Abstract
Foremost amongst the diseases preventable by vaccination is influenza. Worldwide, influenza virus infection is associated with serious adverse events leading to hospitalization, debilitating complications, and death in elderly individuals. Immunization is considered to be the cornerstone for preventing these adverse health outcomes, and vaccination programs are timed to optimize protection during the annual influenza season. Trivalent inactivated influenza virus vaccines are believed to be both effective and cost-saving; however, in spite of widespread influenza vaccination programs, rates of hospitalization for acute respiratory illness and cardiovascular diseases have been increasing in this population during recent annual influenza seasons. From meta-analyses summarizing estimates of influenza vaccine effectiveness from available observational clinical studies, this review aims to examine how effective current influenza vaccine strategies are in the aging and older adult population and to analyze which are the most important biases that interfere with measurements of influenza vaccine effectiveness. Furthermore, consideration is given to strategies that should be adopted in order to optimize influenza vaccine effectiveness in the face of immune exhaustion.
Keywords: influenza vaccine effectiveness, influenza virus infection, immunosenescence, hemagglutinin activity inhibition, innate immunity, hemagglutinin inhibition, older adults
Introduction
Vaccination is considered to be the cornerstone for preventing the morbidity and mortality associated with influenza virus infection. Immunization programs are timed to optimize protection during an influenza season, and in the northern hemisphere a system of annual identification of new strains causing illnesses, selections for vaccines, chick embryo growth, inactivation, processing, packaging, distribution and usage has been in place for decades.1 Current vaccines contain 15 μg of the hemagglutinin of A/H1N1, A/H3N2, and B strains, respectively, and are administered to induce serum antihemagglutinin antibody for prevention of subsequent infection and illness from natural influenza.1,2
In the USA, the Advisory Committee on Immunization Practices recommends universal vaccination (ie, for all persons aged ≥6 months) and the National Advisory Committee on Immunization in Canada as well.2,3 In comparison, vaccination guidelines within the European Union community are much more conservative.4 The yearly influenza vaccination of at-risk individuals is still common practice, and elderly and chronically ill individuals, regardless of age, are the most important target groups,5,6 but with discrepancies between different national government public health recommendations (Austria, Germany, Hungary, and Russia ≥60 years of age; most European countries ≥65 years of age).7 Until now, European policy makers have not found enough evidence to target other groups, but caregivers and health care workers are also strongly advised to receive vaccination.2,4 Only trivalent inactivated influenza virus vaccines are commonly used in Europe;4,8 and live attenuated vaccines, available in the USA since 2003, were only recently approved for use in Europe and are limited to persons aged 2–59 years.4 Trivalent inactivated influenza virus vaccines are therefore widely used throughout the world, with approximately 300 million doses produced each year.9
However, in spite of widespread influenza vaccination programs, vaccine coverage rates are still generally poor and do not meet World Health Organization targets.6 Of course, this could contribute to why influenza infection remains a major public health concern across the world.5,6,10–12 However, it is still unclear, as recently reviewed by Monto,6 if a further increase in vaccine coverage will translate into public health benefits. Thus, annual estimates indicate that influenza infections still cause 3–5 million severe cases, resulting in 250,000 to 350,000 deaths worldwide.1 In the European Union, between 40,000 and 220,000 deaths per year can be attributed to influenza infection, depending on the pathogenicity of the circulating viral strain.5 A review by the National Institutes of Health in 2008 concluded that seasonal influenza virus caused more than 200,000 hospitalizations and 41,000 deaths in the USA every year, and that it was the seventh leading cause of death.13 Older individuals and especially those suffering from chronic medical conditions or immunological disorders account for approximately 90% of all influenza-related deaths.6,13,14 However, mortality is just the tip of the iceberg in terms of disease9,15–18 and economic burden (ie, amounting to $87 billion each year in the USA).19
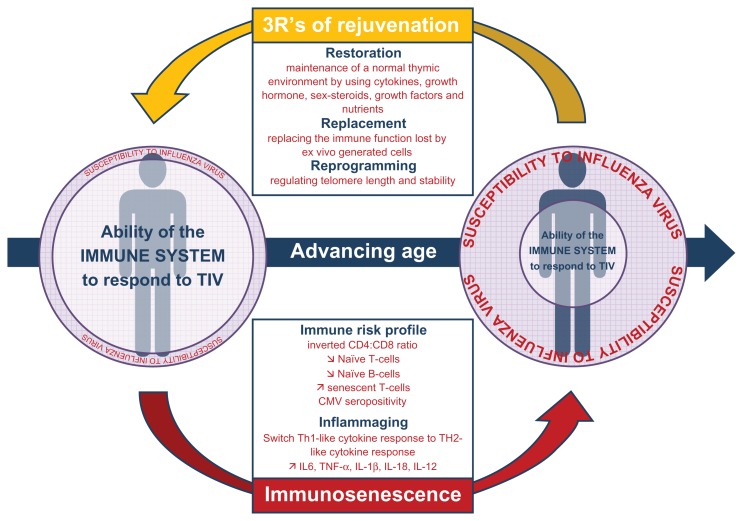
It is largely believed that current influenza vaccines are both effective20 and cost-saving in the aged population.21,22 However, while the trivalent inactivated influenza virus vaccine prevents laboratory-confirmed influenza illness in approximately 70%–90% of healthy adults when the vaccine and circulating virus are antigenically similar,23,24 the picture for older individuals is not as clear. Indeed, few placebo-controlled, randomized clinical trials have been performed, and none have been powered enough to study severe outcomes, including mortality.12 This severely limits what such randomized clinical trials can tell us about the benefits of vaccination.12 The largest and best designed placebo-controlled, randomized clinical trial was done by Govaert et al in The Netherlands during the 1991–1992 influenza season.25 In that study, 1838 healthy volunteers aged ≥60 years were randomly assigned to receive either a placebo or a trivalent inactivated influenza virus vaccine. After stratifying by age, Govaert et al estimated an influenza vaccine effectiveness of 57% in people aged 60–69 years, but of only 23% in volunteers aged ≥70 years. This result suggests that the effect of the vaccine decreases further in this subpopulation, which partly reflects changes in the immune system occurring with advancing age (see Figure 1).10,11
Figure 1.
