Summary
Background
TF is highly expressed in cancerous and atherosclerotic lesions. Monocyte recruitment is a hallmark of disease progression in these pathological states.
Objective
To examine the role of integrin signaling in TF-dependent recruitment of monocytes by endothelial cells.
Methods
The expression of flTF and asTF in cervical cancer and atherosclerotic lesions was examined. Biologic effects of the exposure of primary microvascular endothelial cells (MVEC) to truncated flTF ectodomain (LZ-TF) and recombinant asTF were assessed.
Results
flTF and asTF exhibited nearly identical expression patterns in cancer lesions and lipid-rich plaques. Tumor lesions as well as stromal CD68+ monocytes/macrophages expressed both TF forms. Primary MVEC rapidly adhered to asTF and LZ-TF, and this was completely blocked by anti-β1 integrin antibody. asTF- and LZ-TF-treatment of MVEC promoted adhesion of peripheral blood mononuclear cells (PBMC) under orbital shear conditions and under laminar flow; asTF-elicited adhesion was more pronounced than that elicited by LZ-TF. Expression profiling and western blotting revealed a broad activation of cell adhesion molecules (CAMs) in MVEC following asTF treatment including E-selectin, ICAM-1, and VCAM-1. In transwell assays, asTF potentiated PMBC migration through MVEC monolayers by ~3 fold under MCP-1 gradient.
Conclusions
TF splice variants ligate β1 integrins on MVEC, which induces the expression of CAMs in MVEC and leads to monocyte adhesion and transendothelial migration. asTF appears more potent than flTF in eliciting these effects. Our findings underscore the pathophysiologic significance of non-proteolytic, integrin-mediated signaling by the two naturally occurring TF variants in cancer and atherosclerosis.
Keywords: Tissue Factor, alternative splicing, cervical cancer, atherosclerosis, endothelium, monocyte adhesion
Introduction
Alternatively spliced Tissue Factor (asTF) is a naturally occurring form of Tissue Factor (TF) that lacks the transmembrane domain and exhibits lower pro-coagulant activity compared to decrypted full-length TF (flTF) [1]. We recently reported that human asTF possesses non-proteolytic biologic activity such that it ligates integrins α6β1 and αvβ3 on endothelial cell (EC) surfaces, which triggers fVIIa- and/or PAR-2 independent neovascularization [2]. asTF is more pro-angiogenic than flTF in aortic sprouting assays, and the levels of asTF protein in cervical cancer tissue are in 10–75 nM range, vastly exceeding those in systemic circulation, with asTF’s pro-angiogenic activity manifest at ~1 nM [2]. Plaque neovascularization is a major contributor to its destabilization: vasa vasorum as well as intraplaque vessels are microvascular in nature and plaque infiltration by monocyte/macrophages is strongly associated with abnormal EC morphology [3]. Similarly to atherosclerosis, tumor progression involves heavy infiltration of the tumor site by monocytes that differentiate into M2-type macrophages secreting tumor- and vessel-promoting cytokines [4]. Monocyte-endothelial interactions precede, and are required for monocyte transmigration [5,6]. Integrin ligation by asTF activates multiple kinases, including those comprising the PI3K/Akt pathway [2]. Engagement of PI3K/Akt signaling is known to activate NFkB [7] – a transcription factor involved in upregulation of leukocyte adhesion molecules that play a major role in atherogenesis. Of note, the levels of non-cellular TF are markedly increased in the aqueous humor of patients presenting with proliferative diabetic retinopathy – a condition in which leukocyte adhesion to microvascular EC (MVEC) is a major pathophysiological determinant [8,9].
While flTF and asTF proteins are detectable in organized mural thrombi, some atherosclerotic lesions, and various forms of cancer [1,10], it is not known whether these two naturally occurring TF variants may non-proteolytically contribute to atherogenesis and/or tumorigenesis by eliciting integrin-mediated biologic effects beyond the direct induction of neovascularization. In this study, we examined whether flTF and asTF act as agonists on MVEC – the endothelial subtype most relevant to monocyte egress from the systemic circulation.
Materials and Methods
Antibodies, chemicals, and supplies
Please refer to Online Supplement for the complete list of antibodies, chemicals, and cell culture supplies used in the study.
