Abstract
Aim
To investigate treatment-related pain and the accuracy of navigated laser photocoagulation in the treatment of clinically significant macular edema.
Methods
Focal laser treatment of diabetic macular edema in 54 consecutive patients was digitally planned on fundus images and performed using the navigated laser photocoagulation system Navilas® (OD-OS GmbH, Teltow, Germany). Treatment-related pain was quantified on a visual analog scale directly after treatment and compared with a matched control group who received conventional laser treatment (n = 46). In addition, for Navilas-treated patients, the accuracy of spot placement on color images was analyzed 1 month after treatment.
Results
In total, 5423 laser spots (mean 100 per eye) were analyzed. With navigated treatment, 90% of laser spots were visible on color images, of which 96% were within 100 μm from the target. Eighty percent of the laser spots were placed and visible within the 100 μm target on an intention-to-treat basis for color imaging. Optical coherence topography confirmed that laser effects were limited to the outer retina. Treatment-related pain following navigated laser photocoagulation was significantly lower than that of conventional laser treatment (1.6 vs 4.4 on a visual analog scale, P < 0.001).
Conclusion
Navigated laser effects could be visualized to a high percentage on post-treatment color images, and their location showed a high concordance to targeted areas. Patients reported that treatment-related pain following Navilas laser photocoagulation was significantly lower than pain following conventional laser treatment.
Keywords: diabetic retinopathy, navigated laser therapy, pattern laser, diabetes
Introduction
Diabetic retinopathy (DR) is the most frequent cause of new cases of blindness among adults of working age.1 Diabetic retinopathy can cause vision loss through several mechanisms, with diabetic macular edema (DME) being a major cause.2,3
Laser photocoagulation has played a central role in the treatment of both DR and DME in the past 30 years. The Diabetic Retinopathy Study (DRS) in 1971 marked the progression of photocoagulation clinical trials, which involved more than 1700 patients enrolled across 15 medical centers. A significant result of the DRS and other population-based studies (such as the Early Treatment of Diabetic Retinopathy Study) was the demonstration that retinal laser photocoagulation was a safe and effective treatment for both DR and DME. Since then, laser photocoagulation has become a well-established standard therapy for DME, and it has recently been supplemented by pharmacological interventions, particularly anti-vascular endothelial growth factor treatment.4
Meyer-Schwickerath introduced retinal photocoagulation therapy in the 1950s using the xenon arc photocoagulator, and Campbell et al first described successful retinal laser photocoagulation in 1964 – a tremendous evolution of retinal laser therapy has taken place since this time.5,6 Most importantly, we have seen photocoagulation become a less painful and more accurate treatment of retinal diseases, and several technical advances have been introduced to laser therapy including subthreshold techniques7 and pattern laser generation.8 However, the advantages of computerized precision (“eye tracking”) known from, eg, refractive surgery, have not become available despite several technical approaches towards that goal.9 Of note, in DME a protocol targeting microaneurysms appears to be advantageous versus a non-targeted early treatment diabetic retinopathy study (ETDRS) laser protocol,10 which may hint the importance of targeting precision when performing laser treatments. In addition, essential diagnostics for the planning of laser treatment, such as fluorescein angiography, were mostly separated from the actual treatment device until the development of the navigated laser photocoagulation system Navilas® (OD-OS GmbH, Teltow, Germany).
Navilas is a novel fundus imaging and laser treatment device that allows imaging (infrared, color, and fluorescein angiography) and integrated laser treatment of the retina. It includes pattern laser generation and a laser planning feature.11,12 Besides documentation, safety, and patient comfort, the main theoretical advantages of Navilas use lie in retina navigation, which may help to improve the clinical outcomes of laser treatment. In focal laser treatment, Navilas offers computerized image and target assistance systems, resulting in high precision and a theoretical reproducibility of 60– 110 μm.13 The integration of diagnostic information into the device may help to overcome shortcomings during the process of transferring laser surgery protocols and potential loss of accuracy, helping retinal physicians to achieve better outcomes when treating their patients. An increase in patient comfort and reduction of treatment-related pain may also result from this novel technology.