Development of immunosenescence with advancing age and its impact on the ability of the immune system to respond to influenza virus antigen. Potential impacts of immunotherapy of immunosenescence are also presented.
Abbreviations: CMV, cytomegalovirus; IL, interleukin; TIV, trivalent inactivated influenza vaccine; TNF-α, tumor necrosis factor-alpha.
It has not been possible to resolve this issue for obvious ethical reasons.10 Therefore, current influenza vaccine effectiveness estimates are mainly derived from observational studies, typically using data from research databases or health care utilization data systems.10,26 Based on meta-analyses summarizing these estimates,24,27–32 this review aims to examine how effective current influenza vaccine strategies are and to analyze which are the most important factors modulating the interpretation of study results. Furthermore, consideration is given to current and future strategies to optimize influenza vaccine effectiveness in this higher-risk population.
Literature search strategy
With the aim to identify all meta-analyses estimating the influenza vaccine effectiveness in aging and older adult populations, a literature search was electronically performed in databases via OVID (PUBMED, MEDLINE, EMBASE) from 1990 to 2011 with the following combination of keywords: “(“influenza vaccin*” [MeSH Terms] OR “influenza” [All Fields] AND “vaccine” [All Fields]) OR (“influenza vaccine” [All Fields] OR (“influenza” [All Fields] AND “vaccine” [All Fields]) OR (“influenza vaccine” [All Fields] AND “Humans” [MeSH Terms] AND (“Meta-Analysis” [ptyp]). Appropriate publications from this research were selected on the basis of title/abstract and full text. Only studies of seasonal influenza were considered, and because live attenuated vaccines are not currently available in Europe, the overview was restricted to trivalent inactivated influenza virus vaccines. Studies published in languages other than English or French were not considered. Only randomized and controlled trials with clinical endpoints (efficacy and/or effectiveness versus placebo or no intervention) were considered. Reviews that were not systematic and case-control studies or cohort studies were not included.
What do we know about influenza vaccine effectiveness estimates?
Fedson et al were among the first authors to report influenza vaccine effectiveness against influenza-like illness in terms of hospitalization and death.33 The authors reported that the influenza vaccine prevented 37%–39% of hospital admissions for pneumonia and 27%–30% of all-cause mortality. Subsequent observational studies, as summarized in a 2002 meta-analysis,32 showed similar estimates. According to a recent Cochrane systematic review,28 influenza vaccine effectiveness in a mixed hospital and community population was 41% and 58%, respectively, against laboratory-confirmed influenza. Considering data from the French Sentinel Network, Legrand et al,34 who assessed yearly field vaccine effectiveness during the 1995–2005 epidemic seasons, observed a consistently lower response among older adults (≥65 years) than among people aged 15–64 years. Cases were defined according to criteria of influenza-like illness (sudden onset of fever >39°C with myalgia and respiratory signs). Further examination of influenza vaccine effectiveness in individuals aged ≥65 years was carried out by Rivetti et al.30 These authors performed a review of 64 studies (randomized, quasi-randomized, cohort, and case-control studies assessing efficacy against laboratory-confirmed influenza illness cases or influenza-like illness). Influenza vaccine effectiveness against influenza-like illness was 23% (95% confidence interval [CI] 6–36) and nonsignificant against laboratory-confirmed influenza illness (relative risk [RR] 1.04; 95% CI 0.43–2.51). Well matched vaccines prevented pneumonia (influenza vaccine effectiveness 46%; 95% CI 30–58), hospital admission (influenza vaccine effectiveness 45%; 95% CI 16–64), and deaths from influenza or pneumonia (influenza vaccine effectiveness 42%; 95% CI 17–59). For community-dwelling elderly, trivalent inactivated influenza virus vaccines were not significantly effective against laboratory-confirmed influenza illness (RR 0.19; 95% CI 0.02–2.01), influenza-like illness (RR 1.05; 95% CI 0.6–1.9), or pneumonia (RR 0.88; 95% CI 0.6–1.2). Well matched vaccines prevented hospital admission for influenza and pneumonia (influenza vaccine effectiveness 26%; 95% CI 12–38) and all-cause mortality (influenza vaccine effectiveness 42%; 95% CI 24–55). After adjustment for confounders, vaccine performance was improved for hospitalization for influenza or pneumonia (influenza vaccine effectiveness 27%; 95% CI 21–33), respiratory (influenza vaccine effectiveness 22%; 95% CI 15–28), and cardiac diseases (influenza vaccine effectiveness 24%; 95% CI 18–30), and for all-cause mortality (influenza vaccine effectiveness 47%; 95% CI 39–54). The results also demonstrated that vaccination was most effective for individuals living in institutional settings, and the usefulness of vaccines in the community was modest. Similar results were observed from a study conducted by Nichol et al.20 The influenza vaccine appeared 49% and 32% effective in preventing hospitalizations from pneumonia or influenza and 55% and 64% effective in preventing death from any causes among older adults at low or intermediate risk, respectively. Among older adults at higher risk due to comorbid health conditions, vaccination is 29% and 49% effective in preventing death and hospitalization, respectively. Thus, when influenza vaccine effectiveness among adults is stratified by both age and health status, a different picture emerges in the elderly population. Indeed, in most observational studies, adjustment for underlying health conditions further increased estimates of influenza vaccine effectiveness.4,10,20,28,35–42
