–thick human eye paraffin sections. These were dewaxed with a clearing agent (Histoclear; Thermo Fisher Scientific), rehydrated, and incubated overnight at 4°C with the ADAMTSL4 polyclonal antibody diluted 1:100 in PBS. For immunofluorescence, AlexaFluor 568–labeled goat–anti-rabbit secondary antibody (Invitrogen) was used. Stained slides were viewed using an upright microscope (DMR; Leica, Wetzlar, Germany) and were photographed using a charge-coupled device camera (Q Imaging Retiga EX; Qiagen, Valencia, CA) and image analysis software (Image-Pro; Media Cybernetics, Bethesda, MD).
In Vitro Fibrillin-1 Deposition Assay and Immunocytochemistry
Fibroblasts (5.5 × 104) isolated from fetal bovine nuchal ligament (fBNL cells, <10 passages) were seeded on coverslips in 24-well plates and cultured with DMEM-F12 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Conditioned media were collected from HEK293F cells stably expressing ADAMTSL4 or the empty expression vector (control) cultured in DMEM-F12 medium supplemented with 10% FBS. The pH of the conditioned media was adjusted to 7.0. Conditioned media were mixed with complete fresh medium in a 1:2 ratio and were transferred to fBNL cells at 80% confluence. The optimal ratio of the blended media was determined in initial studies using ratios ranging from 1:1 to 1:4 of conditioned/fresh medium. fBNL cells were cultured in the medium blend for 4 or 5 days, with a change of medium blend after 48 hours. Cells were fixed for 7 minutes with ice-cold methanol, air dried, blocked with 5% normal goat serum in PBS, and incubated with anti–fibrillin-1 monoclonal antibody (MAB1919; Millipore; diluted 1:100) at 4°C overnight to detect fibrillin-1. Goat–anti-mouse AlexaFluor 488 (Invitrogen) was used as a secondary antibody at 6 μg/mL. Nuclei were stained with DAPI (Vector Laboratories, Burlingame, CA). Fibrillin-1 immunofluorescence of the cultures was assessed by three independent observers (LG, HB, and SA). Co-immunofluorescence was performed using anti-ADAMTSL4 and anti-fibrillin-1 monoclonal antibody 1919 to investigate whether ADAMTSL4 was associated with fibrillin microfibrils, using essentially the same conditions as were used for single-antibody immunostaining. For detection of anti-ADAMTSL4, AlexaFluor 568–labeled goat–anti-rabbit secondary antibody was used. Some double-stained cultures were viewed with a laser scanning confocal microscope (TCS-SP; Leica).
RT-PCR Analysis of ADAMTSL4 Expression in Bovine Fibroblasts
Total RNA was isolated from confluent fBNL monolayers using commercial reagent (TriZol; Invitrogen). RNA (430 ng) was used to generate cDNA using reverse transcriptase (Superscript III; Invitrogen) with random hexamers, and 2 μL of this was used as the template in a PCR reaction with the bovine ADAMTSL4 oligonucleotide primers 5′-TCC TTT CAC CTG TCC CTT CAG-3′ (forward) and 5′-GAT GAC ATA CTG ATA GAA AAC ACC TGG-3′ (reverse) or bovine FBN1 primers. The negative control PCR used distilled water as template instead of the reverse-transcribed RNA. The PCR products were analyzed by agarose gel electrophoresis.