We therefore investigated clinical in vivo accuracy and treatment-related pain in patients undergoing focal laser treatment with the Navilas system for diabetic macular edema.
Methods
Navilas
The Navilas device and its operation have been described elsewhere. 11 In brief, Navilas combines imaging, laser application planning, and laser treatment in a single computer- controlled device. It fundamentally differs from most other laser devices by not being added to a slit lamp but is instead a scanning slit-based instrument: it uses approximately 25 images per second in imaging or treatment mode. For focal laser treatment, the field of view is 50°, and this is displayed on a monitor. The optical resolution of the instrument used in this study was 1280 × 1024 pixels resulting in 20–26 pixels/degree. Due to the slit-imaging principle, color images are obtained with high contrast and sharpness.13 Another difference to slit lamp-based laser devices is the touch-screen monitor used for imaging, planning, and treating fundus changes. This allows the retina specialist to plan laser spots on the screen for focal treatments, and then apply semi-automated patterns and single spots as appropriate. The pre-planned targets are stabilized on the fundus and a pre-positioning mode is provided by the system that automatically advances the aiming beam from each pre-planned target to the next. For treatment, the surgeon actuates the laser manually after he or she has verified the target lock.
Patients treated with navigated laser photocoagulation
Consecutive patients were recruited from the outpatient clinic of the Department of Ophthalmology, Ludwig Maximilian University of Munich, Germany. Patients were included if they had diabetes (based on WHO criteria) and were eligible for focal laser treatment as defined by the ETDRS criteria. Written informed consent was obtained from all patients. The device was a fully CE-approved laser at the time of this study. The study conformed to the principles expressed in the Declaration of Helsinki, and approval was obtained from the Ludwig Maximilian University’s Institutional Review Board. Patients were excluded if they suffered from eye diseases involving the posterior pole other than diabetic retinopathy (such as age-related macular degeneration), but they were not excluded for media opacities.
Imaging
Navilas imaging was performed with fully dilated pupils after the clinical examination and before laser therapy, and consisted of taking and saving several color images. Color imaging with Navilas was repeated 1 month after laser treatment and compared with pre-treatment images. Assessment of treatment success was performed by one experienced grader. Additionally, spectral domain optical coherence topography (SD-OCT) was performed 1 month after laser treatment (Heidelberg Engineering, Heidelberg, Germany).
Laser planning and treatment
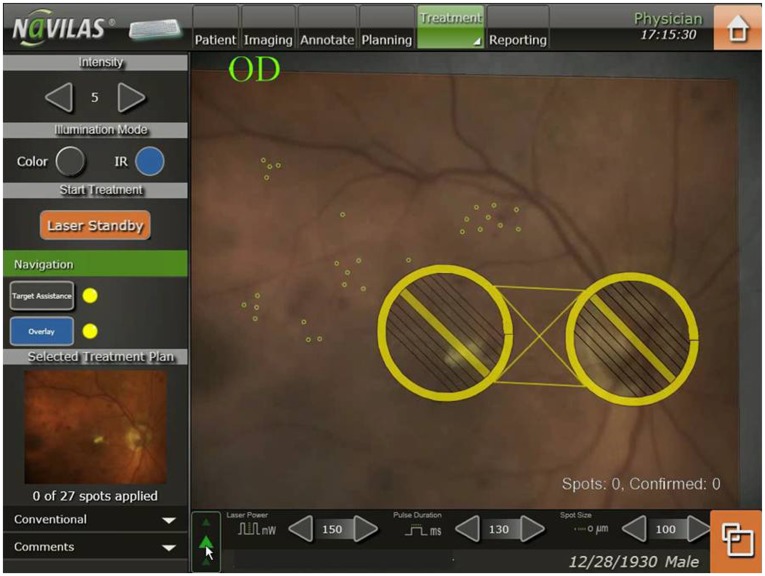
Laser planning and treatment was performed according to the ETDRS principles, not specifically targeting microaneurysms. A color image was obtained with the Navilas device and used to plan laser spot treatments by applying automated patterns and single spots as appropriate on the color image, thus generating a detailed treatment plan (Figure 1). It took less than 5 minutes to plan the treatment for each eye.
