Abstract
Impairments caused by stroke remain the main cause for adult disability. Despite a vigorous research effort, only 1 thrombolytic treatment has been approved in acute stroke (<3h). The limitations of preclinical studies and how these can be overcome have been the subject of various guidelines. However, often these guidelines focus on the acute stroke setting and omit long-term outcome measures, such as behaviour and neuroimaging. The considerations and practicalities of including the serial assessment of these approaches and their significance to establish therapeutic efficacy are discussed here.
Keywords: Stroke, middle cerebral artery occlusion, post-operative care, neurological score, behavioural battery, MRI
1. Introduction
The development of novel treatments for stroke remains a major challenge for scientists and clinicians alike. Although other neurological conditions, such as Parkinson’s disease (PD), have seen the development of pharmacological compounds that at least alleviate the behavioural impairments temporarily, no such success has been achieved for ischaemic stroke, with recombinant tissue-type plasminogen activator (rtPA) remaining the only clinically-approved intervention. This is despite excellent animal models of stroke that more accurately replicate the proximal cause and pathology of the human condition compared to other disease, such as a PD. It is therefore important to understand why compounds showed promise in pre-clinical models, but failed to achieve similar success in clinical trials (Savitz 2007; Wahlgren and Ahmed 2004; Zaleska et al. 2009).
Several initiatives (Chopp et al. 2009; Feuerstein et al. 2008; Fisher 2003; Fisher et al. 2005; Fisher et al. 2009; Liu et al. 2009; Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke 2009) have compiled limitations and omissions in preclinical studies that raised concerns about the studies’ reliability and validity, but also about its outcome measures and the timing of intervention. Their guidelines aim to improve the pre-clinical testing of therapeutic efficacy in stroke by failing unsuccessful interventions, while increasing the likelihood of successful compounds to succeed in the clinic. Nevertheless, the focus is often on a single short-term ex vivo outcome measure, such as histological volume reduction, that differs considerably from clinical outcome measures. Although this might provide an indication that a therapeutic reduces the impact of stroke, this does not necessarily translate into a behavioural benefit.
The validity of measures and their sensitivity to detect therapeutic efficacy are often poorly established. To determine if an intervention was not successful rather than outcome measures not being sensitive to the therapy, ideally within each study a validation of outcome measures using a positive control should be undertaken (Ashioti et al. 2009). Generally, there is a good correlation between ex vivo histological lesion volumes and in vivo behavioural tests or neuroimaging (Ashioti et al. 2007). Nevertheless, brain plasticity can produce recovery on neurological or behavioural tests without a concomitant improvement in lesion volume.
Merely assessing absolute lesion volume disregards the site of the lesion and omits its impact on the remaining brain. Focussing on lesion volume as a whole might average-out any regional neuroprotection. Neuroimaging is emerging though as an integrative assessment that captures the more complete anatomical impact of stroke (Liebeskind 2009). Lesion volume by MRI robustly correlates with behavioural measurements in animals (Ashioti et al. 2007; Lim et al. 2008; Virley et al. 2000) and neurological/neuropsychological assessments in patients (Committeri et al. 2007). Additional measurements of anatomical changes in spared brain regions (i.e. where blood flow has been normal) can further elucidate the secondary impact stroke exerts on other regions, such as the thalamus, but also on the non-injured hemisphere (Modo et al. 2009). Changes in these areas can be crucial to explain behavioural improvement in stroke. Neuroimaging can hence provide a common assessment platform across species and eventually deliver validated surrogate markers of treatment efficacy (Liebeskind 2009).
To ensure that treatment is robust, experiments should not only focus on the acute short-term benefit (<48h), but also the sub-acute (2–14 days) and chronic period (>14 days). It is noteworthy that the lesion volume following 60 minutes of middle cerebral artery occlusion (MCAo) increases up to 6 weeks following insult before it stabilises (Modo et al. 2009). Hence, one would assume that as the lesion evolves the benefit from a continued treatment should become more apparent. Especially interventions impacting on secondary degeneration, such as Wallerian degeneration, will only be evident with long-term assessments (Yu et al. 2009). Therefore, serial in vivo assessments over longer time frames can be more powerful to detect therapeutic effects compared to analysis of a single acute time-point.
2. Neurological Scoring versus Behavioural Test Battery
There is a fundamental distinction between neurological deficits and behavioural impairments. Neurological deficits are specific functions, such as the righting reflex, that are perturbed, whereas behavioural impairments are more complex readouts, such as lack of learning in the water maze. For serial and long-term measurements, one also needs to consider if there is spontaneous recovery of this task. Most tasks will eventually recover long-term, although very specific and detailed analyses will allow the continued detection of a deficit or adaptations (Alaverdashvili et al. 2008). Another essential distinction is between behavioural and functional tests. Behavioural tests are tasks where an overt response of an animal is measured, whereas functional readouts reflect measurements where brain activity is assessed (e.g. fMRI, phMRI, EEG, electrophysiology). Functional tests therefore provide the link between anatomical and behavioural measures. Although functional and behavioural measures are often linked, it is conceivable that changes in the brain’s activity occur (i.e. plasticity) that do not directly translate into a behavioural benefit. For instance, the fMRI response to somatosensory stimulation sub-acutely (1–3 days) indicates activity in the contralateral cortex. However, there is no behavioural recovery associated with this change in activity and indeed animals only recover if activity is restored to areas adjacent to those damaged (Dijkhuizen et al. 2003). Conversely, it is possible that an animal shows “recovery” on a behavioural test due to adaptation that does not necessarily reflect a measurable functional change.
Neurological deficits are often used to probe outcomes in pre-clinical stroke studies. A series of specific tests can be charted into a neurological scale to provide a read-out of overall severity (Modo et al. 2000b). For this readout to be meaningful as a general measure of severity, it is important that each deficit carries equal weight and ideally that it is either impaired (1) or normal (0). The total score hence reflects the magnitude of a general deficit. Several scales that either measure global neurological function (Table 1) or more specific stroke-related deficits are documented in the literature. Tests that are especially important and sensitive to the damage caused by stroke are best assessed separately as a graded scale. For instance, one of the most commonly used stroke-specific scales consists of the 3 point Bederson scale (Bederson et al. 1986) (Table 2). This scale is sensitive to striatal damage and measures a forelimb dysfunction that is dependent on the degree of lesioning. Importantly, this neurological deficit is visible within 45 minutes of occlusion and can be used prior to re-perfusion to surmise if a MCA occlusion was successful.
Table 1. Example of a general neurological scale.