Results
Expression of Recombinant ADAMTSL4 and Validation of Anti-ADAMTSL4 Antibody
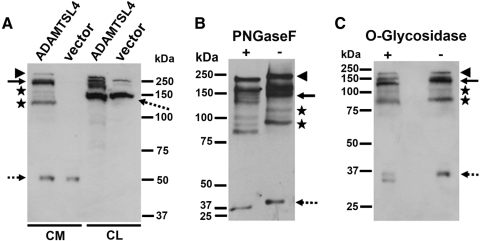
The molecular mass of ADAMTSL4 is predicted to be 116 kDa. Western blot analysis of HEK293F-conditioned medium, but not the medium of vector-transfected cells, identified a major molecular species of 150 kDa reactive with anti-ADAMTSL4 antibody (Fig. 1A, solid arrow). Additional minor species of higher mass ∼240 kDa (Fig. 1A, arrowhead) or lower than expected mass (e.g., 130 kDa, 90 kDa, asterisks) were seen (Fig. 1A). The smallest species visualized, with a mass of 37 kDa (Fig. 1A, dashed arrow), could be a cross-reacting band because it was also seen in the medium of cells transfected with the empty vector, which did not contain additional bands. In the lysates of ADAMTSL4-transfected HEK293F cells, a cluster of bands ranging from 130 to 160 kDa was seen, suggestive of partial or variable glycosylation (Fig. 1A). A strongly reactive band of 100 kDa, seen in both ADAMTSL4-transfected and empty-vector–transfected lysate, was judged to be a cross-reacting protein, whereas weak bands of 140 and 150 kDa in the latter appear to be endogenous ADAMTSL4. The larger than expected species in the medium and the variable species present from 145 to 155 kDa in cell lysate might have resulted from posttranslational modification by N- and O-glycosylation, for which several consensus sites are predicted in ADAMTSL4,23 or from nonreducible complexes containing ADAMTSL4, whereas the smaller species in conditioned medium might have resulted from proteolysis. Similar protein species were seen in the reported analysis of the ADAMTSL4 homolog ADAMTSL6.24 Anti-myc antibody did not provide immunoreactivity in transfected HEK293F cells, suggesting proteolytic loss of the C-terminal myc-His6 tags, which precluded ADAMTSL4 purification using Ni2+ chromatography. The specific reactivity of the ADAMTSL4 antibody toward ADAMTSL4-transfected HEK293F medium was attenuated by the blocking peptides (Supplementary Fig. S1A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental).
Figure 1.
Western blot of recombinant ADAMTSL4. (A) Analysis of medium from HEK293F cells stably transfected with ADAMTSL4 or empty vector shows detectable ADAMTSL4 protein only in ADAMTSL4-transfected cells. Solid arrow: major 140- to 150-kDa species; asterisks: presumed proteolytic fragments. The identities of the 250-kDa species marked by the arrowhead and the species indicated by the broken arrow are unknown, although these species are also attenuated by preblocking the antibody with immunogen peptides (Supplementary Fig. S1A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental). (B) Enzymatic deglycosylation of ADAMTSL4 in conditioned medium of stably transfected HEK293F cells was performed using peptide N-glycosidase F. Increased electrophoretic mobility of anti-ADAMTSL4 reactive species is seen in the presence of peptide N-glycosidase F (+) compared with the untreated control (−). (C) Enzymatic deglycosylation of ADAMTSL4 in conditioned medium of stably transfected HEK293F cells was performed using O-glycosidase (+). Increased electrophoretic mobility of anti-ADAMTSL4 reactive species is seen after O-glycosidase treatment.
PNGase F treatment of ADAMTSL4-containing medium from stably transfected HEK293F cells led to more rapid electrophoretic migration of all immunoreactive bands, together with preservation of their spatial and quantitative relationship to each other (Fig. 1B), suggestive of N-glycosylation. However, the major reactive bands continued to migrate more slowly than the predicted 116-kDa translation product, suggestive of additional posttranslational modification, such as by O-glycosylation, for which several potential sites are predicted in the N-terminal TSR of the protein. Indeed, treatment with O-glycosidase led to more rapid migration of all ADAMTSL4 molecular species (Fig. 1C). Thus, ADAMTSL4 is a glycoprotein containing both N- and O-linked carbohydrate.
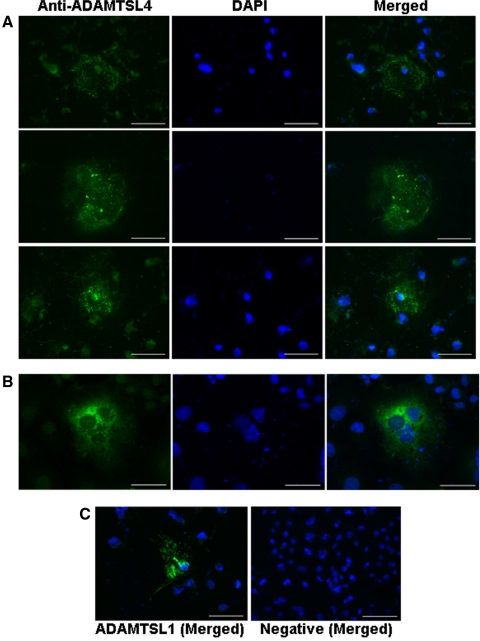
To determine whether, like other ADAMTS-like proteins,21,25,26 ADAMTSL4 bound to the cell-surface or to cell-associated matrix, we used immunofluorescent staining of transiently transfected COS-1 cells with and without previous permeabilization, using the ADAMTSL4 antibody. In nonpermeabilized transfected COS-1 cells, ADAMTSL4 was localized to the extracellular matrix (ECM) underlying cells (subcellular matrix) and to the cell-surface in a finely punctate distribution (Fig. 2A). Staining was seen in only a fraction of cells because transient transfection typically lacks 100% efficiency. In contrast, permeabilization of cells before immunostaining illustrated diffuse cellular staining (Fig. 2B). When the cells were transfected with ADAMTSL1 as a positive control for nonpermeabilized immunostaining, they had punctate distribution in the subcellular matrix, as previously described21 (Fig. 2C, left). Omission of the primary antibody (Fig. 2C, right) or immunostaining of empty vector–transfected cells showed no signal (not shown). Thus, like other ADAMTS-like proteins, ADAMTSL4 appears to be present both in the matrix adjacent to transfected cells (Fig. 2) and in the conditioned medium (Fig. 1).21,25,26
Figure 2.