Figure 1.
Pre-planned focal laser spots (small circles) in the Navilas® system on color image. The large circles indicate non-treatment zones.
For laser application, a navigated, semi-automatic pattern laser application was conducted based on the treatment plan. Treatment spot size was always set at 100 μm, and the treatment time per spot was 100 milliseconds. The power settings of the green (532 nm) frequency doubled neodymium-doped yttrium aluminium garnet (Nd:YAG) laser were adjusted manually from a standard 100 mW so that spots showed a moderate whitening. The pre-positioning mode was used to automatically advance the targeting aiming beam from each targeted retinal position to the next position after the aiming beam and pre-planned target were stabilized on the living fundus. The surgeon actuated the laser manually after verifying the target lock. An alternating infrared to color video fundus visualization mode was applied to allow post-treatment observation of the retinal burn for 2–3 seconds after each application without patient discomfort.
Grading of images
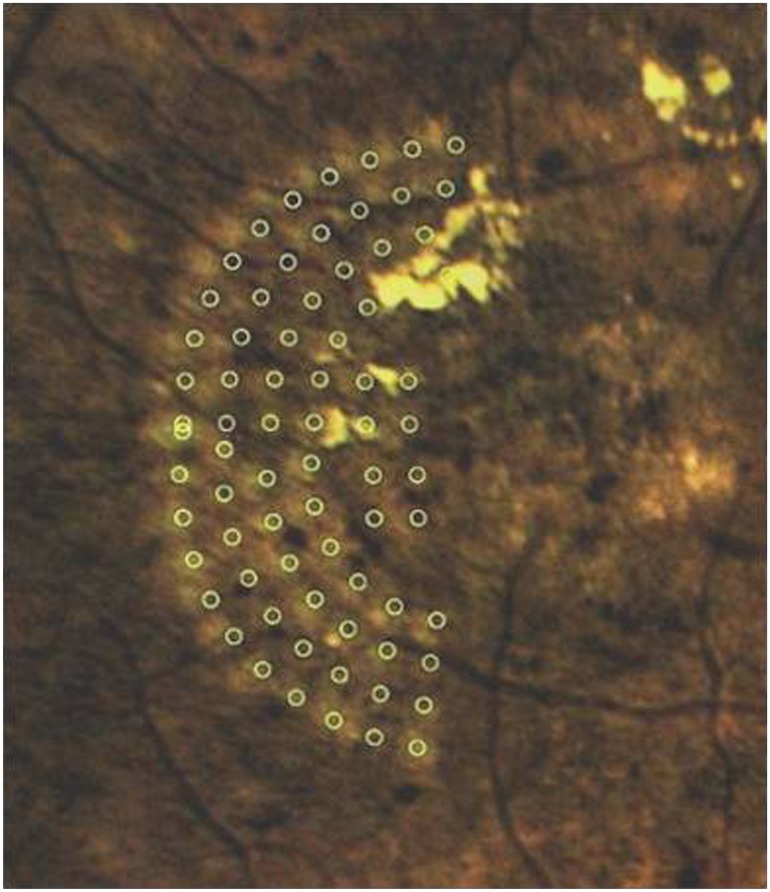
The laser spots visible on the Navilas color images 1 month after treatment were compared to the laser treatment plan by overlaying the laser treatment plan with the post-treatment color image (Figure 2). The resulting image was post-processed using ImageJ (National Institutes of Health, Bethesda, MD) to improve the visibility of laser spots: the contrast was enhanced, the background reduced, and the image was zoomed to the region of interest. The post-processed images (Figure 2) were used to determine whether each treatment spot touched or overlapped the targeted 100 μm laser spot (this was given a binary result: “match” or “no match”). The SD-OCT images were investigated for changes in the inner retinal layers overlying the laser spots. All data were collected in a Microsoft Excel 2000 spreadsheet (Microsoft Corporation, Redmond, WA) and analyzed using SPSS software (v 17.0 for Windows; IBM Corp, Armonk, NY).