Why are the overall benefits of influenza vaccination still hotly debated?
While administrative data sets are recognized as an efficient method to estimate influenza vaccine effectiveness, this is potentially prone to intractable bias,12,43–48 and therefore the validity of estimates derived in this way appears questionable.49 Similarly, observational immunogenicity studies do not seem to provide clearer and stronger outcomes. 50 The significance of an early decrease in primary antibody responses (ie, antihemagglutinin antibody responses induced in previously unvaccinated persons) noted in older adult populations compared with their younger counterparts is also highly debated.51,52 Finally, both approaches highlight serious methodological flaws, ie, variation in definition of influenza cases, use of different clinical endpoints, poor correlates of protection in immunogenicity studies, and potential bias that affect the estimates of influenza effectiveness (being failure to exclude participants with conditions that have an influence on the immune response, those previously vaccinated, and those with high prevaccination titers).11,53
Which factors may interfere with measurement of vaccine effectiveness?
Accurate assessment of influenza vaccine effectiveness can be a challenge due to varying case definitions, use of different clinical endpoints, and poor correlates of protection in immunogenicity studies.9 Diagnostic tests for influenza have varying levels of sensitivity and specificity for influenza-like illness, and this must be considered when interpreting case definitions and the correlation with overall burden of the disease.9 Further complications in diagnosis can occur due to the large number of patients with influenza-like illness who are culture-negative (40%).23,54,55 This further distorts the true extent of the disease burden. When interpreting the findings from observational studies, it is very important to consider not only mortality and hospitalization rates but also other important health outcomes, such as exacerbation of chronic comorbid health conditions, secondary infections that result in physician visits, antibiotic use, and disability.43,45,46,56 Influenza is also a major contributor to functional physical decline,18 and causes exacerbations of pulmonary and cardiovascular disease.9,15 It is also the primary cause of increased mortality among patients with underlying chronic comorbid conditions (ie, acute ischemic heart disease, stroke, and pneumonia) in the winter season.37,57,58
Serologic markers are a complex surrogate of influenza vaccine effectiveness, and notably when birth cohorts are compared. Indeed, the influenza vaccine is constantly changing, and intercohort differences in priming history with regard to one or another of the variants result in extreme heterogeneity in baseline influenza serology.52 Thus, most adults have pre-immunization serum antibody levels and this is because of prior influenza infections and/ or vaccinations, and this affects the serum antihemagglutinin titer and the B cell response to subsequent vaccination. Therefore, the humoral immune responses of young and older adults often differ markedly for some influenza strains (eg, A/H1N1 that circulated between 1918 and 1957 and was reintroduced in 1976) but are more similar for others (eg, A/H3N2 that has circulated since 1968).10 When these differences are taken into account, the elderly appear capable of mounting and maintaining antibody responses similarly to those of younger adults. This is well illustrated by the results of a study conducted by Yu et al,59 who showed that people born in or before 1915 and had brothers and/ or sisters who died during the influenza outbreak of 1918 possessed highly functional, virus-neutralizing antibodies to the 1918 H1N1 virus, nearly 90 years after the pandemic. Therefore, variation in antibody response to vaccination may possibly be attributed to priming experience rather than imaging of the immune system per se. This has also been demonstrated in a recent cross-sectional serological survey conducted in England.60 In 1403 baseline serum samples from 2008 (before the first wave of H1N1 infection), the proportion of samples with an antihemagglutinin titer 1:32 or more (deemed a protective response) ranged from 1.8% in children aged 0–4 years to 31.3% in adults aged 80 years or older. In addition, the proportion of samples with an hemagglutination inhibition titer ≥1:32 between baseline and September 2009 (after the first wave of infection), was 21.3% for children younger than 5 years of age, 42.0% for children aged 5–14 years, and 20.6% for those aged 15–24 years, with