Immunolocalization of ADAMTSL4 in transiently transfected COS-1 cells. Punctate distribution in (A) ECM of nonpermeabilized cells and (B) cytoplasmic staining in permeabilized cells. (A) The plane of focus in (A) is the substratum underneath the imaged cells. The center row demonstrates staining in the subcellular ECM “footprint” left behind by a detached cell; hence, no DAPI stained nuclei are seen here. As a positive control for the staining procedure, and as a representative member of the ADAMTSL family, a punctate staining pattern in ECM was seen in myc-His6–tagged ADAMTSL1-transfected cells immunostained with anti-myc (C, left). Absence of staining in cells where the primary antibody was omitted (C, right). Scale bars, 50 μm.
ADAMTSL4 Is Widely Distributed in the Human Eye
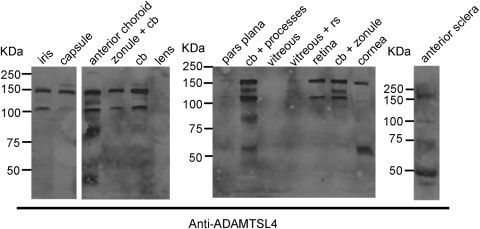
Western blot analysis with ADAMTSL4 antibody demonstrated immunoreactive protein in most ocular components dissected from two normal eyes, with essentially identical results, except for differences in abundance in the lens and sclera, which could be related to efficiency of extraction. The major ∼150-kDa species detected in the eye (Fig. 3) was similar to that identified in transfected cells (Fig. 1). An ∼110-kDa species was seen in most samples that could represent unglycosylated or underglycosylated ADAMTSL4 or proteolytically processed 150-kDa ADAMTSL4 glycoprotein. The 125-kDa species seen in the anterior choroid and ciliary body samples could have had similar origins, whereas the species smaller than 80 kDa seen in choroid, ciliary body, cornea, and sclera most likely represented proteolytic fragments (Fig. 3).
Figure 3.
Western blot analysis of various components dissected from a normal human eye. The source for each sample is indicated above the corresponding lanes. The molecular weight markers (in kDa) are shown at the left of each panel. cb, ciliary body; rs, retinal tissue.
ADAMTSL4 Localizes to Cells and Fibrillar ECM Structures of the Eye
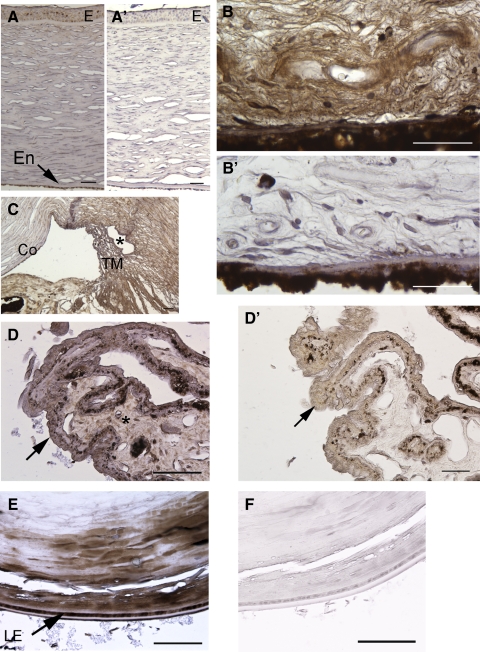
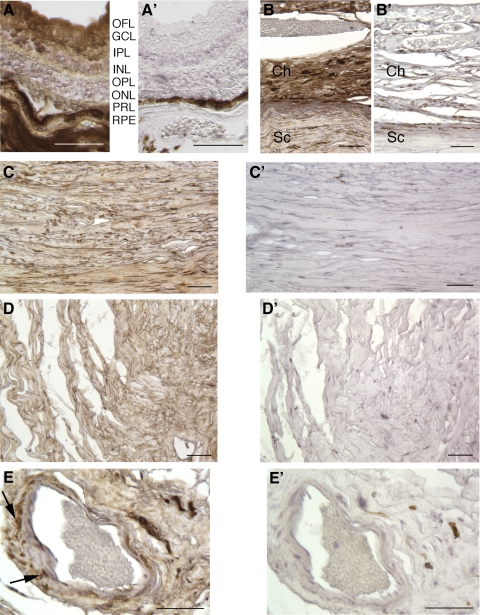
Consistent with Western blot analysis data, immunohistochemistry of sections from the globe showed widespread distribution of the ADAMTSL4 protein, associated with both cells and ECM. In the anterior chamber, staining was seen in the corneal epithelium, stroma, and corneal endothelium (Fig. 4A), fine ECM fibers in the stroma of the iris (Fig. 4B), the trabecular meshwork (Fig. 4C), the stroma of the ciliary body (Fig. 4D), and the periphery of the lens in its equatorial region (Fig. 4E), but not in the lens nucleus. In the posterior chamber, immunostaining was noted in the retina (Fig. 5A), choroid (Fig. 5B), sclera (Fig. 5C), optic nerve (Fig. 5D), and central retinal vessels (Fig. 5E). No staining was seen in the negative control sections (omission of primary antibody; Figs. 4A′, B′, D′, F, 5A′–E′), and there was substantial attenuation of immunostaining signal when the antibody was preincubated with the immunogen peptide (Supplementary Fig. S1B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental).