Figure 2.
Pre-planned focal laser spots (circles) overlaid with post-processed color image 1 month after laser treatment with Navilas®.
Evaluation of treatment-related pain after Navilas treatment
Immediately after treatment, patients were asked to quantify their pain on a visual analog scale (VAS; 0 = no pain; 10 = very bad pain). To evaluate treatment-related pain after Navilas treatment versus conventional treatment, data were collected from the 54 patients treated with the Navilas system as well as from a matched control group of 46 subjects treated with a conventional laser (532 nm wave-length, VISULAS 532s, Carl Zeiss Meditec AG, Jena, Germany; laser settings: 100 mW power, 100 millisecond pulse duration, 100 μm spot size). All patients were treated for DME at the authors’ diabetes clinic during the same period. The two groups were analyzed and compared using SPSS software. For statistical testing, P < 0.05 was considered significant.
Results
In the Navilas group, mean HbA1C was 7.8% (±4.2 SD), while in the matched control group that received conventional laser treatment the mean HbA1C was 7.4 (±3.8 SD). Mean central retinal thickness (CRT) before treatment was 402 μm (±154 μm SD) in the Navilas group and 388 μm (±172 μm SD) in the control group.
Treatment-related pain after Navilas treatment
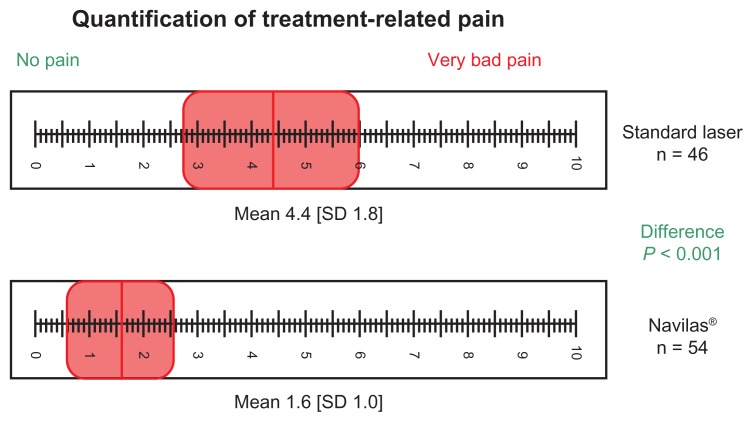
Patients who received Navilas treatment quantified post-treatment pain on the VAS with a mean of 1.6 (SD = 1.0), significantly lower (P < 0.001) than for the control group which had a mean of 4.4 (SD = 1.8) (Figure 3). Significantly fewer (mean 43 ± 36, P < 0.001) laser spots were applied to patients in the conventional laser group than those in the Navilas group.
Figure 3.
Patient’s treatment-related pain after navigated focal laser therapy with Navilas® was quantified by using a VAS.
Abbreviations: SD, standard deviation; VAS, visual analog scale.