Example of a general neurological scale (Modo et al. 2000b). A general neurological scale provides a neurological assessment of a variety of neurological functions. This scale can be used in different neurological conditions to chart an overall neurological deficit. However, it does not provide stroke-specific measures and some of these measures will not necessarily be affected by stroke damage, but would be affected by other types of neurological damage. For instance, the righting reflect is typically intact in animals with stroke (Modo et al. 2000b), but is often impaired in animals with global cerebral ischaemia (Modo et al. 2000a).
| Neurological Test | Description | Normal | Deficit |
|---|---|---|---|
| Spontaneous Motility | The animal is put onto an empty surface in familiar surroundings (the post-operative room) and is expected to move and explore the surroundings. If the animal does not initiate this behaviour after 10 s it is considered to be impaired on spontaneous motility. | 0 | 1 |
| Righting Reflex | The animal is held in a supine position in the hand. The righting reflex is intact if the animal spontaneously turns and returns to its natural position. If the animal, however, fails to turn it has lost its righting reflex. | 0 | 1 |
| Horizontal Bar | The animal’s forelimbs are placed on top of a bar (e.g. pencil), the animal is expected to grasp the bar and to hang on the bar for 3 s. The bar is placed about 30 cm above floor level. A foam pad is placed below the animal to guarantee a soft landing. | 0 | 1 |
| Grasping Reflex | A bar is placed under each of the limbs and the animal is expected to grasp the bar with both forepaws simultaneously. | 0 | 1 |
| Tilted Cage Top | The animal is placed on a tilted cage top (45°). If the animal freezes or if it moves over the edge of the top it is impaired on this task. | 0 | 1 |
| Placing Reaction | The animal is placed on a platform where one side of the body is near the edge. Each limb will be pulled gently in turn below the surface of the platform. The animal is impaired if it fails to re-place the limb on the platform. | 0 | 1 |
| Visual Placing | The animal is held around the torso and lowered slowly above the edge of the cage. If the animal reaches with the forelimbs for the edge of the cage visual placing is intact. If the animal does not reach out with its forelimbs, and does not start to move its forelimbs, it is impaired on this task. | 0 | 1 |
Table 2. The Bederson Scale.
The Bederson Scale (Bederson et al. 1986). This scale assesses a cardinal feature of focal ischaemia and is especially sensitive to striatal damage. Flexion of the contralateral forelimb in stroke is one of the cardinal features of a successful occlusion, but sham occlusion can also transiently produce a similar deficit. Not all animals with an occlusion exhibit circling or body twisting. Nevertheless, these features are a reasonable representation of a group deficit, even if it is not possible to determine with certitude for each animal if they indeed are occluded. It provides a best guess scenario in the absence of other more reliable measures, such as MRI. Modifications of this scale, such as the body swing test (Borlongan et al. 1995), are often used as outcome measures, although some animals will spontaneously recover over time.
| Degree of deficit | Description | Score |
|---|---|---|
| Normal | Both forepaws reach out | 0 |
| Mild | Flexion of contralateral limb | 1 |
| Moderate | Decreased resistance to lateral push without circling | 2 |
| Severe | Decreased resistance to lateral push with circling | 3 |
The most comprehensive approach is to use two scales to measure neurological deficits. One scale should chart the overall neurological impact of the procedure, whereas another scale should measure the degree of deficit that is relevant to stroke. Combinations of general neurological dysfunction with stroke-specific deficits have been described (Hunter et al. 2000). Often these scales, however, give different weight to different tests and therefore the overall score cannot be considered an overall measure of severity. Although neurological scales typically correlate with short-term lesion volumes (Ashioti et al. 2007), they will no longer reflect the degree of histological damage at chronic time points, as most deficits spontaneously recover between a few days to a couple of weeks post-infarction with lesion volumes increasing (Modo et al. 2000b). As such, these neurological scales are only useful in the acute to sub-acute setting. However, appropriate controls consisting of stroke-only, stroke+vehicle and/or sham-surgery need to be incorporated to provide a meaningful assessment of therapeutic effects. Recovery on these scales can at worst merely indicate better spontaneous recovery or that an intervention improves recovery from the surgical procedure itself rather than the stroke. It is therefore best to consider with great caution claims of a persisting recovery, if only neurological scales have been used as read-outs.
Neurological scales typically do not reflect the distress of animals. Neurological scales can hence be misleading in defining humane end-points. Mostly for long-term recovery experiments, it is important to ensure that animals recover fast (Figure 1) and are not persistently distressed or suffering, as this can adversely affect behavioural outcome measures. Especially in the acute setting, neurological tests can be affected if the animals are distressed/suffering. In these cases, the inclusion of a distress scale (Table 3) can be used to determine an animal’s degree of suffering. Based on defined categories on these scales, it is possible to decide whether an animal should be euthanized or not. Although this type of scale can be used as an outcome measure, it does not reflect an improvement in behavioural impairments, but merely the animal’s level of suffering. As such, a distress scale might be considered a complimentary measure to other more damage-specific tests.
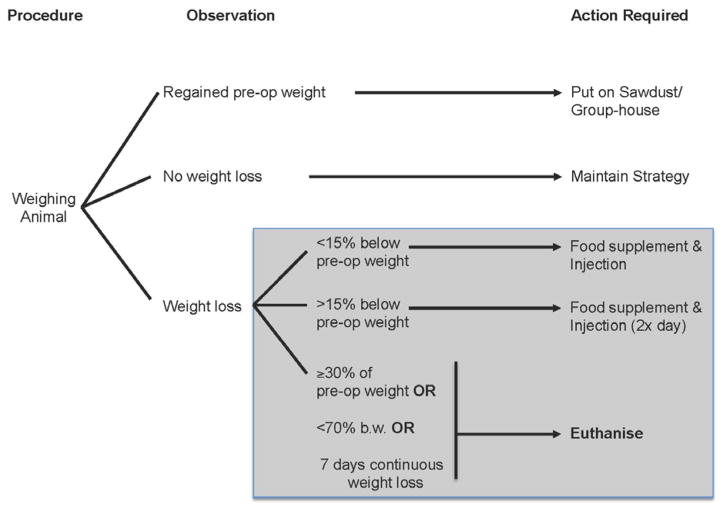
Figure 1.
Flow-chart for post-operative care strategies (Modo et al. 2000b). Animals are assessed daily after stroke. The best time for assessment is early mornings after the animals come out of their active period during which they are most likely to have consumed food. One of the most crucial features of animals’ recovery from stroke is their evolution of body weight. Body weight is a good indicator of the animals’ health status. If animals have re-gaining preoperative weight after surgery, they can be group-housed again. Ideally animals are kept in separate cages overnight to prevent them removing each other’s sutures and to allow an assessment of food intake and defecation. If the animal maintains weight, it is best to maintain the current strategy (i.e. same level of care). In the case of animals undergoing stroke, they typically loose weight (10–15%). If they do not, this often is a good indication that they were not properly occluded or that only minor damage was caused. Different levels of weight loss require different strategies to improve the animal’s recovery. Mashed food, as well as food supplements (e.g. Complan), in addition to injection with gluocsaline (sometimes mixed with Duphalyte) is known to improve post-operative recovery without affecting the evolution of the lesion per se. Typically fresh wet food is provided straight after measuring their weight in the morning and if needed as well just before the dark period. These strategies are designed to ensure the long-term survival of animals. To counteract distress/suffering of animals, additional injections of analgesics should be considered. Nevertheless, if these interfere with the evaluation of a therapeutic, experimenters should discuss alternatives with their local veterinary service.
Table 3. An example of a distress scale.