Figure 4.
Localization of ADAMTSL4 in the anterior segment of a normal human eye. ADAMTSL4 signal is brown, and the section is counterstained with hematoxylin (blue). (A′), (B′), (D′) and (F′) are negative control images corresponding to (A), (B), (D) and (E) were obtained from sections in which the primary antibody was omitted. (A) Cornea. Epithelium (E) and endothelium (En) are indicated. (B) Iris stroma. Signal is primarily seen in fibrillar ECM. (C) Trabecular meshwork (TM). Asterisk: canal of Schlemm. Co, cornea; I, iris. (D) Ciliary body. The unpigmented epithelium is indicated by an arrow. Note that the antibody signal is primarily within the ECM of the ciliary body (see control D′ and Supplementary Fig. S1B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental). (E) Lens shows immunostaining in the lens cortex in the equatorial region. LE, lens epithelium. Scale bars, 50 μm.
Figure 5.
Localization of ADAMTSL4 in the posterior segment of a normal human eye. ADAMTSL4 signal is brown, and the sections are counterstained with hematoxylin (blue). (A′–E′) From sections stained without primary antibody as a negative control. (A–E) Stained sections. (A) Retina. OFL, optic fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor layer, RPE, retinal pigment epithelium. (B) Ch, choroid; Sc, sclera. (C) Sclera. (D) Optic nerve. Note ADAMTSL4 staining in ECM of sclera, choroid, and optic nerve. (E) Retinal blood vessel. Arrows: cellular staining in vascular smooth muscle cells. Scale bars, 50 μm.
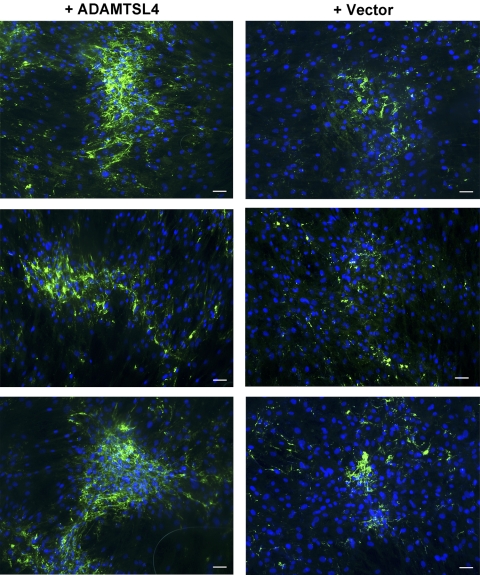
ADAMTSL4 Enhances Fibrillin-1 Deposition in ECM of Cultured Fibroblasts
Identification of ADAMTSL4 mutations in IEL suggested that ADAMTSL4 could have a role in the formation or maintenance of fibrillin-1 microfibrils. Fibroblasts secrete fibrillin-1 into their conditioned medium and deposit it in fibrillar structures representing microfibrils after a period of confluence, where it is detectable by immunofluorescence.27–29 This method has been previously used to illustrate the regulatory roles of heparin,30 fibronectin,31,32 and the ADAMTSL4-related proteins ADAMTSL618 and ADAMTS1019 in microfibril assembly. We used fBNL cells, which are derived from a tissue rich in elastin and microfibrils (nuchal ligament), for this purpose. We consistently observed greater deposition of fibrillin-1 in the presence of ADAMTSL4-conditioned medium than with conditioned medium from cells transfected with the empty vector. This difference included both a greater area of the deposited fibrillar aggregates and a stronger fluorescence intensity (Fig. 6). Given that experiments using ADAMTSL4-conditioned medium and vector-conditioned medium were conducted in parallel, and for the same duration, these data suggest that the presence of ADAMTSL4 accelerates microfibril biogenesis.