Accuracy of navigated retinal laser photocoagulation with Navilas
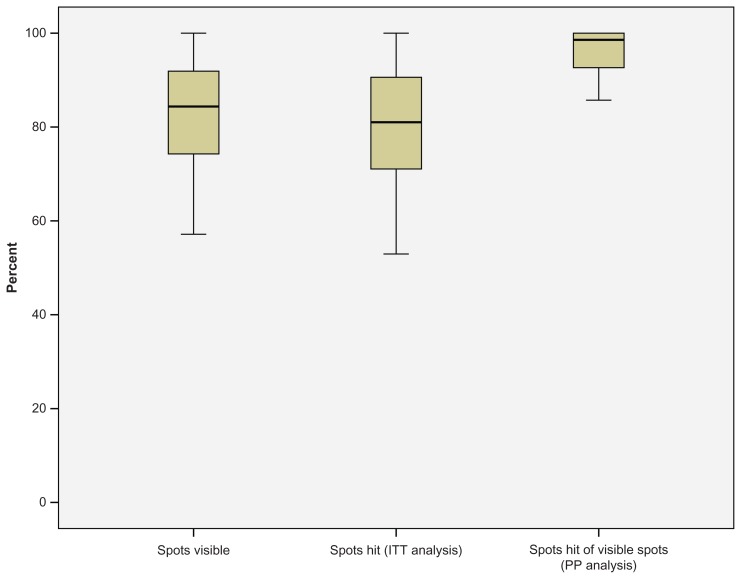
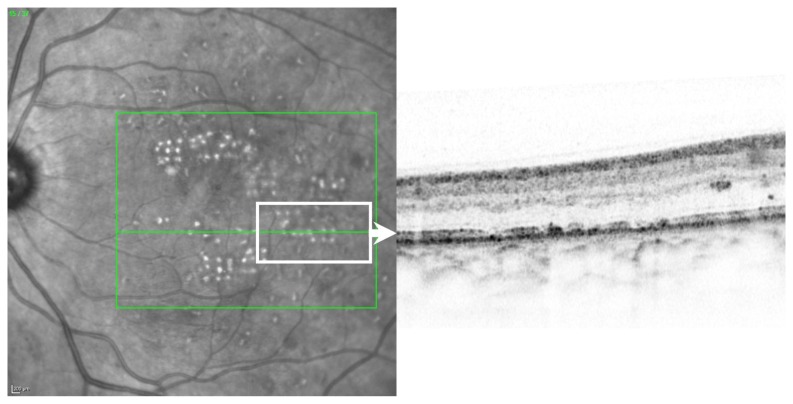
A mean of 100 ± 86 spots per eye were pre-planned for treatment in 54 patients. All patients had clinically significant macular edema (as defined by the ETDRS) caused by type 2 diabetes. In total, 25 right and 29 left eyes were treated. The mean patient age was 61 ± 12 years. In total, 5423 pre-planned laser spots were applied and analyzed, and no complications occurred. Of those laser spots, 4888 (90%) were visible in the 1 month color images (analysis per-protocol, PP). Mean hit-rate of visible spots was 96%. On an intention-to-treat (ITT) analysis based on color image burn evaluation the hit-rate was 80% (Figure 4). Retinal OCT morphology of laser spots confirmed that laser lesions were limited to the outer retina, particularly the retinal pigment epithelium and photoreceptor layer (Figure 5).
Figure 4.
Accuracy of in vivo Navilas® treatment based on n = 54 and 100 ± 86 spots per patient. In total, 5423 pre-planned laser spots were applied and analyzed. Of those laser spots, 4888 (90%) were visible in the 1 month color images. The mean hit-rate of visible spots (color imaging) was 80% on an intention-to-treat (ITT) basis and 96% on a per-protocol (PP) basis.
Figure 5.
Example of the retinal optical coherence topography morphology of laser spots.