An example of a distress scale (Lloyd and Wolfensohn 1999). To determine the distress/suffering of an animal, a distress scale can provide a measurable state that can be used to define humane endpoints. It is important to ensure that animals are not in pain or suffering prior to conducting neurological or behavioural assessments, as these would confound stroke-related impairments. The following categories are distinguished: Normal (0–4; no action); Mild distress (5–9; monitor carefully, consider analgesics); Suffering (10–14; provide relief, observe regularly, consider euthanasia/perfusion); Severe pain or distress (15–20; immediate termination/perfusion); Refine procedure (18–20; consult with veterinary regarding procedure). For each score of 4, an additional point is added to the score.
| Description | Score | |
|---|---|---|
| Clinical Signs | Normal | 0 |
| General lack of grooming, ocular/nasal discharge | 1 | |
| Coat staring | 2 | |
| Pinched features, ridge lines | 4 | |
| Body Weight | Normal – gaining weight | 0 |
| B.W. < 5% reduction | 1 | |
| B.W. 5–15% reduction | 2 | |
| B.W. > 15% reduction | 4 | |
| Disease score | Normal | 0 |
| Loss of forelimb grip | 0.5 | |
| Forelimb paralysis | 1 | |
| Incomplete hind limb paralysis | 2 | |
| Forepaw and hindlimb paralysis | 3 | |
| Loss of righting reflex | 4 | |
| Provoked behavior | Normal | 0 |
| Minor depression or exaggerated response | 1 | |
| Moderate change/isolated | 2 | |
| Very still/lethargic | 4 |
A more robust indication of persistent impairments is achieved by applying behavioural tests (Table 4). The tests typically require the animal to perform a specific task, such as learning to swim to a platform (water maze) or to remove sticky tape from their forepaws (bilateral asymmetry test). They mainly differ from neurological tests in their complexity and often require several functions to integrate (e.g. attention, sensorimotor detection and motor skill for the bilateral asymmetry test). Behavioural tests are often chosen based on the specific anatomical damage that is caused by a stroke. If this damage is predominantly striatal, tests that probe striatal integrity, such as amphetamine-induced rotation, are appropriate. In most cases, stroke affects a variety of anatomical regions (striatum, sensorimotor cortex, thalamus) and hence it is more appropriate to use a battery of tests that canvas damage to these regions. If one would only chose a striatal task, but have more extensive extra-striatal damage, one would potentially fail to detect an improvement on a cortical task due to neuroprotection in the cortex.
Table 4. Overview of behavioural tasks affected by stroke damage.
Overview of behavioural tasks affected by stroke damage. Behavioural tests are designed to uncover damage inflicted by stroke to specific brain regions, but can also uncover damage to areas undergoing secondary damage due to, for instance, Wallerian degeneration. Anatomical areas in italics reflect sites of secondary damage, whereas other regions are directly affected by middle cerebral artery occlusion (MCAo). Degree of damage to these areas is, nevertheless, dependent on the duration and type of occlusion. Most of these tests can be used repeatedly and are fairly resistant to learning effects. A serial assessment on these tasks dramatically improves the statistical power to detect treatments effects. However, behavioural tasks with an asterix are difficult to adapt for serial measurement. For long-term assessments, it is also important to establish if a given behavioural test is resistant to spontaneous recovery. Often with time, brain plasticity and behavioural adaptations allow animals to perform at the level of controls. It is therefore crucial to include appropriate lesion-only controls that determine the persistence of a lesion effect. Sham-surgery controls are also needed for this.
3. Serial in vivo evaluation of stroke impact
Apart of neurological and behavioural assessments, non-invasive neuroimaging can monitor serially the neuropathological impact of stroke (Table 5). Although neuroimaging does not provide the same anatomical detail relative to extensive histopathological assessments, it affords a serial assessment of lesion pathology that can be applied to pre-clinical, as well as clinical studies. By serially assessing the same animal/patient over time, a dramatic improvement in statistical power can be achieved (Figure 2). Merely comparing two groups using a T-test at a single time point necessitates a large group size to reach statistical significance, even with a large effect size. By using a repeated measures approach, the total number of animals needed is dramatically reduced. Therefore this results in a reduction in the number of animals/patients needed to establish therapeutic efficacy. A further refinement of this approach is to use pre-treatment scans to calculate the percentage change over time due to an experimental intervention. This calculation will lead to a reduction in the intra-animal variability and further reduce the number of animals needed to compare different groups (Figure 3). In addition to lesion volumes, other structural changes outside the area of primary infarction, such as Wallerian degeneration, can also be accounted for (i.e. atrophy or hypertrophy) on an animal-by-animal basis. Sophisticated imaging analysis methods can be further developed to, for instance, link changes in the lesion environment with impairments (Lo et al.). These approaches potentially can dramatically improve our understanding of therapeutic efficacy compared to merely assessing lesion volume histologically and therefore should result in a more likely clinical translation.
Table 5. Overview of non-invasive translational imaging techniques to visualise stroke deficits.
Overview of non-invasive translational imaging techniques to visualise stroke deficits. Shaded imaging techniques involve exposure to radioactivity and serial measurements are often difficult to achieve. MRI is gradually emerging as a core assessment technique. However, PET and SPECT are far superior to MRI in assessing specific cellular and molecular aspects of stroke damage (e.g. microglia, apoptosis).