Figure 6.
ADAMTSL4 accelerates fibrillin-1 deposition in the matrix of cultured fibroblasts. Fetal bovine nuchal ligament cells (fBNL) cells were cultured to confluence in media that had previously been conditioned by HEK293F cells stably transfected with ADAMTSL4 expression plasmid or empty vector as indicated. After 4 days of culture, cells were fixed and stained for fibrillin-1 (green), and nuclei were stained with DAPI (blue). Three representative fields (20× magnification) from independent experiments are shown for both vector-conditioned and ADAMTSL4-conditioned medium–treated fBNL cells. Note the enhanced fibrillin-1 fluorescence in cells cultured in the presence of exogenous ADAMTSL4. Scale bars, 50 μm.
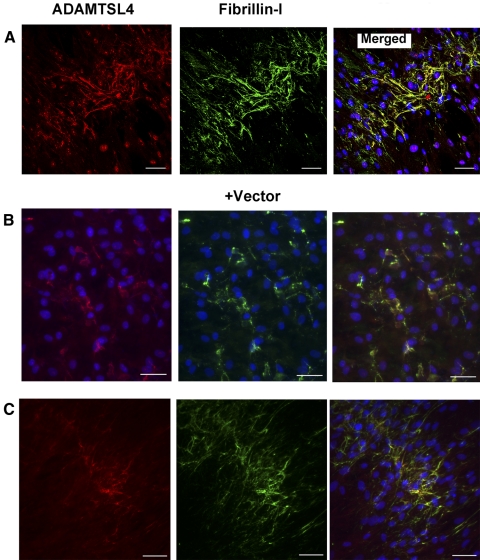
ADAMTSL4 Colocalizes with Fibrillin-1 in Fibroblast Cultures
Using the fBNL cells in the same assay, we performed simultaneous immunofluorescent detection of ADAMTSL4 and fibrillin-1. The data demonstrated that ADAMTSL4 was associated with fibrillin-1–positive structures in ECM (Fig. 7A). In cultures treated with conditioned medium from vector-transfected cells, some costaining of fibrillin-1–positive structures was also seen with the use of ADAMTSL4 antibody (Fig. 7B). HEK293F produced low levels of ADAMTSL4 (Fig. 1B); therefore, we considered the possibility that fBNL cells produced endogenous ADAMTSL4. Using primers specific for bovine ADAMTSL4, we obtained a PCR product of expected size from fBNL cell cDNA (Supplementary Fig. S2A, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental), and its identity was confirmed by DNA sequence analysis. In addition, Western blot analysis using the anti– ADAMTSL4antibody specifically identified a 150-kDa protein band in the fBNL cell lysate; a minor species of 80 kDa was also present (Supplementary Fig. S2B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental). Indeed, immunostaining of confluent fBNL cells grown without vector-conditioned or ADAMTSL4-conditioned medium identified ADAMTSL4 colocalized with fibrillin-1 microfibrils (Fig. 7C), consistent with endogenous ADAMTSL4 production by fBNL cells. Thus, ADAMTSL4 binds fibrillin microfibrils and enhances microfibril biogenesis, properties similar to those previously attributed to its close homolog, ADAMTSL6.24
Figure 7.