Discussion
The ETDRS demonstrated the effectiveness of focal photocoagulation in eyes with macular edema.2 In macular laser photocoagulation (focal or modified grid), the main goal is to solve leakage arising from macular vessels by photocoagulating photoreceptors. This results in a reduced need for oxygen in the outer retina, allowing oxygen to diffuse from the choroid to the inner retina, so reducing hypoxia.14 In addition, increased oxygen tension in the inner retina helps to decrease tissue edema and improve vision by autoregulatory vasoconstriction and reduced hydrostatic pressure in the capillaries and venules.14,15 Therefore, focal photocoagulation, recently supplemented by treatments such as vascular endothelial growth factor treatment, is still the standard treatment. Nevertheless, in combined treatment, the use of modified laser protocols seems to be advantageous,4 and these data underscore the importance of precision laser targeting. Moreover, inadequate application of macular laser photocoagulation may be associated with loss of vision, diminished visual field, increased scotoma, reduced color vision, and reduced contrast sensitivity.16,17
The present study investigated the clinical accuracy of focal retinal laser application using a navigated, semi-automatic pattern laser. By utilizing computerized laser surgeon support, a 96% hit rate was achieved on an analyzable (“per-protocol”) basis and 80% on an ITT basis. A target size of 100 μm spots was applied to all patients, demonstrating the device’s high laser spot application accuracy. The study does have some shortcomings such as missing clinical outcome data, a lack of follow-up, and a limited number of patients. It is also noted that the computerized laser surgeon support systems such as Navilas’s pre-positioning mode, target stabilization, and target assistance require complex and fast real-time image processing, which may result in errors. Although those occurred rarely in our patient series, the final actuating of the laser needs to be performed by the surgeon. In addition, it is possible to continue with manual treatment as a fallback in case the electronic systems do not operate adequately.
The strengths of the system include image quality and remarkable laser spot accuracy on an ITT basis, especially given the limited evaluation properties of color images per se. Still, the present study’s results demonstrate the high accuracy of the Navilas system. The authors believe that the current study demonstrates for the first time that 100 μm pre-defined targets can be hit in vivo with very high precision by a navigated laser system. In an independent study, Kozac et al showed similar results targeting retinal microaneurysms with the device.18 The clinical value of targeting micronaneurysms in DME is disputed,10 but this technique may provide data on accuracy as they are anatomical landmarks on the fundus. In contrast to the work by Freeman’s group we measured the device’s ability to hit predefined fundus spots, which demonstrates accuracy with a given size target. While this approach has higher image processing requirements, it can be more universally applied to other diseases than DME, where improvements in accuracy may also have value, such as in venous occlusions or age-related macular degeneration. Navilas may serve as a platform for the development of new technology and its application in the treatment of those diseases or in special laser applications, such as subthreshold technology.
Patients suffering from DME do not always respond sufficiently to focal laser photocoagulation, which may be because laser treatment cannot always meet the patient’s medical requirements. There are many reasons for this, but it appears to be quite common for patients not to accept or understand the need for laser treatment, and many report that treatment is uncomfortable or painful. The tracking of pain has been found to be a helpful diagnostic tool when evaluating the patient’s treatment-related pain.19,20 By using a VAS the patient bypasses the cognitive level of the brain, resulting in a truer representation of pain.19,20
In our study, patients were asked to quantify treatment-related pain by using a VAS, and those who received navigated focal laser photocoagulation rated this as significantly less painful than conventional slit lamp-based laser photocoagulation (1.6 vs 4.4 respectively on a scale from 1 [no pain] to 10 [very strong pain]). This implicates another potential advantage of navigated laser therapy with Navilas, and the use of devices such as this may result in better patient compliance and acceptance of this treatment option.
In summary, this paper presents data that demonstrates that the Navilas system is accurate when targeting predefined targets in vivo in DME focal laser therapy. This not only suggests that such a computer-supported retinal navigation focal laser therapy is feasible but also demonstrates an accuracy of 80% on an intention-to-treat basis and 96% on a per-protocol basis. In addition, patients reported significantly less treatment-related pain following navigated focal laser photocoagulation compared to patients treated with a conventional laser. This may result in a higher acceptance of macular laser therapy in DME patients, which may help to improve treatment outcomes.
Acknowledgments
The authors thank Mrs S Guthmann and Mrs N Ehsani for expert technical assistance with OCT imaging.
Footnotes
Disclosure
Marcus Kernt and Aljoscha S Neubauer are consultants for OD-OS GmbH.