| Technique | Scan Type | Pathology | References |
|---|---|---|---|
| Magnetic Resonance Imaging (MRI) | T2 | Ischaemic Lesion | (Berger et al. 1998; Bihel et al. 2009; Siemonsen et al. 2009) |
| Haemorrhage | (Chen et al. 2008; Tsubokawa et al. 2007) | ||
| Diffusion | Ischaemic Lesion | (Sood et al. 2007; Srivastava et al. 2008; Taheri et al. 2009) | |
| Perfusion | Blood Flow | (Ebinger et al. 2009; Ma et al. 2009; Poppe et al. 2009; Rivers et al. 2006; Zaro-Weber et al. 2009) | |
| Diffusion Tensor | White Matter Damage | (Bihel et al. 2009; Lindberg et al. 2009; Yu et al. 2009) | |
| Functional | Loss of Activity | (Carvalho et al. 2008; Geisler et al. 2006; Murata et al. 2006) | |
| Spectroscopy | Metabolite Changes (↓NAA) | (Kang et al. 2009; Lei et al. 2009; Munoz Maniega et al. 2008) | |
| Angiogram | Vessel Occlusion | (Khan et al. 2009) | |
| Electron Paramagentic Resonance Imaging | Brain Oxygen | (Shen et al. 2009) | |
| Computer Tomography (CT) | Structural | Ischaemic Lesion | (Andersen et al. 2009) |
| Haemorrhage | (Andersen et al. 2009) | ||
| Angiogram | Vessel Occlusion | (Delgado Almandoz et al. 2009; Khan et al. 2009; Maas et al. 2009; Nikolova et al. 2009; Puetz et al. 2009) | |
| Perfusion | Blood Flow | (Blau et al. 2000) | |
| Positron Emission Tomography (PET) | 15O | Blood Flow | (Zaro-Weber et al. 2009) |
| 11C-PK11195 | Microglia | (Rojas et al. 2007; Schroeter et al. 2009) | |
| 18F-fluorodeoxyglucose | Metabolism | (Martin et al. 2009; Schroeter et al. 2009) | |
| 18F-fluoroacetate | Glial Metabolism | (Marik et al. 2009) | |
| 18F-Flumazenil | Penumbra | (Massaweh et al. 2009) | |
| 18F-5-fluoropentyl-2-methyl- malonic acid | Apoptosis | (Reshef et al. 1998) | |
| Single Photon Emission Tomography (SPECT) | 99mTc-HMPAO | Blood Flow | (Kaakinen et al. 2006; Umemura et al. 2000) |
| Transcranial Ultrasound (TUS) | 99mTc-ethylcysteinate dimer | Blood Flow | (Murakami et al. 2008) (Holzer et al. 2009; Khan et al. 2009; Nikolova et al. 2009; Schreiber et al. 2009) |
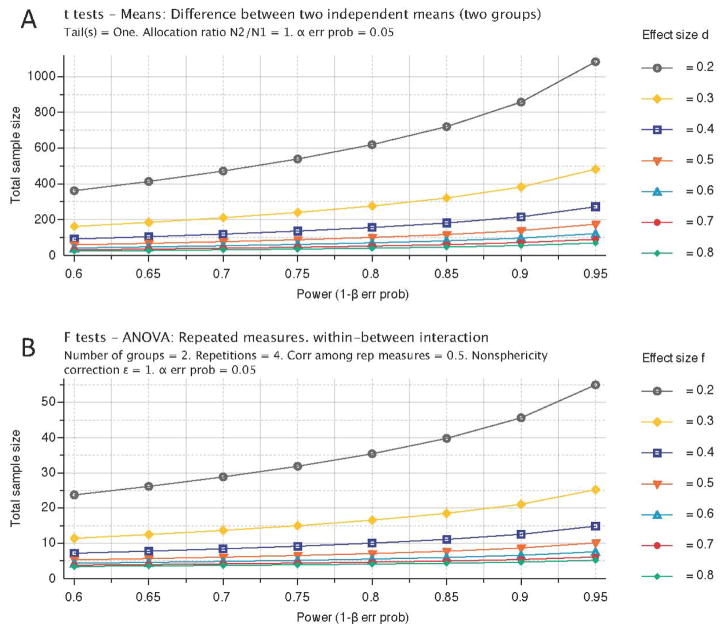
Figure 2.
Reducing animal numbers through serial assessments. A comparison of T- and F-test statistics using power calculations (generated using G*Power software 3.0.10, freely available at www.psycho.uni-duesseldorf.de/aap/projects/gpower/) indicate that an almost 20-fold reduction in sample size can be achieved with a similar effect size (0.3) by repeatedly (3×) measuring the same subjects. For instance, to compare two groups (i.e. treated versus untreated) at a single time point with an effect size of 0.5 at the 95% power level, a total sample size of 88 subjects is needed. In contrast, if there are 3 serial measurements on these subjects using a repeated measure Analysis of Variance (ANOVA), a total sample of only 12 is required. The variance used for the power calculations here is based on the Modo et al. (Modo et al. 2009) MRI lesion volume data that yielded an effect size of 0.5 at the final time point.
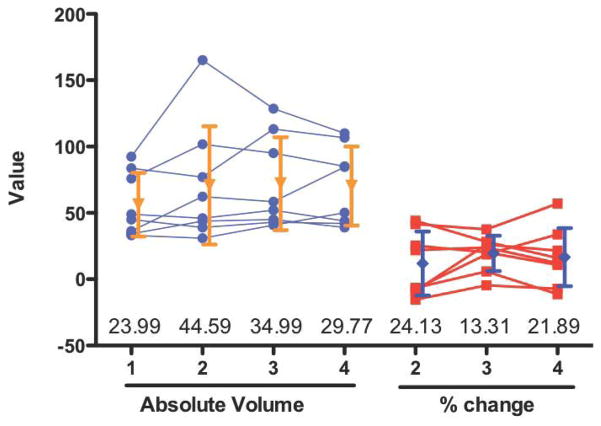
Figure 3.
Reduction of group variability through serial measurement. If baseline information can be acquired prior to animals undergoing any treatment, this data can be used to calculate the percentage (%) change of these animals at later time points. For instance, here MRI-based lesion volume data (expressed as mm3 for absolute and % change) from Modo et al. (Modo et al. 2009) is presented for 8 animals at 4 time-points. The standard deviation for % change measures is reduced, as the baseline intra-animal variability is accounted for (values at the bottom of the graph indicate the standard deviation for each time point). The reason for this reduction in variability is that % change removes the initial distance between animals (i.e. initial intra-animal variability). Removing this intra-animal variability will increase the power of a statistical comparison (i.e. reduces the number of animals needed for each group).
A major challenge to reconciling pre-clinical with clinical studies to date has been the administration of therapeutic agents in animals before occlusion or immediately after occlusion. This scenario is unlikely to occur in a clinical situation. However, with imaging it is now possible to investigate a therapeutic effect based on particular imaging characteristics rather than estimated time passed since occlusion. A pre-treatment scan can establish baseline pathology as in a clinical trial and monitor its progression over days. These pre-treatment scans can define inclusion and exclusion criteria for both preclinical and clinical studies. So far, in most animal studies, it has not been possible to determine very reliable inclusion or exclusion criteria based on neurological scales, as even sham surgery can produce similar transient signs of occlusion. Nevertheless, having clearly defined objective inclusion criteria will reduce variance within each group, as only animals with a clearly defined pathology will be included. Similar inclusion and exclusion criteria can in principle be applied in animal and human studies.
Apart of structural information, all non-invasive imaging techniques that are used clinically can also provide preclinical information about cerebral blood flow (Zaro-Weber et al. 2009) and in some cases penumbral tissue (Massaweh et al. 2009; Rivers et al. 2006). Being able to detect the molecular or cellular components involved in ischaemic brain damage will also increasingly afford a more targeted intervention (Rojas et al. 2007). As many of these aspects are detectable within minutes of occlusion (Table 6), it is possible to determine therapeutic windows during which particular treatments will be effective. Although a variety of relevant and important information regarding the stroke damage can potentially be derived from neuroimaging, especially by combining various approaches, time constraints on each of these limit their application (Felberg and Naidech 2003). One of the major challenges for years to come therefore will be to develop a robust and informative imaging paradigm that will be sufficiently fast to provide relevant physiological information to conduct efficacy studies (i.e. define inclusion/exclusion criteria).
Table 6. Overview of the detection of pathological changes caused by stroke on MRI scans.