ADAMTSL4 colocalizes with fibrillin-1 microfibrils in ECM of cultured fibroblasts. Combined immunofluorescence for ADAMTSL4 (red) and fibrillin-1 (green) was performed after culture of fBNL cells in the presence of ADAMTSL4-conditioned medium or without the addition of conditioned medium. (A) Confocal microscopy. In ADAMTSL4 medium–treated cells, confocal images show that ADAMTSL4 distribution overlaps with fibrillin-1 (yellow, merged image). (B) Conventional fluorescence microscopy. Cells were incubated with vector-conditioned medium. ADAMTSL4 was also detected in these cultures and colocalized with fibrillin-1 immunostaining (yellow), suggesting the production of ADAMTSL4 by fBNL cells or HEK293F cells. (C) fBNL cultures were grown without the addition of any conditioned medium. Note the presence of ADAMTSL4 on fibrillin-1 microfibrils, indicating the production of ADAMTSL4 by fBNL cells (see Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-5955/-/DCSupplemental). Scale bars, 50 μm.
Discussion
Recent progress in the genetics of ectopia lentis suggests the involvement of three members of the ADAMTS superfamily, including ADAMTSL4, in the formation, maintenance, or both of the zonule.12,15,17 Previously, ADAMTSL4 mRNA was shown by RT-PCR to be expressed in many human organs and tissues.12,23 However, the restricted effect of ADAMTSL4 mutations suggested that it had a critical and specific function in the genesis or maintenance of the zonule, which was not compensated by other family members or alternative pathways. Accordingly, we focused on ADAMTSL4 distribution in the eye and on its potential functional relationship with fibrillin-1, the major component of the zonule fibers.
Initially, we expressed ADAMTSL4 in mammalian cells and could demonstrate that it was posttranslationally modified by glycosylation and proteolytic cleavage and that, after secretion, it was localized to the subcellular ECM and conditioned medium, similar to other ADAMTS-like proteins.21,25,33 Expression of human ADAMTSL4 also permitted validation of the ADAMTSL4 antibody as a specific reagent for its identification in tissue extracts by Western blot analysis and tissue sections by immunohistochemistry. The data obtained using these two methods showed that ADAMTSL4 was widely distributed in the eye. Although it was present in the ciliary body and lens capsule in the equatorial region, the structures most relevant to ectopia lentis,2,34,35 ADAMTSL4 antibody also conspicuously stained fibrillar structures in the ECM of other parts of the eye. In addition to specialized ocular structures, we found ADAMTSL4 in the wall of arteries, where fibrlllin-1 is abundant, and in optic nerve ECM. These observations are consistent with previously reported widespread distribution of the ADAMTSL4 mRNA using RT-PCR12,23 and relevant to ADAMTSL4 mutations in ectopia lentis et pupillae.13 Fibrillin-1 is widely expressed in the eye,4 and its reported distribution is similar to that of ADAMTSL4. Although coimmunostaining for ADAMTSL4 and fibrillin-1 in cultured cells demonstrated their colocalization, costaining of eye sections proved to be technically unsatisfactory, possibly because of differing fixation and tissue processing optima for each antibody.
Because ectopia lentis can occur as a consequence of decreased fibrillin-1 (i.e., FBN1 haploinsufficiency) in MFS and dominant IEL, we asked whether ADAMTSL4 functioned in the biogenesis of fibrillin-1 microfibrils. Indeed, the recessive inheritance and diversity of reported ADAMTSL4 mutations11,12 strongly suggested a loss of function effect in recessive IEL. Fibroblasts and other mesenchymal cells that express fibrillin-1 deposit it into their ECM as fibrillar structures in culture once they achieve confluence or high density. Immunostaining of control fBNL cultures demonstrated initial assembly of fibrillin-1 microfibrils after 3 days at confluence. Accordingly, in several independent experiments, we examined the deposition of endogenous fibrillin-1 in cultures supplemented with exogenous ADAMTSL4 and compared them with those supplemented with vector-only medium at 4 days. We observed a consistent increase of fibrillin-1 immunostaining in ECM after ADAMTSL4 supplementation. Furthermore, we observed that ADAMTSL4 was incorporated into ECM structures that stained positive with fibrillin-1 antibodies.