no difference between baseline and September in older age groups. However, while the antigen mismatch has important effects on the serum antibody level,59,61,62 this does not necessarily mean that the immune response is not effective.63–65 Indeed, as recently showed by Sasaki et al,65 the inferior antibody response to influenza vaccination in the elderly is primarily due to reduced quantities of vaccine-specific antibodies rather than a lack of antibody avidity and activity. Instead, this clear inverse relationship between preimmunization serum antibody levels and antibody increase after vaccination leads to an underestimation of influenza vaccine effectiveness when the antihemagglutinin titer is used as a surrogate maker of protection and the increase in antihemagglutinin titer as a measure for predicting vaccine efficacy.10,11,50
Evidence of bias in estimates of influenza vaccine effectiveness
All meta-analyses summarizing estimates of influenza vaccine effectiveness have questioned the quality and interpretation of available data.24,27–32 This suggests that biases may be partially or even completely responsible for estimates of influenza vaccine effectiveness.10,12,26,43,45–47,66,67 Jackson et al have assessed the risk of death from any cause and hospitalization for pneumonia or influenza in relation to vaccination in periods before, during, and after influenza seasons.45 The results indicated preferential receipt of vaccine by relatively healthier seniors. Furthermore, adjustment for comorbid health conditions did not control for this bias. These findings have also been noticed by others.12,26,45–48 Thus, a curvilinear relationship between predictors of mortality and vaccination has been depicted,67 with the propensity to obtain vaccine waning with the risk of mortality due to comorbid conditions. Furthermore, functional limitations, such as requiring assistance for bathing for example, have been demonstrated to be associated with a decreased likelihood of vaccination, even in aged persons free of comorbid conditions.46 Overall, these findings suggest that near the end of life, disability appears to be a contributing factor in the decision to receive or not to receive the vaccine.10 Thus, pre-influenza season analyses seem to introduce biases that may not be present in influenza season analyses.41 Indeed, observational studies generally select subjects who are appropriate candidates for the intervention, who all have similar access to the intervention. Thus, persons known to have a short life expectancy may not be offered the vaccine, or may have fewer opportunities to get vaccinated compared with individuals who are relatively healthy throughout the vaccination season.20 In addition, the retrospective assessment of functional status is also a finding leading to a healthy vaccinee bias in observational studies.46 Further findings from Fireman et al showed that after having adjusted for risk factors (ie, older age, chronic conditions, and self-reported health status) mortality before, during, and after nine influenza seasons increased by a similar trajectory over time in both healthier individuals and subgroups at high-risk of death.67 Interestingly, a recent study44 has returned to one of the linked population databases by which vaccine effectiveness against hospitalization and death in the elderly was first assessed.33 Exploring influenza vaccine effectiveness across six influenza seasons (including periods before, during, and after peak activity), the authors demonstrated that changes in immunization habits relative to the preceding years may be a readily accessible and recognizable bias.44 This study illustrates the profound noncomparability of immunized and nonimmunized elderly which is not corrected, but rather is exacerbated, by adjustment for standard confounders. Thus, individuals who were previously immunized and continued to receive the influenza vaccine experienced the lowest hospitalization and mortality rates across all analysis periods; those previously immunized and then not undergoing immunization had a significantly higher likelihood of both hospitalization and death that was evident in advance of the influenza period. Conversely, those who were previously and again immunized were at significantly lower risk at all times. Further, those choosing to be newly vaccinated were at significantly higher risk for hospitalization but not death before and during the influenza season. All these findings provide strong evidence that selection bias is a fatal flaw in many observational estimates in the elderly, and these results do not provide valid evidence on which to estimate the true benefit that may be derived from influenza vaccination campaigns. Finally, accurate assessment of influenza vaccine effectiveness from observational studies is a more arduous and challenging task than initially expected. This was recently confirmed by systematic reviews of existing literature (randomized and quasi-randomized controlled trials comparing influenza vaccines with placebo or no intervention in naturally occurring influenza or relevant reviews) in healthy elderly and older adults with comorbidities or the institutionalized elderly.4,10,24,28,44,50
Can we properly differentiate a true vaccine effect from biases?