Overview of the detection of pathological changes caused by stroke on MRI scans (based on Sen (Ebinger et al. 2009)). Within minutes of stroke onset, it is possible to detect pathological changes in the area of stroke due to a loss of blood flow and changes in the extracellular matrix that are reflected on perfusion and diffusion scans, respectively. A mismatch between perfusion (area larger than final lesion volume) and diffusion scans has been suggested to reflect penumbral tissue (Ma et al. 2009). Gradually further physiological changes occur that eventually can result in a complete tissue loss. However, at these different stages various imaging protocols and hallmarks exist to use imaging to reach a differential diagnosis and potentially select an appropriate intervention.
| Time | Sequence | Characteristic | Aetiology |
|---|---|---|---|
| 2–3 min | Diffusion | ↓ADC | ↓ proton motion |
| Perfusion | ↓CBF, CBV, MTT | ↓ CBF | |
| 0–2 h | T2 | Absent flow | Occlusion |
| T1 | Arterial enhancement | Slow flow | |
| 2–4 h | T2 | Sulcal effacement | Edema |
| T1 | Slightly hyperintense | Incomplete infarction | |
| 8h | T2 | Hyperintense | Edema |
| 16–24 h | T1 | Hypointense | Edema |
| >5 days | T2 | Hyperintense | Tissue loss |
It is important here to potentially distinguish efficacy and mode-of-action studies. In efficacy studies, the main focus will be to establish if there is a benefit of an intervention and hence the aim is to merely distinguish subjects with or without a treatment. In mode-of-action studies, specific mechanisms through which an intervention is working are under investigation. These will require more detailed studies that involve a variety of imaging techniques to probe specific processes in vivo (e.g. apoptosis). However, it is likely that for mode-of-action studies more traditional ex vivo measures will remain superior to in vivo imaging studies for the coming years.
4. Perspective
Many investigations into the therapeutic efficacy in stroke focus on acute histological outcome measures. Although these studies are less time-consuming, they often fail to implement a clinically-relevant experimental design. Nevertheless, these fast and reliable measures should be regarded as screening tools for potential therapeutic candidates. To establish candidates for clinical translation, serial assessments over a longer time frame, with the inclusion of both behavioural and in vivo neuropathological measures, will yield more appropriate indications of therapeutic potential. Especially, the use of neuroimaging is increasingly mandated, as it will afford the same outcome measures to be used in preclinical and clinical studies. Although these measures can improve the quality of our preclinical studies and decrease the risk of ineffective therapies being prematurely tested in patients, it does still not guarantee a successful clinical translation. Closer links between clinical and preclinical studies are required to ensure that similar approaches are used to establish therapeutic efficacy. Only if we can close the methodological gap between animal and human studies are we likely to achieve similar efficacy.
Acknowledgments
The generous grant support from the Medical Research Council (MRC - G0802552 & G0800846), Biotechnological and Biological Sciences Research Council (BBSRC - BB/D014808/1), European Framework VII (ENCITE - 201842) and the National Institute for Biomedical Imaging and Biotechnology (NIBIB - 1 P20 EB007076-01) are gratefully acknowledged. MM is supported by a Research Council of the United Kingdom (RCUK) fellowship.
Abbreviations
- CT
Computer tomography
- EEG
Electroencephalography
- fMRI
functional Magnetic Resonance Imaging
- MCA
Middle Cerebral Artery
- MRI
Magnetic Resonance Imaging
- PD
Parkinson’s Disease
- PET
Positron Emission Tomography
- phMRI
pharmacological Magnetic Resonance Imaging
- rtPA
recombinant tissue-type Plasminogen Activator
- SPECT
Single Photon Emission Computer Tomography
References
- Alaverdashvili M, Foroud A, Lim DH, Whishaw IQ. “Learned baduse” limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: a new analysis of the effect of gestures on success. Behav Brain Res. 2008;188:281–290. doi: 10.1016/j.bbr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Alaverdashvili M, Whishaw IQ. Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur J Neurosci. 2008;28:311–322. doi: 10.1111/j.1460-9568.2008.06315.x. [DOI] [PubMed] [Google Scholar]
- Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, Jones TA. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170:229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40:2068–2072. doi: 10.1161/STROKEAHA.108.540112. [DOI] [PubMed] [Google Scholar]
- Ashioti M, Beech JS, Lowe AS, Bernanos M, McCreary A, Modo MM, Williams SC. Neither in vivo MRI nor behavioural assessment indicate therapeutic efficacy for a novel 5HT(1A) agonist in rat models of ischaemic stroke. BMC Neurosci. 2009;10:82. doi: 10.1186/1471-2202-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashioti M, Beech JS, Lowe AS, Hesselink MB, Modo M, Williams SC. Multi-modal characterisation of the neocortical clip model of focal cerebral ischaemia by MRI, behaviour and immunohistochemistry. Brain Res. 2007;1145:177–189. doi: 10.1016/j.brainres.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Babu CS, Ramanathan M. Pre-ischemic treatment with memantine reversed the neurochemical and behavioural parameters but not energy metabolites in middle cerebral artery occluded rats. Pharmacol Biochem Behav. 2009;92:424–432. doi: 10.1016/j.pbb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Badin RA, Modo M, Cheetham M, Thomas DL, Gadian DG, Latchman DS, Lythgoe MF. Protective effect of post-ischaemic viral delivery of heat shock proteins in vivo. J Cereb Blood Flow Metab. 2009;29:254–263. doi: 10.1038/jcbfm.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More Than Numbers: The Power of Graphs in Meta-Analysis. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn340. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Berger R, Jensen A, Hossmann KA, Paschen W. Effect of mild hypothermia during and after transient in vitro ischemia on metabolic disturbances in hippocampal slices at different stages of development. Brain Res Dev Brain Res. 1998;105:67–77. [PubMed] [Google Scholar]
- Bihel E, Pro-Sistiaga P, Letourneur A, Toutain J, Saulnier R, Insausti R, Bernaudin M, Roussel S, Touzani O. Permanent or transient chronic ischemic stroke in the non-human primate: behavioral, neuroimaging, histological, and immunohistochemical investigations. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau S, Jubeh TT, Haupt SM, Rubinstein A. Drug targeting by surface cationization. Crit Rev Ther Drug Carrier Syst. 2000;17:425–465. [PubMed] [Google Scholar]
- Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res. 1995;676:231–234. doi: 10.1016/0006-8993(95)00150-o. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Yu G, Matsukawa N, Xu L, Hess DC, Sanberg PR, Wang Y. Acute functional effects of cyclosporine-A and methylprednisolone treatment in adult rats exposed to transient ischemic stroke. Life Sci. 2005;76:1503–1512. doi: 10.1016/j.lfs.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Brown AW, Marlowe KJ, Bjelke B. Age effect on motor recovery in a post-acute animal stroke model. Neurobiol Aging. 2003;24:607–614. doi: 10.1016/s0197-4580(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Carvalho SM, Pontes-Neto OM, Fabio SR, Leite JP, Santos AC, de Araujo DB. Rapid BOLD fMRI signal loss in the primary motor cortex of a stroke patient. Arq Neuropsiquiatr. 2008;66:885–887. doi: 10.1590/s0004-282x2008000600022. [DOI] [PubMed] [Google Scholar]
- Chen YF, Chang YY, Liu JS, Lui CC, Kao YF, Lan MY. Association between cerebral microbleeds and prior primary intracerebral hemorrhage in ischemic stroke patients. Clin Neurol Neurosurg. 2008;110:988–991. doi: 10.1016/j.clineuro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, Li Y, Hess DC, Borlongan CV. Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009 doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota-Scanderbeg A, Piccardi L, Guariglia C, Pizzamiglio L. Neural bases of personal and extrapersonal neglect in humans. Brain. 2007;130:431–441. doi: 10.1093/brain/awl265. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G. Neocortical localization of tactile/proprioceptive limb placing reactions in the rat. Brain Res. 1992;573:44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, Oleinik A, Lev MH, Gonzalez RG, Romero JM. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger M, De Silva DA, Christensen S, Parsons MW, Markus R, Donnan GA, Davis SM. Imaging the penumbra - strategies to detect tissue at risk after ischemic stroke. J Clin Neurosci. 2009;16:178–187. doi: 10.1016/j.jocn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Felberg RA, Naidech AM. The 5 Ps of acute ischemic stroke treatment: parenchyma, pipes, perfusion, penumbra, and prevention of complications. South Med J. 2003;96:336–342. doi: 10.1097/01.SMJ.0000063573.56033.A6. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, Finklestein SP, Pangalos MN, Poole M, Stiles GL, Ruffolo RR, Walsh FL. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- Fisher M. Recommendations for Advancing Development of Acute Stroke Therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–1546. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- Fisher M, Albers GW, Donnan GA, Furlan AJ, Grotta JC, Kidwell CS, Sacco RL, Wechsler LR. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann-Bard P, Boulouard M. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behav Neurosci. 2009;123:224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- Freret T, Chazalviel L, Roussel S, Bernaudin M, Schumann-Bard P, Boulouard M. Long-term functional outcome following transient middle cerebral artery occlusion in the rat: correlation between brain damage and behavioral impairment. Behav Neurosci. 2006;120:1285–1298. doi: 10.1037/0735-7044.120.6.1285. [DOI] [PubMed] [Google Scholar]
- Geisler BS, Brandhoff F, Fiehler J, Saager C, Speck O, Rother J, Zeumer H, Kucinski T. Blood-oxygen-level-dependent MRI allows metabolic description of tissue at risk in acute stroke patients. Stroke. 2006;37:1778–1784. doi: 10.1161/01.STR.0000226738.97426.6f. [DOI] [PubMed] [Google Scholar]
- Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Brundin P, Johansson BB. Paw-reaching, sensorimotor, and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–895. doi: 10.1161/01.str.24.6.889. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Sorensen JC, Mattsson B, Zimmer J, Johansson BB. Influence of an enriched environment and cortical grafting on functional outcome in brain infarcts of adult rats. Exp Neurol. 1995;133:96–102. doi: 10.1006/exnr.1995.1011. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Sinha K, Chaudhary G. Transient focal ischemia induces motor deficit but does not impair the cognitive function in middle cerebral artery occlusion model of stroke in rats. J Neurol Sci. 2002;203–204:267–271. doi: 10.1016/s0022-510x(02)00303-9. [DOI] [PubMed] [Google Scholar]
- Haelewyn B, Freret T, Pacary E, Schumann-Bard P, Boulouard M, Bernaudin M, Bouet V. Long-term evaluation of sensorimotor and mnesic behaviour following striatal NMDA-induced unilateral excitotoxic lesion in the mouse. Behav Brain Res. 2007;178:235–243. doi: 10.1016/j.bbr.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Hayase M, Kitada M, Wakao S, Itokazu Y, Nozaki K, Hashimoto N, Takagi Y, Dezawa M. Committed neural progenitor cells derived from genetically modified bone marrow stromal cells ameliorate deficits in a rat model of stroke. J Cereb Blood Flow Metab. 2009;29:1409–1420. doi: 10.1038/jcbfm.2009.62. [DOI] [PubMed] [Google Scholar]
- Holmberg P, Liljequist S, Wagner A. Secondary brain injuries in thalamus and hippocampus after focal ischemia caused by mild, transient extradural compression of the somatosensori cortex in the rat. Curr Neurovasc Res. 2009;6:1–11. doi: 10.2174/156720209787466073. [DOI] [PubMed] [Google Scholar]
- Holzer K, Sadikovic S, Esposito L, Bockelbrink A, Sander D, Hemmer B, Poppert H. Transcranial Doppler ultrasonography predicts cardiovascular events after TIA. BMC Med Imaging. 2009;9:13. doi: 10.1186/1471-2342-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Hurwitz BE, Dietrich WD, McCabe PM, Alonso O, Watson BD, Ginsberg MD, Schneiderman N. Amphetamine promotes recovery from sensory-motor integration deficit after thrombotic infarction of the primary somatosensory rat cortex. Stroke. 1991;22:648–654. doi: 10.1161/01.str.22.5.648. [DOI] [PubMed] [Google Scholar]
- Hurwitz BE, Dietrich WD, McCabe PM, Watson BD, Ginsberg MD, Schneiderman N. Sensory-motor deficit and recovery from thrombotic infarction of the vibrissal barrel-field cortex. Brain Res. 1990;512:210–220. doi: 10.1016/0006-8993(90)90628-o. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Gornicka-Pawlak E, Kozlowska H, Domanska-Janik K, Gielecki J, Lukomska B. Structural and functional characteristic of a model for deep-seated lacunar infarct in rats. J Neurol Sci. 2008;273:40–48. doi: 10.1016/j.jns.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Kaakinen T, Heikkinen J, Dahlbacka S, Alaoja H, Laurila P, Kiviluoma K, Salomaki T, Romsi P, Tuominen H, Biancari F, Lepola P, Nuutinen M, Juvonen T. Fructose-1,6-bisphosphate supports cerebral energy metabolism in pigs after ischemic brain injury caused by experimental particle embolization. Heart Surg Forum. 2006;9:E828–835. doi: 10.1532/HSF98.20061079. [DOI] [PubMed] [Google Scholar]
- Kadam SD, Mulholland JD, Smith DR, Johnston MV, Comi AM. Chronic brain injury and behavioral impairments in a mouse model of term neonatal strokes. Behav Brain Res. 2009;197:77–83. doi: 10.1016/j.bbr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BT, Jang DP, Lee JH, Jung DI, Gu SH, Lim CY, Kim YB, Quan FS, Kim HJ, Woo EJ, Cho ZH, Park HM. Detection of cerebral metabolites in a canine model of ischemic stroke using 1H magnetic resonance spectroscopy. Res Vet Sci. 2009;87:300–306. doi: 10.1016/j.rvsc.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Khan S, Rich P, Clifton A, Markus HS. Noninvasive detection of vertebral artery stenosis: a comparison of contrast-enhanced MR angiography, CT angiography, and ultrasound. Stroke. 2009;40:3499–3503. doi: 10.1161/STROKEAHA.109.556035. [DOI] [PubMed] [Google Scholar]