Taken together, the molecular genetic relationship between ADAMTSL4 and fibrillin-1 deficiency that results in IEL, the codistribution of ADAMTSL4 and fibrillin-1 in cultured cells, and the enhancement of fibrillin-1 deposition in ECM by ADAMTSL4 strongly suggest that ADAMTSL4 is a fibrillin-1–binding protein that facilitates microfibril assembly. Intriguingly, in a recent mass spectrometric analysis of zonule and skin microfibrils,1 neither ADAMTSL4 nor ADAMTS10, another ADAMTS protein recently shown to be associated with microfibrils,19 was detected. It is possible that these proteins are of low abundance, are expressed at higher levels during zonule development, or were not retained in the microfibril preparations used for the analysis. The recent demonstration that the ADAMTSL4 homolog ADAMTSL6 bound fibrillin-1 directly18 is consistent with the findings reported here, although a genetic disorder resulting from ADAMTSL6 mutations is unknown. However, the demonstrated direct binding of ADAMTSL6 to fibrillin-1, and the resultant enhancement of microfibril assembly suggest that it should be considered a potential candidate gene for IEL or Weill-Marchesani syndrome. Fibrillin-1 microfibrils have been implicated as having both a regulatory role in the control of TGFβ activity and a mechanical role in cell and tissue anchorage.36–40 Thus, in the eye, where the zonule has a critical mechanical role, the lack of ADAMTSL4 leads to ectopia lentis. It is possible that ADAMTSL4 is specifically required for a mechanical role in the eye in relation to tissue microfibrils or that it is not as critical in microfibril assembly at extraocular sites because of the participation of other ADAMTS proteins. Further studies that will require purified ADAMTSL4 will be undertaken to investigate a possible direct interaction between ADAMTSL4 and fibrillin-1 and to investigate mechanisms by which it enhances microfibril biogenesis.
Supplementary Material
Acknowledgments
The authors thank Mary G. Rayborn for sections.
Footnotes
Supported by National Institutes of Health Grants EY021151, AR53890, and HL107147 (SSA); the National Marfan Foundation (SSA) and Grant EY14240 (JGH); Foundation Fighting Blindness Histopathology and Research Center grant (JGH); an unrestricted grant from Research to Prevent Blindness; and a grant from Vision Institute, Brazil (LG).
Disclosure: L.A.R. Gabriel, None; L.W. Wang, None; H. Bader, None; J.C. Ho, None; A.K. Majors, None; J.G. Hollyfield, None; E.I. Traboulsi, None; S.S. Apte, None
References
- 1. Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122 [DOI] [PubMed] [Google Scholar]
- 2. Hanssen E, Franc S, Garrone R. Synthesis and structural organization of zonular fibers during development and aging. Matrix Biol. 2001;20:77–85 [DOI] [PubMed] [Google Scholar]
- 3. Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wheatley HM, Traboulsi EI, Flowers BE, et al. Immunohistochemical localization of fibrillin in human ocular tissues: relevance to the Marfan syndrome. Arch Ophthalmol. 1995;113:103–109 [DOI] [PubMed] [Google Scholar]
- 5. Kielty CM, Sherratt MJ, Marson A, Baldock C. Fibrillin microfibrils. Adv Protein Chem. 2005;70:405–436 [DOI] [PubMed] [Google Scholar]
- 6. Robinson PN, Arteaga-Solis E, Baldock C, et al. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;43:769–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ades LC, Holman KJ, Brett MS, Edwards MJ, Bennetts B. Ectopia lentis phenotypes and the FBN1 gene. Am J Med Genet A. 2004;126A:284–289 [DOI] [PubMed] [Google Scholar]
- 8. Lonnqvist L, Child A, Kainulainen K, Davidson R, Puhakka L, Peltonen L. A novel mutation of the fibrillin gene causing ectopia lentis. Genomics. 1994;19:573–576 [DOI] [PubMed] [Google Scholar]
- 9. Neuhann TM, Artelt J, Neuhann TF, Tinschert S, Rump A. A homozygous microdeletion within ADAMTSL4 in patients with isolated ectopia lentis: evidence of a founder mutation. Invest Ophthalmol Vis Sci. 2011;52:695–700 [DOI] [PubMed] [Google Scholar]
- 10. Aragon-Martin JA, Ahnood D, Charteris DG, et al. Role of ADAMTSL4 mutations in FBN1 mutation-negative ectopia lentis patients. Hum Mutat. 2010;31:E1622–E1631 [DOI] [PubMed] [Google Scholar]
- 11. Greene VB, Stoetzel C, Pelletier V, et al. Confirmation of ADAMTSL4 mutations for autosomal recessive isolated bilateral ectopia lentis. Ophthalmic Genet. 2010;31:47–51 [DOI] [PubMed] [Google Scholar]
- 12. Ahram D, Sato TS, Kohilan A, et al. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Hum Genet. 2009;84:274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christensen AE, Fiskerstrand T, Knappskog PM, Boman H, Rodahl E. A novel ADAMTSL4 mutation in autosomal recessive ectopia lentis et pupillae. Invest Ophthalmol Vis Sci. 2010;51:6369–6373 [DOI] [PubMed] [Google Scholar]