References
- 1.Icks A, Trautner C, Haastert B, Berger M, Giani G. Blindness due to diabetes: population-based age-and sex-specific incidence rates. Diabet Med. 1997;14(7):571–575. doi: 10.1002/(SICI)1096-9136(199707)14:7<571::AID-DIA384>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study repor t number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 3.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766–785. [PubMed] [Google Scholar]
- 4.Soheilian M, Ramezani A, Obudi A, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116(6):1142–1150. doi: 10.1016/j.ophtha.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Campbell CJ, Noyori KS, Rittler MC, Innis RE, Koester CJ. The application of fiber laser techniques to retinal surgery. Arch Ophthalmol. 1964;72:850–857. doi: 10.1001/archopht.1964.00970020852022. [DOI] [PubMed] [Google Scholar]
- 6.Meyer–Schwickerath G. Light coagulation; a method for treatment and prevention of the retinal detachment. Albrecht Von Graefes Arch Ophthalmol. 1954;156(1):2–34. German. [PubMed] [Google Scholar]
- 7.Nakamura Y, Mitamura Y, Ogata K, Arai M, Takatsuna Y, Yamamoto S. Functional and morphological changes of macula after subthreshold micropulse diode laser photocoagulation for diabetic macular oedema. Eye (Lond) 2010;24(5):784–788. doi: 10.1038/eye.2009.207. [DOI] [PubMed] [Google Scholar]
- 8.Bolz M, Kriechbaum K, Simader C, et al. In vivo retinal morphology after grid laser treatment in diabetic macular edema. Ophthalmology. 2010;117(3):538–544. doi: 10.1016/j.ophtha.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Naess E, Molvik T, Ludwig D, et al. Computer–assisted laser photocoagulation of the retina – a hybrid tracking approach. J Biomed Opt. 2002;7(2):179–189. doi: 10.1117/1.1461831. [DOI] [PubMed] [Google Scholar]
- 10.Fong DS, Strauber SF, Aiello LP, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125(4):469–480. doi: 10.1001/archopht.125.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kernt M, Cheuteu R, Vounotrypidis E, et al. Focal and panretinal photocoagulation with a navigated laser (NAVILAS®) Acta Ophthalmol. 2011;89(9):e662–e664. doi: 10.1111/j.1755-3768.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 12.Kozak I, Kim JS, Oster SF, Chhablani J, Freeman WR. Focal navigated laser photocoagulation in retinovascular disease: clinical results in initial case series. Retina. 2011 doi: 10.1097/IAE.0b013e318227ab5b. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 13.Kernt M, Neubauer AS, Liegl R, et al. Cytoprotective effects of a blue light-filtering intraocular lens on human retinal pigment epithelium by reducing phototoxic effects on vascular endothelial growth factor-alpha, Bax, and Bcl-2 expression. J Cataract Refract Surg. 2009;35(2):354–362. doi: 10.1016/j.jcrs.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 14.Arnarsson A, Stefánsson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000;41(3):877–879. [PubMed] [Google Scholar]
- 15.Stefansson E, Landers MB, 3rd, Wolbarsht ML. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc. 1981;79:307–334. [PMC free article] [PubMed] [Google Scholar]
- 16.Han DP, Mieler WF, Burton TC. Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113(5):513–521. doi: 10.1016/s0002-9394(14)74722-1. [DOI] [PubMed] [Google Scholar]
- 17.Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 2007;27(7):816–824. doi: 10.1097/IAE.0b013e318042d32c. [DOI] [PubMed] [Google Scholar]
- 18.Kozak I, Oster SF, Cortes MA, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology. 2011;118(6):1119–1124. doi: 10.1016/j.ophtha.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hussainy S, Dodson PM, Gibson JM. Pain response and follow-up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye (Lond) 2008;22(1):96–99. doi: 10.1038/sj.eye.6703026. [DOI] [PubMed] [Google Scholar]
- 20.Roberts CJ, MacLeod JD, Elkington AR. Ocular pain: a casualty study. The spectrum and prevalence of pain in acute eye disease. Eye (Lond) 1997;11(Pt 3):342–344. doi: 10.1038/eye.1997.72. [DOI] [PubMed] [Google Scholar]