- Knieling M, Metz GA, Antonow-Schlorke I, Witte OW. Enriched environment promotes efficiency of compensatory movements after cerebral ischemia in rats. Neuroscience. 2009;163:759–769. doi: 10.1016/j.neuroscience.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Lei H, Berthet C, Hirt L, Gruetter R. Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J Cereb Blood Flow Metab. 2009;29:811–819. doi: 10.1038/jcbfm.2009.8. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS. Imaging the future of stroke: I. Ischemia. Ann Neurol. 2009;66:574–590. doi: 10.1002/ana.21787. [DOI] [PubMed] [Google Scholar]
- Lim SH, Lee JS, Lee JI, Im S, Ko YJ, Kim HW. The quantitative assessment of functional impairment and its correlation to infarct volume in rats with transient middle cerebral artery occlusion. Brain Res. 2008;1230:303–309. doi: 10.1016/j.brainres.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Lindberg PG, Bensmail D, Bussel B, Maier MA, Feydy A. Wallerian Degeneration in Lateral Cervical Spinal Cord Detected with Diffusion Tensor Imaging in Four Chronic Stroke Patients. J Neuroimaging. 2009 doi: 10.1111/j.1552-6569.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhen G, Meloni BP, Campbell K, Winn HR. rodent stroke model guidelines for preclinical stroke trials. Journal of Experimental Stroke and Translational Medicine. 2009;2:2–27. doi: 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd MH, Wolfensohn SE. Practical use of distress scoring systems in the application of humane endpoints. In: CFMHDBM, editor. Humane endpoints in animal experiments for biomedical research. London, UK: Royal Society for Medicine Press Ltd; 1999. pp. 48–53. [Google Scholar]
- Lo R, Gitelman D, Levy R, Hulvershorn J, Parrish T. Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage. 49:9–18. doi: 10.1016/j.neuroimage.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Lonzo LM, Nunez SY, Dockstader T, Mathieu-Costello O, Zivin JA. Effect of ischemic cerebral volume changes on behavior. Behav Brain Res. 1997;87:59–67. doi: 10.1016/s0166-4328(96)02269-3. [DOI] [PubMed] [Google Scholar]
- Ma H, Zavala JA, Teoh H, Churilov L, Gunawan M, Ly J, Wright P, Phan T, Arakawa S, Davis SM, Donnan GA. Fragmentation of the Classical Magnetic Resonance Mismatch “Penumbral” Pattern With Time. Stroke. 2009 doi: 10.1161/STROKEAHA.109.555011. [DOI] [PubMed] [Google Scholar]
- Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 2009;1280:107–116. doi: 10.1016/j.brainres.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik J, Ogasawara A, Martin-McNulty B, Ross J, Flores JE, Gill HS, Tinianow JN, Vanderbilt AN, Nishimura M, Peale F, Pastuskovas C, Greve JM, van Bruggen N, Williams SP. PET of glial metabolism using 2-18F-fluoroacetate. J Nucl Med. 2009;50:982–990. doi: 10.2967/jnumed.108.057356. [DOI] [PubMed] [Google Scholar]
- Martin A, Rojas S, Pareto D, Santalucia T, Millan O, Abasolo I, Gomez V, Llop J, Gispert JD, Falcon C, Bargallo N, Planas AM. Depressed glucose consumption at reperfusion following brain ischemia does not correlate with mitochondrial dysfunction and development of infarction: an in vivo positron emission tomography study. Curr Neurovasc Res. 2009;6:82–88. doi: 10.2174/156720209788185650. [DOI] [PubMed] [Google Scholar]
- Massaweh G, Schirrmacher E, la Fougere C, Kovacevic M, Wangler C, Jolly D, Gravel P, Reader AJ, Thiel A, Schirrmacher R. Improved work-up procedure for the production of [(18)F]flumazenil and first results of its use with a high-resolution research tomograph in human stroke. Nucl Med Biol. 2009;36:721–727. doi: 10.1016/j.nucmedbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Mattsson B, Sorensen JC, Zimmer J, Johansson BB. Neural grafting to experimental neocortical infarcts improves behavioral outcome and reduces thalamic atrophy in rats housed in enriched but not in standard environments. Stroke. 1997;28:1225–1231. doi: 10.1161/01.str.28.6.1225. discussion 1231–1222. [DOI] [PubMed] [Google Scholar]
- McGill JK, Gallagher L, Carswell HV, Irving EA, Dominiczak AF, Macrae IM. Impaired functional recovery after stroke in the stroke-prone spontaneously hypertensive rat. Stroke. 2005;36:135–141. doi: 10.1161/01.STR.0000149629.32525.b7. [DOI] [PubMed] [Google Scholar]
- Michalski D, Kuppers-Tiedt L, Weise C, Laignel F, Hartig W, Raviolo M, Schneider D, Hobohm C. Long-term functional and neurological outcome after simultaneous treatment with tissue-plasminogen activator and hyperbaric oxygen in early phase of embolic stroke in rats. Brain Res. 2009 doi: 10.1016/j.brainres.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Modo M, Beech JS, Meade TJ, Williams SC, Price J. A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. Neuroimage. 2009;47(Suppl 2):T133–142. doi: 10.1016/j.neuroimage.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modo M, Sowinski P, Hodges H. Conditional discrimination learning in rats with global ischaemic brain damage. Behav Brain Res. 2000a;111:213–221. doi: 10.1016/s0166-4328(00)00160-1. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33:2270–2278. doi: 10.1161/01.str.0000027693.50675.c5. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of dead stem cells in rats with stroke damage. Neuroreport. 2003;14:39–42. doi: 10.1097/00001756-200301200-00007. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. J Neurosci Methods. 2000b;104:99–109. doi: 10.1016/s0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Munoz Maniega S, Cvoro V, Chappell FM, Armitage PA, Marshall I, Bastin ME, Wardlaw JM. Changes in NAA and lactate following ischemic stroke: a serial MR spectroscopic imaging study. Neurology. 2008;71:1993–1999. doi: 10.1212/01.wnl.0000336970.85817.4a. [DOI] [PubMed] [Google Scholar]
- Murakami M, Fujioka S, Hirata Y, Kuratsu J. Low-dose of statin treatment improves cerebrovascular reactivity in patients with ischemic stroke: single photon emission computed tomography analysis. J Stroke Cerebrovasc Dis. 2008;17:16–22. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Murata Y, Sakatani K, Hoshino T, Fujiwara N, Kano T, Nakamura S, Katayama Y. Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and BOLD contrast functional MRI in stroke patients. Stroke. 2006;37:2514–2520. doi: 10.1161/01.STR.0000239698.50656.3b. [DOI] [PubMed] [Google Scholar]
- Nikolova S, Moyanova S, Hughes S, Bellyou-Camilleri M, Lee TY, Bartha R. Endothelin-1 induced MCAO: dose dependency of cerebral blood flow. J Neurosci Methods. 2009;179:22–28. doi: 10.1016/j.jneumeth.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Pazos AJ, Orezzoli SL, McCabe PM, Dietrich WD, Green EJ. Recovery of vibrissae-dependent behavioral responses following barrelfield damage is not dependent upon the remaining somatosensory cortical tissue. Brain Res. 1995;689:224–232. doi: 10.1016/0006-8993(95)00579-f. [DOI] [PubMed] [Google Scholar]