- 14. Faivre L, Dollfus H, Lyonnet S, et al. Clinical homogeneity and genetic heterogeneity in Weill-Marchesani syndrome. Am J Med Genet. 2003;123A:204–207 [DOI] [PubMed] [Google Scholar]
- 15. Dagoneau N, Benoist-Lasselin C, Huber C, et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 2004;75:801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faivre L, Gorlin RJ, Wirtz MK, et al. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003;40:34–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morales J, Al-Sharif L, Khalil DS, et al. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am J Hum Genet. 2009;85:558–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsutsui K, Manabe RI, Yamada T, et al. A disintegrin and metalloproteinase with thrombospondin motifs-like-6 (ADAMTSL-6) is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J Biol Chem. 2010;285:4870–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kutz WE, Wang LW, Bader HL, et al. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J Biol Chem. 2011;286:17156–17167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirohata S, Wang LW, Miyagi M, et al. Punctin, a novel ADAMTS-like molecule (ADAMTSL-1) in extracellular matrix. J Biol Chem. 2002;277:12182–12189 [DOI] [PubMed] [Google Scholar]
- 22. Somerville RP, Longpre JM, Jungers KA, et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513 [DOI] [PubMed] [Google Scholar]
- 23. Buchner DA, Meisler MH. TSRC1, a widely expressed gene containing seven thrombospondin type I repeats. Gene. 2003;307:23–30 [DOI] [PubMed] [Google Scholar]
- 24. Tsutsui K, Manabe RI, Yamada T, et al. A disintegrin and metalloproteinase with thrombospondin motifs-like-6 (ADAMTSL-6) is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J Biol Chem. 2010;285:4870–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall NG, Klenotic P, Anand-Apte B, Apte SS. ADAMTSL-3/punctin-2, a novel glycoprotein in extracellular matrix related to the ADAMTS family of metalloproteases. Matrix Biol. 2003;22:501–510 [DOI] [PubMed] [Google Scholar]
- 26. Koo BH, Hurskainen T, Mielke K, et al. ADAMTSL3/punctin-2, a gene frequently mutated in colorectal tumors, is widely expressed in normal and malignant epithelial cells, vascular endothelial cells and other cell types, and its mRNA is reduced in colon cancer. Int J Cancer. 2007;121:1710–1716 [DOI] [PubMed] [Google Scholar]
- 27. Dallas SL, Keene DR, Bruder SP, et al. Role of the latent transforming growth factor beta binding protein 1 in fibrillin-containing microfibrils in bone cells in vitro and in vivo. J Bone Miner Res. 2000;15:68–81 [DOI] [PubMed] [Google Scholar]
- 28. Dzamba BJ, Keene DR, Isogai Z, et al. Assembly of epithelial cell fibrillins. J Invest Dermatol. 2001;117:1612–1620 [DOI] [PubMed] [Google Scholar]
- 29. Hollister DW, Godfrey M, Sakai LY, Pyeritz RE. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990;323:152–159 [DOI] [PubMed] [Google Scholar]
- 30. Tiedemann K, Batge B, Muller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparan sulfate, implications for microfibrillar assembly. J Biol Chem. 2001;276:36035–36042 [DOI] [PubMed] [Google Scholar]
- 31. Kinsey R, Williamson MR, Chaudhry S, et al. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704 [DOI] [PubMed] [Google Scholar]
- 32. Sabatier L, Chen D, Fagotto-Kaufmann C, et al. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koo BH, Goff CL, Jungers KA, et al. ADAMTS-like 2 (ADAMTSL2) is a secreted glycoprotein that is widely expressed during mouse embryogenesis and is regulated during skeletal myogenesis. Matrix Biol. 2007;26:431–441 [DOI] [PubMed] [Google Scholar]
- 34. Gloor BP. Proceedings: the incorporation of 3H-labeled amino acids in the zonule of the mouse during development. Exp Eye Res. 1973;17:401–402 [DOI] [PubMed] [Google Scholar]
- 35. McCulloch C. The zonule of Zinn: its origin, course, and insertion, and its relation to neighboring structures. Trans Am Ophthalmol Soc. 1954;52:525–585 [PMC free article] [PubMed] [Google Scholar]
- 36. Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. Regulation of limb patterning by extracellular microfibrils. J Cell Biol. 2001;154:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411 [DOI] [PubMed] [Google Scholar]
- 39. Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbβ and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramirez F, Sakai LY, Dietz HC, Rifkin DB. Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19:151–154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.