Abstract
MicroRNAs have recently been identified as regulators that modulate target gene expression and are suggested to be involved in the development and progression of endometriosis. This study was undertaken to analyze the expression level of microRNA-199a (miR-199a) in paired ovarian endometrioma and eutopic endometrium from women with endometriosis, and to investigate the contribution of miR-199a to the invasive capability of endometrial stromal cells (ESCs). Cell adhesion, migration and Matrigel invasion assays were carried out to measure the invasiveness of ESCs. Bioinformatics prediction, reporter gene assay, PCR, western blotting and ELISA were performed to identify miR-199a targets and related signaling pathways. The results showed that the expression level of miR-199a was lower in the eutopic endometrium from women with endometriosis, and even lower in the paired ovarian endometrioma, compared with the expression in normal controls. Moreover, ectopic expression of miR-199a attenuated ESC adhesion, migration and invasiveness. MiR-199a targeted and inhibited IkappaB kinase beta (IKKβ) in ESCs. Accompanied by IKKβ reduction, miR-199a suppressed nuclear factor-kappa B (NF-κB) pathway activation and interleukin-8 (IL-8) production in ESCs. All these findings suggest that miR-199a, down-regulated in endometriosis, attenuates the invasive capability of ESCs in vitro partly through IKK/NF-κB pathway suppression and reduced IL-8 expression. In conclusion, miR-199a could be involved in the pathogenesis of endometriosis.
Keywords: endometriosis, miR-199a, invasion, nuclear factor-kappa B, interleukin-8
Introduction
Endometriosis, characterized by the presence and growth of functional endometrial-like tissues outside the uterus, is a common, chronic and estrogen-dependent gynecological disorder associated with pelvic pain and infertility. An understanding of the pathogenesis of endometriosis has steadily improved, but the molecular mechanisms mediating endometriosis are just beginning to be elucidated. In particular, epigenetic factors have been suggested as a regulatory source promoting endometriosis development, including the expression and function of microRNAs (miRNAs) (Guo, 2009; Qian et al., 2009; Teague et al., 2010; Hawkins et al., 2011).
MiRNAs are small non-coding RNAs of 20–22 nucleotides, which post-transcriptionally regulate gene expression and can control a broad spectrum of normal and pathological cellular functions (Djuranovic et al., 2011; Huntzinger and Izaurralde, 2011). First discovered in 2003 (Lagos-Quintana et al., 2003; Lim et al., 2003), microRNA-199a (miR-199a) has been shown to be down-regulated in several cancerous tissues and to contribute to various malignant processes, such as tumor invasion, metastasis and angiogenesis (Shen et al., 2010; Cheung et al., 2011). Furthermore, miR-199a can target IkappaB kinase beta (IKKβ) (Chen et al., 2008), a co-factor required for nuclear factor-kappa B (NF-κB) pathway activation, which may drive the expression of several genes related to malignant transformation. Recently, multiple published studies have identified differentially expressed miRNAs in endometriotic tissues (Pan et al., 2007; Toloubeydokhti et al., 2008; Burney et al., 2009; Ohlsson Teague et al., 2009; Filigheddu et al., 2010; Hawkins et al., 2011; Ramon et al., 2011), and the results of one research study suggest that miR-199a may decrease in endometriosis (Pan et al., 2007). However, to the best of our knowledge, the expression level of miR-199a in endometriosis has not been validated and the role of miR-199a in the disease remains largely unknown.
Numerous in vitro and in vivo studies have suggested that NF-κB plays an important role in regulating key cell processes that may contribute to the initiation and progression of endometriosis, such as cell adhesion, migration, invasion and angiogenesis (Gonzalez-Ramos et al., 2010; Zhang et al., 2010, 2011). NF-κB is composed of homo- and heterodimers of five members of the Rel family including NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB and c-Rel (Rel). NF-κB p50/p65 is generally considered to be the predominant and inducible form of NF-κB in most cells, and the general term NF-κB traditionally refers to the p50/p65 heterodimer (McKay and Cidlowski, 1999; Karin et al., 2004). For this reason, throughout the remainder of this text, the term NF-κB will refer to the NF-κB p50/p65 heterodimer unless otherwise specified. In normal resting cells, NF-κB is sequestered in the cytoplasm by virtue of binding to inhibitor of kappa B (IκB). IKKβ is the major kinase controlling canonical pathway of NF-κB activation, in which phosphorylation of IκB by IKK releases NF-κB to enter nucleus, where it binds to cognate sequences in the promoter region of multiple genes (Karin, 1999).
Interleukin-8 (IL-8) is an NF-κB inducible protein, which functions as a potent angiogenic agent and a major chemotactic protein for neutrophils and T cells (Heidemann et al., 2003; Himmel et al., 2011). In addition to its angiogenic and chemotactic properties, IL-8 stimulates the proliferation and metastasis of various cell types including lung caner, melanoma and renal cell carcinoma (Luppi et al., 2007; Gabellini et al., 2009; Huang et al., 2010). IL-8 expression has been shown to be elevated in both endometrium and peritoneal fluid of women with endometriosis (Ulukus et al., 2009; Kuroda et al., 2010). Moreover, IL-8 stimulates the proliferation of endometrial stromal cells (ESCs) (Arici et al., 1998). It also enhances the adhesion of ESCs to fibronectin and increases the invasiveness of ESCs to the extracellular matrix (ECM) (Garcia-Velasco and Arici, 1999; Mulayim et al., 2004), thus it may be operative in the attachment of endometrial fragments to the peritoneal lining and their development to endometrial lesions.