To differentiate vaccine effects from biases, Fireman et al have proposed a “difference in differences” approach.67 In other words, if the influenza vaccine really does prevent deaths, then in a large population there should be a detectable difference between the difference in the odds of prior vaccination between decedents and survivors that is observed on days when influenza is circulating and the difference in the odds of prior vaccination between decedents and survivors that would be expected on the same calendar dates if influenza were not circulating. Hence, influenza vaccine effectiveness reduced all-cause mortality among aged adults by 4.6% during the 1996–2005 laboratory-confirmed influenza seasons.67 Whereas it has been found by Simonsen et al that influenza infections accounted at most for 10% of all deaths during the influenza season,12 many cohort studies reported a 50% reduction in the total risk of death in winter.20,38 However, as recently discussed by Nichol,56 the accurate excess deaths/winter-time deaths ratio attributable to influenza is challenging to estimate, due to the lack of available measures at the individual level. This ratio uses a numerator that underestimates influenza-associated deaths due to the difficulty in understanding the true disease burden caused by influenza and a denominator that overestimates deaths during the influenza season.56,68 As a result, this ratio does not accurately reflect the absolute mortality burden attributable to influenza, and is therefore a misleading number for judging the plausibility of influenza vaccine mortality benefit. Similarly, in a recent large population-based nested case-control study, which incorporated a seasonal analysis, influenza vaccination was not significantly associated with a reduction in the risk of community-acquired pneumonia in the aged population.48 While the influenza vaccine effectiveness estimate is consistent with approximations from two recent meta-analyses demonstrating influenza vaccine effectiveness against pneumonia hospitalization,30,32 the width of its confidence interval demonstrates imprecision and a lack of statistical power.10
Thus, the influenza vaccine benefits “controversy” arises from questions about whether residual confounding and biases in observational studies have resulted in influenza vaccine effectiveness estimates that misjudge the true benefit. Without dramatic modification, the current adjustment methods will not adequately control for bias, and the controversy will undoubtedly continue. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates. Future studies should include exploring the strengths and limitations of various comparison periods for model validation, the influence of important potential confounders, and other methods to quantify the impact of potential residual confounding such as sensitivity analyses.56 Complementarily, approaches for reducing bias should include obtaining more accurate information on confounders, such as functional status and life expectancy, avoiding all-cause death in favor of outcomes such as pneumonia or influenza-related pneumonia, and include prospective ascertainment of influenza-specific outcomes to improve study sensitivity to detect a true vaccine effect.26 However, no analytic technique explored could adjust for change in immunization habit, and therefore improved methods to achieve valid interpretation of protection in the elderly are needed.44 In order to improve influenza vaccine effectiveness, age-related changes in the immune system should also be considered. However, while immunosenescence is undoubtedly a very real and important phenomenon that adversely influences vaccine response,49,69–71 how it should be measured and how exactly it influences changes in clinical protection is still poorly understood.10,72
Vaccination of elderly in the face of immune exhaustion: next steps?
This review has demonstrated that the achievement of an accurate assessment of influenza vaccine effectiveness is fraught with considerable methodological and epidemiological challenges. Alternatively, as current trivalent inactivated influenza virus vaccines do not offer optimal direct protection to older adult populations, protecting them indirectly through the effect of herd immunity or enhancing their immune response in order to offer higher and broader protection could be interesting strategies (see Figure 1).9,72,73
Beneficial effects of herd immunity in this ever increasing aging population?
While most vaccines are designed primarily to protect immunized individuals directly, the knockon effect on close contacts, neighbors, and even at the community level when sufficient numbers of the population have been immunized, is protection from the disease. This process, which has been measured and termed “herd immunity,” results in a lower infection rate among unimmunized individuals for infections that are transmitted from person-to-person, such as influenza.73,74 Thus, not everyone needs to be immunized to control the disease, and individuals who fail or reject vaccination or for whom vaccination is less effective, ineffective, or contraindicated would be protected indirectly. The protection afforded by vaccination of older individuals is frequently incomplete because of impaired immune function and/or comorbid conditions,73 so vaccination of health care workers (including ancillary staff and informal caregivers) has been recommended as an additional or alternative strategy. 73 High vaccine coverage rates of influenza immunization in health care workers in extended care facilities may result in lower patient mortality and influenza-like illness.75–80 However, a recent systematic review of randomized controlled trials performed by Thomas et al75,76 concluded that no effect was shown for specific outcomes (ie, laboratory-confirmed influenza, pneumonia, and death from pneumonia) while an effect was shown for nonspecific outcomes (ie, influenza-like illness, general practitioner consultations for influenza-like illness, and all-cause of mortality in 60+ individuals). However, these nonspecific outcomes are difficult to interpret, and these studies suffer from similar limitations and biases to those we have previously mentioned. Moreover, some health care workers remain unvaccinated because they do not perceive influenza infection to be disabling and life-threatening, doubt vaccine efficacy, and are seriously concerned about side effects.81 The vaccine coverage rate was 51% in hospitals in which health care workers were routinely offered vaccine compared with only 5% in those in which they were not.82 This probably explains why mandatory immunization would be the best strategy for increasing the vaccine coverage rate in the health care worker population.81