- Poppe AY, Coutts SB, Kosior J, Hill MD, O’Reilly CM, Demchuk AM. Normal magnetic resonance perfusion-weighted imaging in lacunar infarcts predicts a low risk of early deterioration. Cerebrovasc Dis. 2009;28:151–156. doi: 10.1159/000225908. [DOI] [PubMed] [Google Scholar]
- Puetz V, Sylaja PN, Hill MD, Coutts SB, Dzialowski I, Becker U, Gahn G, von Kummer R, Demchuk AM. CT Angiography Source Images Predict Final Infarct Extent in Patients with Basilar Artery Occlusion. AJNR Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef A, Sperling O, Zoref-Shani E. Opening of ATP-sensitive potassium channels by cromakalim confers tolerance against chemical ischemia in rat neuronal cultures. Neurosci Lett. 1998;250:111–114. doi: 10.1016/s0304-3940(98)00458-3. [DOI] [PubMed] [Google Scholar]
- Risedal A, Nordborg C, Johansson BB. Infarct volume and functional outcome after pre- and postoperative administration of metyrapone, a steroid synthesis inhibitor, in focal brain ischemia in the rat. Eur J Neurol. 1999;6:481–486. doi: 10.1046/j.1468-1331.1999.640481.x. [DOI] [PubMed] [Google Scholar]
- Rivers CS, Wardlaw JM, Armitage PA, Bastin ME, Carpenter TK, Cvoro V, Hand PJ, Dennis MS. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke. 2006;37:98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- Rojas S, Martin A, Arranz MJ, Pareto D, Purroy J, Verdaguer E, Llop J, Gomez V, Gispert JD, Millan O, Chamorro A, Planas AM. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab. 2007;27:1975–1986. doi: 10.1038/sj.jcbfm.9600500. [DOI] [PubMed] [Google Scholar]
- Romanova GA, Silachev DN, Shakova FM, Kvashennikova YN, Viktorov IV, Shram SI, Myasoedov NF. Neuroprotective and antiamnesic effects of Semax during experimental ischemic infarction of the cerebral cortex. Bull Exp Biol Med. 2006;142:663–666. doi: 10.1007/s10517-006-0445-0. [DOI] [PubMed] [Google Scholar]
- Roulston CL, Callaway JK, Jarrott B, Woodman OL, Dusting GJ. Using behaviour to predict stroke severity in conscious rats: post-stroke treatment with 3′, 4′-dihydroxyflavonol improves recovery. Eur J Pharmacol. 2008;584:100–110. doi: 10.1016/j.ejphar.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–25. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Serdaroglu M, Schreiber F, Skalej M, Heinze HJ, Goertler M. Simultaneous occurrence and interaction of hypoperfusion and embolism in a patient with severe middle cerebral artery stenosis. Stroke. 2009;40:e478–480. doi: 10.1161/STROKEAHA.109.549378. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Dennin MA, Walberer M, Backes H, Neumaier B, Fink GR, Graf R. Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer [11C]PK11195- and [18F]FDG-PET study. J Cereb Blood Flow Metab. 2009;29:1216–1225. doi: 10.1038/jcbfm.2009.36. [DOI] [PubMed] [Google Scholar]
- Shen J, Sood R, Weaver J, Timmins GS, Schnell A, Miyake M, Kao JP, Rosen GM, Liu KJ. Direct visualization of mouse brain oxygen distribution by electron paramagnetic resonance imaging: application to focal cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1695–1703. doi: 10.1038/jcbfm.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemonsen S, Mouridsen K, Holst B, Ries T, Finsterbusch J, Thomalla G, Ostergaard L, Fiehler J. Quantitative t2 values predict time from symptom onset in acute stroke patients. Stroke. 2009;40:1612–1616. doi: 10.1161/STROKEAHA.108.542548. [DOI] [PubMed] [Google Scholar]
- Soderstrom I, Strand M, Ingridsson AC, Nasic S, Olsson T. 17beta-estradiol and enriched environment accelerate cognitive recovery after focal brain ischemia. Eur J Neurosci. 2009;29:1215–1224. doi: 10.1111/j.1460-9568.2009.06662.x. [DOI] [PubMed] [Google Scholar]
- Sood A, Knudsen K, Sood R, Wahner-Roedler DL, Barnes SA, Bardia A, Bauer BA. Publication bias for CAM trials in the highest impact factor medicine journals is partly due to geographical bias. J Clin Epidemiol. 2007;60:1123–1126. doi: 10.1016/j.jclinepi.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Mehrotra G, Bhargava SK, Agarwal S, Tripathi RP. Studies on the time course of apparent diffusion coefficient and signal intensities on T2- and diffusion-weighted MR Imaging in acute cerebral ischemic stroke. J Med Phys. 2008;33:162–170. doi: 10.4103/0971-6203.44479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Spatiotemporal correlations between blood-brain barrier permeability and apparent diffusion coefficient in a rat model of ischemic stroke. PLoS One. 2009;4:e6597. doi: 10.1371/journal.pone.0006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J Neurosci Methods. 2009;181:18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa T, Jadhav V, Solaroglu I, Shiokawa Y, Konishi Y, Zhang JH. Lecithinized superoxide dismutase improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Stroke. 2007;38:1057–1062. doi: 10.1161/01.STR.0000257978.70312.1d. [DOI] [PubMed] [Google Scholar]
- Umemura A, Suzuka T, Yamada K. Quantitative measurement of cerebral blood flow by (99m)Tc-HMPAO SPECT in acute ischaemic stroke: usefulness in determining therapeutic options. J Neurol Neurosurg Psychiatry. 2000;69:472–478. doi: 10.1136/jnnp.69.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virley D, Beech JS, Smart SC, Williams SC, Hodges H, Hunter AJ. A temporal MRI assessment of neuropathology after transient middle cerebral artery occlusion in the rat: correlations with behavior. J Cereb Blood Flow Metab. 2000;20:563–582. doi: 10.1097/00004647-200003000-00015. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies--the need for new approaches. Cerebrovasc Dis. 2004;17(Suppl 1):153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, Liu J. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow Metab. 2008;28:1936–1950. doi: 10.1038/jcbfm.2008.82. [DOI] [PubMed] [Google Scholar]
- Willing AE, Jiang L, Nowicki P, Poulos S, Milliken M, Cahill DW, Sanberg PR. Effects of middle cerebral artery occlusion on spontaneous activity and cognitive function in rats. Int J Neurosci. 2002;112:503–516. doi: 10.1080/00207450290025617. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, Li K. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage. 2009;47:451–458. doi: 10.1016/j.neuroimage.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Zaleska MM, Mercado ML, Chavez J, Feuerstein GZ, Pangalos MN, Wood A. The development of stroke therapeutics: promising mechanisms and translational challenges. Neuropharmacology. 2009;56:329–341. doi: 10.1016/j.neuropharm.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. The performance of MRI-based cerebral blood flow measurements in acute and subacute stroke compared with 15O-water positron emission tomography: identification of penumbral flow. Stroke. 2009;40:2413–2421. doi: 10.1161/STROKEAHA.108.540914. [DOI] [PubMed] [Google Scholar]
- Zou LY, Cheung RT, Liu S, Li G, Huang L. Melatonin reduces infarction volume in a photothrombotic stroke model in the wild-type but not cyclooxygenase-1-gene knockout mice. J Pineal Res. 2006;41:150–156. doi: 10.1111/j.1600-079X.2006.00349.x. [DOI] [PubMed] [Google Scholar]