In this study, we hypothesized that miR-199a may be involved in the pathogenesis of endometriosis by controlling endometrial cell invasiveness via regulating IKK/NF-κB pathway activity and IL-8 secretion. Therefore, the expression levels of miR-199a in endometrial tissues from women with or without endometriosis were compared, and the invasion ability of miR-199a transfected ESCs was measured. Further, we have identified a miR-199a regulated pathway that is likely to contribute to the invasiveness of ESCs.
Materials and Methods
Human subject characteristics
The study included 12 women with endometriosis (mean age: 31.6 years; range 24–38). All the women underwent surgical examination of the abdominal cavity and a complete excision of the endometriotic tissue was performed. The presence of the disease was confirmed by the surgical findings and the post-operative pathological examination. Among these cases, 12 (100%) have ovarian endometriosis (defined as biopsy-proven endometrioma), 5 (41.7%) have peritoneal endometriosis (defined as biopsy-proven serosal implant), 2 (16.7%) have rectovaginal endometriosis (defined as posterior cul de sac obliteration due to endometriotic lesions) and 1 (8.3%) have uterine leiomyomas. The endometriosis was identified as Stages III–IV disease according to the Revised American Fertility Society classification system. The control group was composed of 12 women (mean age: 34.4 years; range 22–42) without endometriosis, who underwent surgery for uterine prolapse (8.3%), uterine leiomyomata (33.3%), tubal factor infertility (41.7%) or tubal sterilization (16.7%). The absence of the disease was confirmed after surgical examination of the abdominal cavity. The menstrual phase was identified according to the day of the reproductive cycle and the histological analysis of the endometrium. Of women with endometriosis, 10 (83.3%) were in the proliferative phase and 2 (16.7%) were in the secretory phase of the menstrual cycle. In the control group, nine (75%) controls were in the proliferative phase and three (25%) were in the secretory phase of the menstrual cycle. Patients with irregular menstruation or women who had been pregnant or breast-feeding in the previous 6 months were excluded from the study. None of the women had received hormonal treatment for at least 3 months before the study. Informed consent was obtained from all patients and controls using protocols approved by Institutional Review Board of Shanghai Jiaotong University.
Tissue collection, cell culture and transfection
Paired ovarian endometriomas and endometrial biopsies (eutopic endometrium) were obtained from 12 patients with endometriosis, and control endometrium was collected from 12 patients without endometriosis. A portion of eutopic endometrial tissues from women with endometriosis were used for isolation and culturing of primary ESCs as previously described (Brosens et al., 1999; Mulayim et al., 2004). The isolated cells were cultured in Dulbecco's Modified Eagle's Medium/Ham's Nutrient Mixture F-12 containing 10% fetal bovine serum (FBS) and incubated at 37°C in a humidified 5% CO2 incubator. MiR-199a mimics and negative control (NC) miRNA were synthesized by Shanghai GenePharma Company. The sequence of mimics and NC are as follows: miR-199a mimics 5′-CCCAGUGUUCAGACUACCUGUUC-3′ (forward), 5′-ACAGGUAGUCUGAACACUGGGUU-3′ (reverse); NC 5′-UUCUCCGAACGUGUCACGUTT-3′ (forward), 5′-ACGUGACACGUUCGGAGA ATT-3′ (reverse). MiR-199a mimics (50 nM) or NC (50 nM) were transfected into ESCs using lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions.
Quantitative real-time RT–PCR analysis
Total RNA was isolated by TRIzol Reagent (Invitrogen). TaqMan kit (Applied Biosystems, Foster City, CA, USA) specified for quantification of miRNA was used to assess the expression of miR-199a and U6. SYBR Green quantitative RT–PCR (qRT-PCR) was performed to detect IL-8 and glyceraldehyde-3-phosphatedehydrogenase (GAPDH). Relative expression levels were calculated using the 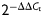 method. Primers used were as follows: for GAPDH, 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse); for IL-8, 5′-TCAGAGACAGCAGAGCACACAAGC-3′ (forward) and 5′-CACACAGTGAGATGGTTCCTTCCG-3′ (reverse).
method. Primers used were as follows: for GAPDH, 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse); for IL-8, 5′-TCAGAGACAGCAGAGCACACAAGC-3′ (forward) and 5′-CACACAGTGAGATGGTTCCTTCCG-3′ (reverse).
Cell adhesion assay
At 48 h post-transfection, ESCs (2 × 104) were allowed to adhere to the Matrigel (BD Bioscience, Bedford, MA, USA) coated well at 37°C for 1 h. After incubation, each well was washed five times with phosphate-buffered saline (PBS) and the cells remaining attached to the Matrigel were fixed, stained and counted under a microscope at ×200 magnification.
Cell migration assay
At 48 h post-transfection, cells (5 × 104) were placed into the upper wells of the Boyden chamber (Millicell, 8-µm pore size, 12-mm diameter; Millipore, Billerica, MA, USA). The medium containing 20% FBS was added to the lower chamber. After 8 h of incubation, cells that had invaded through the 8-μm pore size membrane were fixed, stained and counted under a microscope at ×200 magnification.
Matrigel invasion assay
At 48 h post-transfection, cells (5 × 104) were placed into the upper wells of the Boyden chamber that had been coated with 50 μl of Matrigel (1:3 dilution in serum-free medium). Medium supplemented with 20% serum was added to the outer cup. After 24 h of incubation, cells that had invaded through the Matrigel and the 8-μm pore size membrane were fixed, stained and counted.