Providing stronger stimulus for weakening immune response
Stimulation of a primary immune response following vaccination involves the activation of naive lymphocytes by antigen and their differentiation into memory T and B cells and antibody-secreting plasma B cells. Long-term immunity is assured by memory cells in the blood and lymph nodes, as well as by long-lived plasma cells and memory T cells in the bone marrow.83 As depicted in Figure 1, with immunosenescence, older individuals have fewer naive B and T cells, more memory cells, and an ever increasing number of senescent cells which are known to exert a regulatory role in vivo.10,50,73,83 Assuming the immune response becomes gradually weaker with age and immune cells requires a stronger stimulus, novel vaccine formulations have been tested.8,50 While currently licensed adjuvant (MF59, AS03), intradermal, or higher-dose vaccines (15 μg of hemagglutinin versus 30 or 60 μg) enhance vaccine immunogenicity modestly, it is not yet clear how this will translate into protection against all usual influenza-associated outcomes. Safety and tolerability evaluations showed that solicited injection site reactions and systemic adverse events were however more frequent but were typically mild and transient.8 Moreover, these alternative strategies mainly focus on the initial steps of the immunological process of the vaccine response, and therefore overstimulate the naive cell pool that is reduced the most during the immunosenescence process,83 without consideration of the pool that affects the immune response the most, ie, senescent cells.73
Identify the problem and fix it
Because thymic atrophy is the main preceding event in all cases of immunosenescence,10 different ways have been explored regarding how best to rejuvenate the peripheral T cell pool and delay or reverse the immune decline.72 The different approaches of rejuvenating can be categorized into the “3Rs” of rejuvenation, ie, restoration, replacement, and reprogramming (see Figure 1).72 Restoration strategies aim to maintain a normal thymic environment by using cytokines, growth hormone, sex steroids, growth factors, and nutrients that are considered as potential immune rejuvenators. Replacement strategies aim to restore lost immune function by several techniques, including the transfusion of autologous blood derived from an individual during their early life and transfused when they are much older. Such an approach involves the long-term storage of blood-derived lymphocytes, much as is currently done for cord blood stem cell banking. Alternatively, restoration can involve transferring ex vivo- generated naive T cells into individuals with defective thymopoiesis or applying the adoptive transfer procedure usually used in hemopoietic stem cell transplantation. Finally, and probably the most “ revolutionary” treatment, could involve reprogramming the ageing immune system. Thus, pharmacologic approaches to enhance telomerase are currently being addressed as a possible means for prevention or retardation of replicative senescent cells.50 In addition, it has also been proposed to physically remove senescent cells from the circulation and/or induce apoptosis with the hope of inducing homeostatic expansion of a more functional population of memory T cells.10
Although the 3Rs have only been achieved in experimental systems, they raise questions regarding their future translation into the clinic.72 How could immunosenescent individuals or those who are at risk for immunosenescence be identified? What would be an appropriate point of intervention depending on one’s susceptibility rating? Even though a selection of promising parameters could provide a guideline for an individual’s immunosenescence status, no testable hypothesis to approach the difficult definition of immunosenescence is currently available.73
Conclusion
Rising death and hospitalization rates due to influenza over recent decades in spite of a substantial increase of trivalent inactivated influenza virus vaccine coverage rates among the elderly greatly contrast with initial described benefits from influenza vaccination in this population. Beyond the lack of methods able to deal properly with bias that interfere with the accuracy of influenza vaccine effectiveness measurement, this review calls for a greater understanding of how age-related changes and their interaction with common chronic comorbid conditions interfere with the vaccine response. In addition, and in order to validate future influenza vaccines and/or immunological therapeutic approaches enhancing protection in this population in head-to-head clinical trials, there is still no gold standard against which to predict the impact of aging on vaccine response. Moreover, while immunosenescence undoubtedly interferes with the ability of the immune system to respond properly to vaccination, predicting individual responsiveness using strong biological makers that distinguish between healthy and immunosenescent states is also desirable.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization (WHO) Influenza (Seasonal) Fact sheet N°211. Apr, 2009. [Accessed November 30, 2011]. [updated 2011]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/
- 2.Centers for Disease Control and Prevention (CDC) Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011;60(33):1128–1132. [PubMed] [Google Scholar]
- 3.National Advisory Committee on Immunization. Statement on seasonal influenza vaccine for 2011–2012. [Accessed October 30, 2011];Canada Communicable Disease Report (CCDR) 2011 37(ACS5):1–55. doi: 10.14745/ccdr.v37i00a05. [updated 2011]. Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/11vol37/acs-dcc-5/assets/pdf/acs-dcc-5-eng.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michiels B, Govearts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine. 2011;29(49):9159–9170. doi: 10.1016/j.vaccine.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention Control. Seasonal human influenza and vaccination. [Accessed October 30, 2011]. [updated 2011]. Available from: http://www.ecdc.europa.eu/en/press/Press%20Releases/071012_PR_SI_Prof_Fact_Sheet.pdf.