Reporter gene assay
A sequence containing miR-199a-predicted target within the IKKβ 3′ (untranslated region) UTR CGCCTTGTCTGCACACTGGAGGTCCTCCATT or a mutant sequence lacking any complementarity with miR-199a seed sequence CGCCTTGTCTGCTGTGACCAGGTCCTCCATT were cloned in the 3′UTR of the luciferase gene, generating Luc.IKKβ and Luc.control vectors, respectively. All the luciferase report plasmids used in the experiment were confirmed by restriction enzyme digestion and DNA sequence analysis. ESCs were transfected with these constructs (200 ng/ml), using lipofectamine 2000, in the presence of miR-199a or NC miRNA (50 nM). After 48 h, luciferase activity was measured using Dual-Light luminescent reporter gene assay.
Western blot analysis
At 48 h post-transfection, total cell lysates were extracted by radio immunoprecipitation assay buffer, while cytoplasmic and nuclear protein extracts were prepared using the Nuclear and Cytoplasmic Extraction Reagents Kit (Pierce, Appleton, WI, USA) according to the supplier's instructions. Antibodies against IKKβ, NF-κB (p65), IκB-α, IκB-α-Ser32P were purchased from Cell Signaling Technology, Danvers, MA, USA, and antibodies against GAPDH and nucleolin were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Western blotting was performed as previously described (Dai et al., 2009).
Immunofluorescence
At 48 h post-transfection, cells were fixed for 5 min in 4% paraformaldehyde in PBS followed by permeabilization with cold methanol for 10 min. Cells were then blocked with 2% bovine serum albumin and 1% goat serum and incubated with rabbit anti-p65 antibody followed by secondary Alexa Fluor 555-conjugated anti-rabbit immunoglobulin G. Cell nuclei were also stained with diamidino-2-phenylindole hydrochloride (DAPI) for 5 min at 37°C. Immunofluorescent samples were examined under confocal laser scanning microscope. The staining intensity of the p65 in the nuclear area was quantified by using Image J software (National Institutes of Health, Bethesda, MD, USA).
ELISA assay
At 48 h post-transfection, cell culture supernatants were collected, centrifuged, aliquoted and immediately stored at −80°C. A commercially available ELISA (R&D Systems, Hornby, ON, Canada) was used to determine the IL-8 in the supernatants.
Statistical analysis
Each experiment comparing the effects of different treatments used the same endometrial sample, and each experiment was repeated at least three times on different specimens. Data were presented as mean ± SD and analyzed by SPSS software using non-parametric statistical analysis (Mann–Whitney U-test for independent comparisons and Wilcoxon signed-ranks test for paired comparisons). A P < 0.05 was defined as statistically significant.
Results
MiR-199a is reduced in paired eutopic endometrium and ovarian endometrioma from patients with endometriosis
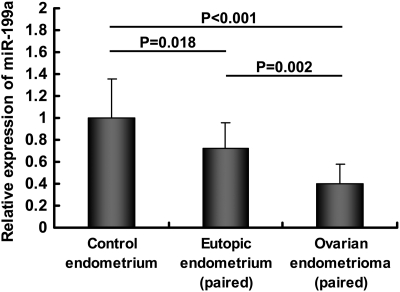
TaqMan qRT-PCR analysis was used to compare the miR-199a expression levels in 12 paired eutopic endometrium and ovarian endometrioma from patients with endometriosis and 12 normal control endometrium. As shown in Fig. 1, the expression level of miR-199a was lower in the eutopic endometrium from women with endometriosis, and even lower in the paired ovarian endometrioma, compared with the expression in normal controls.
Figure 1.
The relative expression levels of miR-199a in 12 paired eutopic endometrium and ovarian endometrioma from patients with endometriosis and in 12 endometrium from women without endometriosis (control endometrium). Data are expressed as mean ± SD miR-199a expression is presented as fold change relative to the women without endometriosis group (control endometrium = 1). Mann–Whitney U-test was used to draw comparisons between endometrial tissues from controls and endometriosis patients; while Wilcoxon signed-ranks test was used to draw comparisons between paired ovarian endometrioma and eutopic endometrium from endometriosis patients.
Ectopic expression of miR-199a attenuates ESC invasiveness
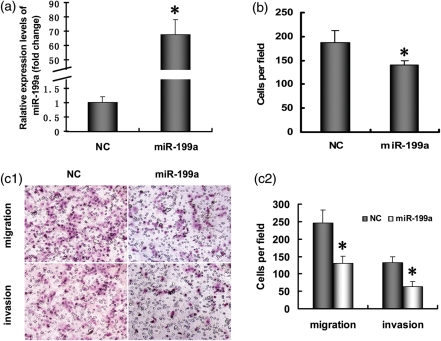
Cell adhesion, migration and Matrigel invasion assays were carried out to measure the invasiveness of ESCs. The potential effect of miR-199a on cell adhesion to ECM was assessed by using Matrigel, which contains almost all of the ECM components. At 48 h post-transfection, ESCs were harvested and plated to wells precoated with Matrigel. We found that the number of adherent ESCs was significantly decreased after miR-199a transfection (Fig. 2b). Cell migration and Matrigel invasion assays were used to evaluate the change in migration and invasion ability of ESCs after miR-199a transfection, and the results showed that miR-199a significantly decreased the migration and invasion activity of ESCs (see Fig. 2c1 and c2).
Figure 2.
MiR-199a suppressed ESC invasiveness. (a) qRT-PCR analysis of miR-199a in ESCs at 24 h post-transfection. (b) Cell adhesion assay. At 48 h post-transfection, cells were added to a precoated 96-well plate containing Matrigel and allowed to adhere for 1 h at 37°C. The number of attached cells was counted under a microscope at ×200 magnification. (c) Cell migration and invasion assay. (c1) Representative hematoxylin and eosin staining fields of migrated or invaded cells on the membrane (c2) Average number of the migrated or invaded cells from triplicate cultures. The results are shown as the mean ± SD from triplicate cultures. (*P<0.05 versus NC group).
MiR-199a targets and inhibits IKKβ in ESCs
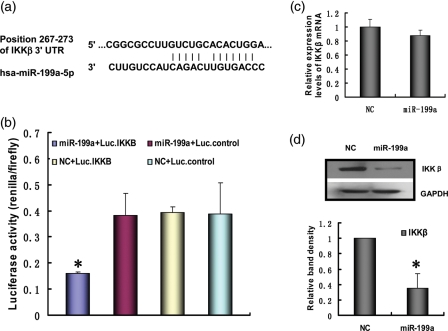
Computational prediction of targets by TargetScan software identified IKKβ as a miR-199a target. Figure 3a shows the alignment between miR-199a and a highly conserved region within the 3′UTR of human IKKβ, which represents a putative target sequence that can confer inhibition of translation by miR-199a. MiR-199a or NC miRNA were co-transfected with Luc.IKKβ or Luc.control into ESCs. As expected, overexpression of miR-199a effectively attenuated luciferase activity of Luc.IKKβ, while NC miRNA had no effect on the reporter constructs. Besides, both miR-199a and NC miRNA had no effect on the luciferase activity of Luc.control (Fig. 3b). These results suggest that miR-199a has an inhibitory effect on the predicted binding site. We then introduced miR-199a into ESCs and analyzed the expression of IKKβ. Overexpression of miR-199a reduced the expression of IKKβ protein, but has little effect on IKKβ mRNA, implying the translation-inhibitory effect of miR-199a (Fig. 3c and d). The results are consistent with the prior research (Song et al., 2010), which shows that the mRNA levels of the target genes are not affected but the protein levels are affected by miR-199a.
Figure 3.
MiR-199a targets and inhibits IKKβ in ESCs. (a) The alignment between hsa-miR-199a and the 3′UTR of IKKβ, identified by TargetScan software. (b) The miR-199a target region, or a mutant, was cloned into the 3′UTR of a luciferase gene. These constructs were introduced into ESCs, in addition to miR-199a mimics or NC. After 48 h, luciferase activity was measured, averaged and plotted. The y-axis represents the renilla luciferase activity normalized to firefly luciferase activity. (*P<0.05 versus the other three groups) (c) The IKKβ mRNA level in transfected ESCs was analyzed by qRT-PCR at 24 h post-transfection. (d) The IKKβ protein level in transfected ESCs was analyzed by western blot at 48 h post-transfection. The results are shown as the mean ± SD from triplicate cultures. (*P<0.05 versus NC group).
Negative regulation of the NF-κB pathway by miR-199a
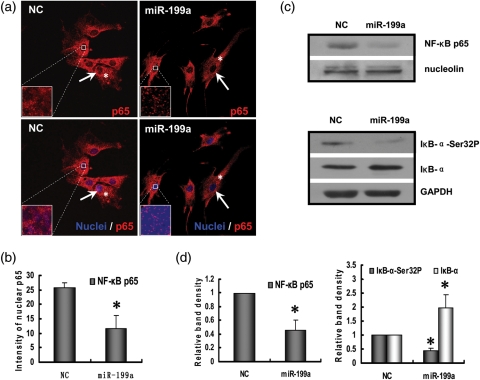
Previous studies have shown that IKKβ can mediate NF-κB pathway activation (Hernandez et al., 2010; Lin et al., 2011). To determine the effect of IKKβ down-regulation induced by miR-199a, we examined the localization of NF-κB by immunofluorescence in ESCs following miR-199a transfection. Cells were fixed and immunostained for the p65 subunit of NF-κB, and cell nuclei were identified by co-staining with DAPI. NF-κB p65 was present in both nuclei and cytoplasm of the NC transfected cells, while p65 subunit labeling was significantly reduced in the nuclei of the miR-199a transfected cells (Fig. 4a and b). To further validate miR-199a inhibition of NF-κB nuclear translocation, we isolated the nuclear fraction of cells transfected with miR-199a and immunoblotted for the NF-κB p65 protein subunit. Densitometric analysis revealed a decreased level of p65 protein in the nuclear lysates of miR-199a transfected cells (Fig. 4c and d). NF-κB translocation and transcriptional activity are inhibited by its association with IκB-α protein. Western blot analysis of the whole cellular lysates of ESCs after miR-199a transfection showed a reduction of IκB-α phosphorylation. Concomitantly, the level of endogenous IκB-α protein increased, as expected, upon miR-199a transfection, corresponding to the decrease of IκB-α phosphorylation (Fig. 4c and d).
Figure 4.
MiR-199a inhibits NF-κB cellular localization, IκB-α phosphorylation and NF-κB pathway activation in ESCs. ESCs were transfected with miR-199a mimics or NC for 48 h. (a) Cells were fixed and stained for the p65 subunit of NF-κB (red, p65; blue, nuclei). Arrows indicate NF-κB p65 immunostaining in the cell nucleus, and asterisks indicate NF-κB p65 in the cytoplasm. (b) Summarized data show the immunofluorescent staining intensity of p65 in the nuclear area of ESCs transfected with NC or miR-199a. (*P<0.05 versus NC group) (c) ESCs were lysed after transfection, and nuclear extracts were prepared and immunoblotted for either the p65 subunit of NF-κB or nucleolin. Whole cell lysates of transfected ESCs were immunoblotted for phospho-IκB-α, total IκB-α and GAPDH. Nucleolin and GAPDH were used as the loading control. (d) Summarized data show the average protein of NF-κB p65, phospho-IκB-α and total IκB-α (normalized to the protein level of GAPDH or nucleolin) corresponding to the bands shown in the western blots. The results are shown as the mean ± SD from triplicate cultures. (*P<0.05 versus NC group).