- 6.Monto AS. Seasonal influenza and vaccination coverage. Vaccine. 2010;28(Suppl 4):D33–44. doi: 10.1016/j.vaccine.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Michel JP, Lang PO, Baeyens JP. Flu vaccination policy in old adults: need for harmonization of national public health recommendations throughout Europe. Vaccine. 2009;27(2):182–183. doi: 10.1016/j.vaccine.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 8.Parodi V, de Florentiis D, Martini M, Ansaldi F. Inactivated influenza vaccines: recent progress and implication for the elderly. Drugs Aging. 2011;28(2):93–106. doi: 10.2165/11586770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Monto AS, Ansaldi F, Aspinall R, et al. Influenza control in the 21st century: optimizing protection for older adults. Vaccine. 2009;27(37):5043–5053. doi: 10.1016/j.vaccine.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Lang PO, Govind S, Mitchell WA, Siegrist CA, Aspinall R. Vaccine effectiveness in older individuals: What has been learned from influenza-vaccine experience. Ageing Res Rev. 2011;10(3):389–395. doi: 10.1016/j.arr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Lang PO, Govind S, Mitchell WA, et al. Influenza vaccine effectiveness in aged individuals: the role played by cell-mediated immunity. Eur Geriatr Med. 2010;1(2):233–238. [Google Scholar]
- 12.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7(10):658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 13.Beigel JH. Influenza. Crit Care Med. 2008;36(9):2660–2666. doi: 10.1097/CCM.0b013e318180b039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Influenza vaccines. Wkly Epidemiol Rec. 2005;80(33):279–287. [PubMed] [Google Scholar]
- 15.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl 1):S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg HB, Piedra PA. Immunization against viral respiratory disease: a review. Pediatr Infect Dis J. 2004;23(Suppl 11):S254–S261. doi: 10.1097/01.inf.0000144756.69887.f8. [DOI] [PubMed] [Google Scholar]
- 17.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2(11):659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 18.Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med. 1998;158(6):645–650. doi: 10.1001/archinte.158.6.645. [DOI] [PubMed] [Google Scholar]
- 19.Molinari NA, Ortega-Sanchez IR, Messonier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 21.Deans GD, Stiver HG, McElhaney JE. Influenza vaccines provide diminished protection but are cost-saving in older adults. J Intern Med. 2010;267(2):220–227. doi: 10.1111/j.1365-2796.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 22.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Influenza vaccination health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am J Prev Med. 2006;31(1):72–79. doi: 10.1016/j.amepre.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2004;53(RR-06):1–40. [Google Scholar]
- 24.Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007:CD001269. doi: 10.1002/14651858.CD001269.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665. [PubMed] [Google Scholar]
- 26.Nelson JC, Jackson ML, Weiss NS, Jackson LA. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among senior. J Clin Epidemiol. 2009;62(7):687–694. doi: 10.1016/j.jclinepi.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366(9492):1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010:CD004876. doi: 10.1002/14651858.CD004876.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2011;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 30.Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123(7):518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20(13–14):1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 33.Fedson DS, Wajda A, Nicol JP, Hammond GW, Kaiser DL, Roos LL. Clinical effectiveness of influenza vaccination in Manitoba. JAMA. 1993;270(16):1956–1961. [PubMed] [Google Scholar]
- 34.Legrand J, Vergu E, Flahault A. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine. 2006;24(44–46):6605–6611. doi: 10.1016/j.vaccine.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 35.Mullooly JP, Bennett MD, Hornbrook MC, et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med. 1994;121(12):947–952. doi: 10.7326/0003-4819-121-12-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Nordin J, Mullooly J, Poblete S, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001;184(6):665–670. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- 37.Nichol KL, Nordin J, Mullooly J, et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 38.Voordouw BC, van der Linden PD, Simonian S, van der Lei J, Sturkenboom MC, Stricker BH. Influenza vaccination in community-dwelling elderly: impact on mortality and influenza-associated morbidity. Arch Intern Med. 2003;163(9):1089–1094. doi: 10.1001/archinte.163.9.1089. [DOI] [PubMed] [Google Scholar]
- 39.Hak E, Verheij TJ, Grobbee DE, Nichol KL, Hoes AW. Confounding by indication in non-experimental evaluation of vaccine effectiveness: the example of prevention of influenza complications. J Epidemiol Community Health. 2002;56(12):951–955. doi: 10.1136/jech.56.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hak E, Buskens E, van Essen GA, et al. Clinical effectiveness of influenza vaccination in persons younger than 65 years with high-risk medical conditions: the PRISMA study. Arch Intern Med. 2005;165(3):274–280. doi: 10.1001/archinte.165.3.274. [DOI] [PubMed] [Google Scholar]
- 41.Hak E, Hoes AW, Nordin J, Nichol KL. Benefits of influenza vaccine in US elderly – appreciating issues of confounding, bias and precision. Int J Epidemiol. 2006;35(3):800–802. doi: 10.1093/ije/dyl068. [DOI] [PubMed] [Google Scholar]