MiR-199a inhibits the IL-8 secretion from ESCs
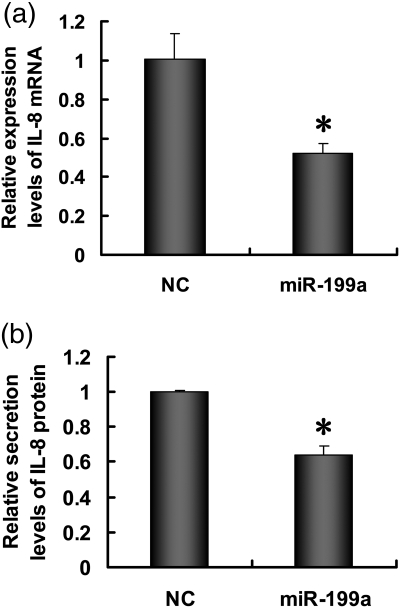
IL-8 is an NF-κB inducible gene (Heidemann et al., 2003; Himmel et al., 2011). To explore whether NF-κB pathway inhibition by miR-199a transfection has an effect on the IL-8 expression, we measured both the mRNA and protein level of IL-8 in miR-199a transfected ESCs. qRT-PCR analysis showed that miR-199a transfection significantly inhibited the mRNA level of IL-8 (Fig. 5a). The level of secreted IL-8, as measured by ELISA, is also reduced significantly after transfection (Fig. 5b).
Figure 5.
MiR-199a represses the IL-8 production from ESCs. (a) ESCs were transfected with miR-199a mimics or NC for 24 h, and IL-8 mRNA expression was analyzed by qRT-PCR. (b) ESCs were transfected with miR-199a mimics or NC for 48 h, and IL-8 protein secretion in the culture medium was analyzed by ELISA. Results are presented as mean ± SD from three separate experiments (*P<0.05 versus NC group).
Discussion
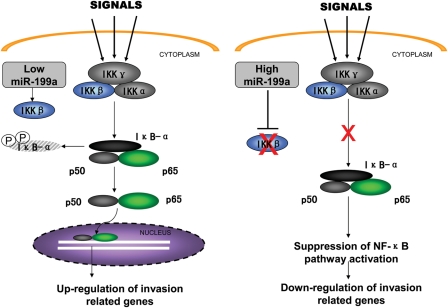
In the current study, we found that miR-199a was down-regulated in the ovarian endometrioma and eutopic endometrium from women with endometriosis compared with normal controls. Furthermore, we showed that miR-199a could inhibit ESC adhesion, migration and invasion, which may be involved in the processes of endometriotic lesion development. Additionally, we demonstrated that IKKβ is a direct target of miR-199a in ESCs. Lastly, our data also showed that miR-199a-induced IKKβ reduction is accompanied by NF-κB pathway suppression and reduced IL-8 secretion, which may be the underlying basis of ESC invasiveness (Fig. 6).
Figure 6.
Overview of the negative regulation of the IKK/NF-κB pathway by miR-199a. ESCs have high levels of IKKβ expression, and when stimulated, NF-κB activation leads to cytokine production and cell invasion. While miR-199a was transfected into ESCs, it used the inhibitory machinery to reduce the expression of its target gene IKKβ, thereby suppressing the IKK/NF-κB pathway activation and attenuating cytokine production (for example IL-8) and cell invasiveness.
MiRNAs are small, non-coding RNAs that are frequently dysregulated in female reproductive pathologies, including endometriosis (Pan et al., 2007; Toloubeydokhti et al., 2008; Burney et al., 2009; Carletti and Christenson, 2009; Ohlsson Teague et al., 2009; Filigheddu et al., 2010; Hawkins et al., 2011; Ramon et al., 2011). In the present report, we observed that the expression level of miR-199a was lower in the eutopic endometrium from women with endometriosis, and even lower in the paired ovarian endometrioma, compared with the expression in normal controls. The differential expression of miRNAs in endometriosis has been previously evaluated. In agreement with our results, Pan et al. (2007) have identified that miR-199a is down-regulated in endometriotic tissues in comparison with normal endometrium. However, down-regulation of miR-199a was not reported by other studies on miRNA expression in endometriosis (Burney et al., 2009; Ohlsson Teague et al., 2009; Filigheddu et al., 2010; Hawkins et al., 2011; Ramon et al., 2011). This is likely due to the difference between the study designs, methods of analysis, menstrual cycle phase at the time of biopsy and patient cohorts used in their study when compared with ours.
Although endometriosis is a well-known disease, the pathogenesis remains controversial. One of the currently accepted models of peritoneal endometriotic lesion development is that displaced endometrial tissue in retrograde menstrual fluid progresses through a process of adhesion, invasion, proliferation and neovascularization in order to become established at an ectopic site (Giudice and Kao, 2004; Hull et al., 2008). Also, altered immune surveillance, stem cells, genetic predisposition, environmental and hormonal factors could be involved in the pathogenesis (Koninckx et al., 1994; Matsuura et al., 1999; Gargett and Masuda, 2010; Giudice, 2010). A considerable body of evidence indicates that miR-199a may play a critical role in regulating cell adhesion, migration and invasion (Shen et al., 2010; Song et al., 2010; Cheung et al., 2011). MiR-199a inhibits the invasion and metastasis of testicular cancer cell by targeting podocalyxin-like protein 1 (Cheung et al., 2011). Moreover, decreased expression of miR-199a in hepatocellular carcinoma contributes to increased cell invasion by functional deregulation of discoidin domain receptor 1 activity (Shen et al., 2010). However, miR-199a is highly expressed in gastric cancer and positively regulates cell migration and invasion partly by targeting mitogen-activated protein kinase kinase kinase 11 (Song et al., 2010). To the best of our knowledge, there 's no published data on the effect of miR-199a on ESCs. In this report, we describe that miR-199a transfection inhibits ESC adhesion to ECM, which suggests that miR-199a may play a key role in regulating cell attachment to ectopic sites. Moreover, in vitro invasion and migration assay reveal that miR-199a attenuates both cell migration and invasion, which suggests that miR-199a may be involved in cell implantation in ectopic sites. Thus, our results suggest that miR-199a may be involved in endometrial implants formation and endometriosis development. Recent studies indicate that miR-199a family may also be associated with other disorders of the female reproductive system (Daikoku et al., 2008; Nam et al., 2008; Eitan et al., 2009). MiR-199a is related to the progression and prognosis of ovarian cancer (Nam et al., 2008). In addition, miR-199a* has been suggested to be involved in endometrial cancer development by targeting cyclooxygenase-2 (COX-2) (Daikoku et al., 2008). Moreover, increased expression of miR-199a* corresponds to the resistance of platinum-based chemotherapy in ovarian cancer (Eitan et al., 2009). Therefore, exploring the function of miR-199a may lead to the development of effective therapies against these disorders.
One of the best ways to understand miRNA function is via the elucidation of functional targets, which usually involves analysis of changes in target proteins following either a gain or loss of function of the specific miRNA. IKKβ has been validated as a potential miR-199a target in ovarian cancer cells (Chen et al., 2008). However, the targets of certain miRNAs could be tissue-specific, in other words, certain miRNAs can regulate different types of cells through down-regulation of different target genes (Dahiya et al., 2008; Inomata et al., 2009). Although IKKβ is a target of miR-199a in ovarian cancer (Chen et al., 2008), it has not been determined whether IKKβ is also a direct target of miR-199a in ESCs. To answer this question, we examined the regulation of miR-199a at the binding site of the 3′UTR of IKKβ mRNA in comparison to seed region mutants using dual luciferase reporter gene assays. The results indicate that IKKβ is also a target gene of miR-199a in ESCs. Moreover, miRNA can guide post-transcriptional regulation of protein synthesis by means of targeted RNA degradation or translational arrest (Huntzinger and Izaurralde, 2011). Our results show that the protein level of IKKβ could be down-regulated by miR-199a but the mRNA level was almost unaffected, even in higher miRNA transfection dosages (data not shown). This suggests a translation-inhibitory effect of miR-199a rather than induction of RNA degradation. Our results are consistent with the prior research (Song et al., 2010), which shows that the mRNA levels of the target genes are not affected but the protein levels are affected by miR-199a. However, the results of another paper show that miR-199a could significantly degrade the mRNA of the target gene discoidin domain receptor 1, although miR-199a is partially complementary to the 3′UTR of the target gene (Shen et al., 2010). The mechanisms underlying these phenomena may need further research.
IKKβ, one of the catalytic subunits of IKK complex, is responsible for the phosphorylation of the NF-κB inhibitors and necessary for the canonical pathway activation of NF-κB (Karin, 1999). It has been shown that down-regulating IKKβ activity, either by a small molecule kinase inhibitor or by short hairpin RNA depletion, could inhibit the activation of NF-κB signaling and attenuate ovarian cancer aggressiveness (Hernandez et al., 2010). Our results are consistent with this report, indeed miR-199a-induced IKKβ protein down-regulation is accompanied with both decreased IκB-α phosphorylation and lowered NF-κB nuclear translocation, which suggests that miR-199a may inhibit NF-κB pathway activation through down-regulating IKKβ protein expression. Numerous findings suggest that NF-κB may be a major culprit in the pathophysiology of endometriosis (Guo, 2007; Wieser et al., 2007; Gonzalez-Ramos et al., 2010). Constitutive activation of NF-κB has been demonstrated in endometriotic lesions (Gonzalez-Ramos et al., 2007). NF-κB transcriptional activity modulates key cell processes contributing to the initiation and progression of endometriosis (Gonzalez-Ramos et al., 2010). NF-κB activation promotes endometriotic lesion development while NF-κB inhibition reduces endometriosis symptoms in women (Huber et al., 2004; Wieser et al., 2007). Thus, it is possible that miR-199a could attenuate ESC invasiveness partly through NF-κB pathway inhibition.