- 42.Vila-Corcoles A, Rodriguez T, de Diego C, et al. Effect of influenza vaccine status on winter mortality in Spanish community-dwelling elderly people during 2002–2005 influenza periods. Vaccine. 2007;25(37–38):6699–6707. doi: 10.1016/j.vaccine.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson LA. Influenza vaccination and mortality benefits: new insights, new opportunity. Vaccine. 2009;27(45):6300–6304. doi: 10.1016/j.vaccine.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Hottes TS, Skowronski DM, Hiebert B, et al. Influenza vaccine effectiveness in the elderly based on administrative databases: change in immunization habit as a marker for bias. PLoS One. 2011;6(7):e22618. doi: 10.1371/journal.pone.0022618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 46.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35(2):345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 47.Jackson LA. Benefits of examining influenza vaccine associations outside of influenza season. Am J Respir Crit Care Med. 2008;178(5):439–440. doi: 10.1164/rccm.200805-805ED. [DOI] [PubMed] [Google Scholar]
- 48.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested casecontrol study. Lancet. 2008;372(9636):398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 49.Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–429. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McElhaney JE. Influenza vaccine response in older adults. Ageing Res Rev. 2011;10(3):379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccine in the elderly: a quantitative review. Vaccine. 2006;24(8):6808–6811. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 52.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989;7(5):385–394. doi: 10.1016/0264-410x(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 53.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197(4):490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- 54.Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50(5):747–751. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebell MH, Alfonso A. A systematic review of clinical decision rules for the diagnosis of influenza. Ann Fam Med. 2011;9(1):69–77. doi: 10.1370/afm.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nichol KL. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine. 2009;27(45):6305–6311. doi: 10.1016/j.vaccine.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Reichert T, Simonsen L, Shama A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 58.Fleming DM, Cross KW, Pannell RS. Influenza and its relationship to circulatory disorders. Epidemiol Infect. 2005;133(2):255–262. doi: 10.1017/s0950268804003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X, Tsibane T, McGraw PA, et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455(7212):532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375(9720):1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 61.Lang PO. Inactivated influenza vaccines: pre-vaccination haemagglutinin-antibody titres influence the vaccine response but not necessarily the vaccine effectiveness. Drugs Aging. 2011;28(6):505. doi: 10.2165/11592350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3(8):e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 2011;204(12):1879–1885. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 64.Feng J, Gulati U, Zhang X, et al. Antibody quantity versus quality after influenza vaccination. Vaccine. 2009;27(45):6358–6362. doi: 10.1016/j.vaccine.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121(8):3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jefferson T. Influenza vaccination: policy versus evidence. BMJ. 2006;333(7574):912–915. doi: 10.1136/bmj.38995.531701.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter M. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;170(5):650–656. doi: 10.1093/aje/kwp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson WW, Moore MR, Weintraub E, et al. Estimating influenza-associated deaths in the United States. Am J Public Health. 2009;99(Suppl 2):S225–S230. doi: 10.2105/AJPH.2008.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21(3):201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 70.Fulop T, Pawelec G, Castle S, Loeb M. Immunosenescence and vaccination in nursing home. Clin Infect Dis. 2009;48(4):443–448. doi: 10.1086/596475. [DOI] [PubMed] [Google Scholar]
- 71.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–193. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 72.Govind S, Lapenna A, Lang PO, Aspinall R. Immunotherapy of immunosenescence: who and how? Open Longev Sci. 2012 In press. [Google Scholar]
- 73.Lang PO, Aspinall R. Immunosenescence and herd immunity: with an ever increasing aging population do we need to rethink vaccine schedules? Expert Rev Vaccines. 2012 doi: 10.1586/erv.11.187. In press. [DOI] [PubMed] [Google Scholar]
- 74.Lang PO, Govind S, Michel JP, Aspinall R, Mitchell WA. Immunosenescence: Implications for vaccination programmes in adults. Maturitas. 2011;68(4):322–330. doi: 10.1016/j.maturitas.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev. 2010:CD005187. doi: 10.1002/14651858.CD005187.pub3. [DOI] [PubMed] [Google Scholar]
- 76.Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who work with the elderly: systematic review. Vaccine. 2010;29(2):344–356. doi: 10.1016/j.vaccine.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 77.Lemaitre M, Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc. 2009;57(9):1580–1586. doi: 10.1111/j.1532-5415.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 78.Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ. 2006;333(7581):1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet. 2000;355(9198):93–97. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- 80.Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175(1):1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talbot TR, Dellit TH, Hebden J, Sama D, Cuny J. Factors associated with increased healthcare worker influenza vaccination rates: results from a national survey of university hospitals and medical centers. Infect Control Hosp Epidemiol. 2010;31(5):456–462. doi: 10.1086/651666. [DOI] [PubMed] [Google Scholar]
- 82.Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect. 2009;58(6):446–458. doi: 10.1016/j.jinf.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Weiskopf D, Weinberger D, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22(11):1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]