In vitro and in vivo studies show that NF-κB inhibition could reduce endometriosis development and maintenance partly by reducing proinflammatory and invasion mediators (Tagashira et al., 2009; Veillat et al., 2009). IL-8, a major NF-κB inducible gene, is one of these mediators, and NF-κB can regulate IL-8 expression in various kinds of cells, such as bronchial epithelial cells, endothelial cells and colorectal cancer cells (Abolhassani et al., 2008; Fang et al., 2010; Kanoh et al., 2011). In the present study, both IL-8 mRNA and protein down-regulation are observed after miR-199a transfection, and are accompanied with reduced NF-κB nuclear translocation and activation. These results suggest that miR-199a may function, in part, through the NF-κB pathway to down-regulate IL-8 expression. IL-8 is involved in ESC adhesion, invasion and growth (Arici et al., 1998; Garcia-Velasco and Arici, 1999; Mulayim et al., 2004). In ESCs, IL-8 enhances the adhesion of ESCs to fibronectin, and thus may be involved in the attachment of endometrial fragments to the peritoneal lining (Garcia-Velasco and Arici, 1999). Additionally, IL-8 increases the invasiveness of ESCs to the ECM by up-regulating matrix metalloproteinase (MMP)-9 and MMP-2 activity (Mulayim et al., 2004). Moreover, IL-8 induces the proliferation of ESCs as a potential autocrine growth factor (Arici et al., 1998). Thus, inhibition of ESC invasiveness by miR-199a may be partly attributed to IL-8 down-regulation.
Collectively, our present results have revealed that miR-199a is down-regulated in endometriosis, and the expression level of miR-199a affects the invasive ability of ESCs in vitro. Furthermore, it seems that miR-199a attenuates ESC invasiveness partly through IKKβ/NF-κB pathway suppression and reduced IL-8 expression (Fig. 6). Similar regulation is present in ovarian cancer cells: miR-199a could affect the NF-κB activity in ovarian cancer cells by targeting IKKβ (Chen et al., 2008). Besides, it was also involved in the formation of a pro-inflammatory environment in ovarian cancer, which may have implications in tissue repair, chemoresistance and tumor progression (Yin et al., 2010). In addition, miR-199a* has been suggested to be involved in endometrial cancer development by targeting COX-2 (Daikoku et al., 2008). Therefore, our results provide further evidence that the miR-199a family has been implicated in a variety of pathological conditions of the female reproductive system, which indicates that miR-199a may be a potential therapeutic target of these disorders. However, until now, the exact mechanisms underlying the effect of miR-199a are just beginning to be elucidated. Whether other signaling pathways can also be controlled by miR-199a remains to be determined in future studies.
Authors' roles
L.D. performed experiments and contributed toward writing the manuscript. L.G. performed experiments and interpreted data. W.D. contributed toward making an experimental design and revision of the article.
Funding
This work was supported by Leading Academic Discipline Program, 211 Project for Shanghai Jiao Tong University School of Medicine (the third phase; 211-2008-7).
References
- Abolhassani M, Aloulou N, Chaumette MT, Aparicio T, Martin-Garcia N, Mansour H, Le Gouvello S, Delchier JC, Sobhani I. Leptin receptor-related immune response in colorectal tumors: the role of colonocytes and interleukin-8. Cancer Res. 2008;68:9423–9432. doi: 10.1158/0008-5472.CAN-08-1017. [DOI] [PubMed] [Google Scholar]
- Arici A, Seli E, Zeyneloglu HB, Senturk LM, Oral E, Olive DL. Interleukin-8 induces proliferation of endometrial stromal cells: a potential autocrine growth factor. J Clin Endocrinol Metab. 1998;83:1201–1205. doi: 10.1210/jcem.83.4.4743. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625–631. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti MZ, Christenson LK. MicroRNA in the ovary and female reproductive tract. J Anim Sci. 2009;87:E29–E38. doi: 10.2527/jas.2008-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Alvero AB, Silasi DA, Kelly MG, Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T, Mor G. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27:4712–4723. doi: 10.1038/onc.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HH, Davis AJ, Lee TL, Pang AL, Nagrani S, Rennert OM, Chan WY. Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene. 2011;30:3404–3415. doi: 10.1038/onc.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih Ie M, Zhang Y, Wood W, III, Becker KG, Morin PJ. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One. 2008;3:e2436. doi: 10.1371/journal.pone.0002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Gu L, Ding C, Qiu L, Di W. TWEAK promotes ovarian cancer cell metastasis via NF-kappaB pathway activation and VEGF expression. Cancer Lett. 2009;283:159–167. doi: 10.1016/j.canlet.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331:550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan R, Kushnir M, Lithwick-Yanai G, David MB, Hoshen M, Glezerman M, Hod M, Sabah G, Rosenwald S, Levavi H. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009;114:253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol. 2010;2010:369549. doi: 10.1155/2010/369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A, Zupi G, Del Bufalo D. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45:2618–2627. doi: 10.1016/j.ejca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Arici A. Interleukin-8 stimulates the adhesion of endometrial stromal cells to fibronectin. Fertil Steril. 1999;72:336–340. doi: 10.1016/s0015-0282(99)00223-x. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramos R, Donnez J, Defrere S, Leclercq I, Squifflet J, Lousse JC, Van Langendonckt A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007;13:503–509. doi: 10.1093/molehr/gam033. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramos R, Van Langendonckt A, Defrere S, Lousse JC, Colette S, Devoto L, Donnez J. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil Steril. 2010;94:1985–1994. doi: 10.1016/j.fertnstert.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Guo SW. Nuclear factor-kappab (NF-kappaB): an unsuspected major culprit in the pathogenesis of endometriosis that is still at large? Gynecol Obstet Invest. 2007;63:71–97. doi: 10.1159/000096047. [DOI] [PubMed] [Google Scholar]
- Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15:587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hsu SC, Davidson B, Birrer MJ, Kohn EC, Annunziata CM. Activation of NF-kappaB signaling by inhibitor of NF-kappaB kinase beta increases aggressiveness of ovarian cancer. Cancer Res. 2010;70:4005–4014. doi: 10.1158/0008-5472.CAN-09-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel ME, Crome SQ, Ivison S, Piccirillo C, Steiner TS, Levings MK. Human CD4+ FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol. 2011;41:306–312. doi: 10.1002/eji.201040459. [DOI] [PubMed] [Google Scholar]
- Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AV, Huber JC, Kolbus A, Imhof M, Nagele F, Loizou D, Kaufmann U, Singer CF. Systemic HCG treatment in patients with endometriosis: a new perspective for a painful disease. Wien Klin Wochenschr. 2004;116:839–843. doi: 10.1007/s00508-004-0296-5. [DOI] [PubMed] [Google Scholar]
- Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC, Smith SK, Tavare S, Print CG, Charnock-Jones DS. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol. 2008;173:700–715. doi: 10.2353/ajpath.2008.071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- Kanoh S, Tanabe T, Rubin BK. Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with LPS and resolves airway inflammation in the ferret. Chest. 2011;140:980–990. doi: 10.1378/chest.10-2908. [DOI] [PubMed] [Google Scholar]
- Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Braet P, Kennedy SH, Barlow DH. Dioxin pollution and endometriosis in Belgium. Hum Reprod. 1994;9:1001–1002. doi: 10.1093/oxfordjournals.humrep.a138623. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Kitade M, Kikuchi I, Kumakiri J, Matsuoka S, Kuroda M, Takeda S. Peritoneal vascular density assessment using narrow-band imaging and vascular analysis software, and cytokine analysis in women with and without endometriosis. J Minim Invasive Gynecol. 2010;17:21–25. doi: 10.1016/j.jmig.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003;9:175–179. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science. 2003;299:1540. doi: 10.1126/science.1080372. [DOI] [PubMed] [Google Scholar]
- Lin CH, Cheng HW, Ma HP, Wu CH, Hong CY, Chen BC. Thrombin induces NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells by a Rac1-dependent PI3K/Akt pathway. J Biol Chem. 2011;286:10483–10494. doi: 10.1074/jbc.M110.112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Ohtake H, Katabuchi H, Okamura H. Coelomic metaplasia theory of endometriosis: evidence from in vivo studies and an in vitro experimental model. Gynecol Obstet Invest. 1999;47(Suppl 1):18–20. doi: 10.1159/000052855. discussion 20–22. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Mulayim N, Savlu A, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Regulation of endometrial stromal cell matrix metalloproteinase activity and invasiveness by interleukin-8. Fertil Steril. 2004;81(Suppl 1):904–911. doi: 10.1016/j.fertnstert.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, Kim JW, Kim S. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- Qian K, Hu L, Chen H, Li H, Liu N, Li Y, Ai J, Zhu G, Tang Z, Zhang H. Hsa-miR-222 is involved in differentiation of endometrial stromal cells in vitro. Endocrinology. 2009;150:4734–4743. doi: 10.1210/en.2008-1629. [DOI] [PubMed] [Google Scholar]
- Ramon LA, Braza-Boils A, Gilabert-Estelles J, Gilabert J, Espana F, Chirivella M, Estelles A. microRNAs expression in endometriosis and their relation to angiogenic factors. Hum Reprod. 2011;26:1082–1090. doi: 10.1093/humrep/der025. [DOI] [PubMed] [Google Scholar]
- Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Zeng H, Li J, Xiao L, He Y, Tang Y, Li Y. miR-199a regulates the tumor suppressor mitogen-activated protein kinase kinase kinase 11 in gastric cancer. Biol Pharm Bull. 2010;33:1822–1827. doi: 10.1248/bpb.33.1822. [DOI] [PubMed] [Google Scholar]
- Tagashira Y, Taniguchi F, Harada T, Ikeda A, Watanabe A, Terakawa N. Interleukin-10 attenuates TNF-alpha-induced interleukin-6 production in endometriotic stromal cells. Fertil Steril. 2009;91:2185–2192. doi: 10.1016/j.fertnstert.2008.04.052. [DOI] [PubMed] [Google Scholar]
- Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–165. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- Toloubeydokhti T, Pan Q, Luo X, Bukulmez O, Chegini N. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod Sci. 2008;15:993–1001. doi: 10.1177/1933719108324132. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ulukus M, Ulukus EC, Tavmergen Goker EN, Tavmergen E, Zheng W, Arici A. Expression of interleukin-8 and monocyte chemotactic protein 1 in women with endometriosis. Fertil Steril. 2009;91:687–693. doi: 10.1016/j.fertnstert.2007.12.067. [DOI] [PubMed] [Google Scholar]
- Veillat V, Lavoie CH, Metz CN, Roger T, Labelle Y, Akoum A. Involvement of nuclear factor-kappaB in macrophage migration inhibitory factor gene transcription up-regulation induced by interleukin-1 beta in ectopic endometrial cells. Fertil Steril. 2009;91:2148–2156. doi: 10.1016/j.fertnstert.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Wieser F, Cohen M, Gaeddert A, Yu J, Burks-Wicks C, Berga SL, Taylor RN. Evolution of medical treatment for endometriosis: back to the roots? Hum Reprod Update. 2007;13:487–499. doi: 10.1093/humupd/dmm015. [DOI] [PubMed] [Google Scholar]
- Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Xu ZM, Dai HY, Ji XQ, Duan YY, Zhang CM, Qin DY. Application of the nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate for the treatment of endometriosis: an in vitro study. Fertil Steril. 2010;94:2942–2944. doi: 10.1016/j.fertnstert.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ, Wang XF, Li C. Pyrrolidine dithiocarbamate inhibits nuclear factor-kappaB pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stroikmal cells. Mol Hum Reprod. 2011;17:175–181. doi: 10.1093/molehr/gaq090. [DOI] [PubMed] [Google Scholar]