Tissue specimens, immunohistochemistry, and immunofluorescence studies
Use of de-identified banked specimens was approved by the Institutional Review Board of Leiden University Medical Center (Leiden, The Netherlands), Mount Sinai School of Medicine (New York, USA), and Charite Clinic (Berlin, Germany). Serial sections of formalin-fixed, paraffin-embedded specimens were deparaffinized and analyzed using standard immunohistochemical techniques as described [1,11]; images were captured using BX51 microscope and DP72 camera (Olympus). Immunofluorescence studies are detailed in Online Supplement.
Cell culture
Primary human cardiac MVEC were purchased from Lonza. Cells were grown in EBM medium supplemented with antibiotics (Lonza). Primary human retinal MVEC were purchased from Cell Systems and maintained in MCDB-131 medium (Cell Systems). For experiments, MVEC between passages 3 and 6 were used. THP-1 cells (ATCC) were maintained in complete RPMI medium; peripheral blood mononuclear cells (PBMC) were isolated from whole blood as previously described [12].
RT-PCR
Please refer to Online Supplement for the list of reagents and primers employed for RT-PCR.
Flow cytometry
MVEC were grown to confluence and detached using 2 mM EDTA in PBS. Cells were centrifuged, washed in PBS, blocked in 1% BSA, incubated with anti-β1/β1 antibodies for 30 min, washed in PBS, and incubated with anti-mouse goat-IgG (Alexa-488) for 30 min. Cells were then washed with PBS, and flow cytometry was performed using Epics XL (Beckman Coulter) with 525 band pass filter.
Human TF proteins
Recombinant human asTF mature peptide with an N-terminal His-tag followed by the enterokinase cleavage site (DDDDK) was produced in E. Coli and purified as previously described [2]; asTF purity and identity were confirmed by Coomassie staining and western blotting, respectively (not shown); asTF’s biologic activity was preserved following the cleavage of the His-tag and removal of enterokinase (Online Supplement). Recombinant human flTF extracellular domain with the GCN4 leucine zipper domain at the C-terminus (LZ-TF) was previously described [13].
MVEC adhesion assay
asTF and LZ-TF (100 ng/well) were used to coat 96-well tissue culture plates; 10% BSA (100 μl/well) served as control. MVEC were trypsinized, neutralized using serum-containing medium, washed, added to 96-well plates at 20,000 cells/well and left to adhere under 5% CO2 at 37°C for 2 hrs. Following the incubation, non-adherent cells were removed by washing the wells twice with PBS. The adherent cells were fixed in methanol, stained with crystal violet (Sigma), and counted at 10X using phase-contrast inverted microscope (Olympus) in three random fields excluding the edges.
Monocyte-MVEC interaction assays
Orbital shear assay
MVEC were grown to confluence in 96-well plates, after which LZ-TF/asTF (final concentration 50 nM) was added to the wells for 4 hrs; equal volumes of 50% glycerol in PBS served as the vehicle control. Functional blocking studies of LZ-TF/asTF were carried out using 6B4 antibody (100 μg/ml) that hinders TF association with integrins [2]. PBMC/THP-1 cells were labeled with 1 μM Calcein-AM for 30 min, washed in serum-free medium, and placed in 96-well plates added at 1.5 × 105 cells/well on an orbital shaker set at 90 rpm in a humidified incubator at 37°C and 5% CO2 for 1 hr. Following the incubation, plates were washed with PBS to remove non-adherent cells and lysed with 0.1% Triton-X in PBS for 15 min. Fluorescence was measured at Ex-485 and Em-535 in Omega Fluorimeter (BMG Labtech).
Parallel plate flow assay
MVEC were seeded in 35-mm tissue culture dishes and allowed to reach confluence over 3–4 days, following which LZ-TF/asTF (final concentration – 50 nM) was added to the medium for 4 hrs; equal volumes of 50% glycerol in PBS served as the vehicle control. Cells were washed with serum-free medium and assembled onto the flow chamber (Glycotech); subsequently, PBMC/THP-1 cells were perfused through the chamber at 0.5 × 106 cells/ml in RPMI-1640 media at 37°C using a syringe infusion pump (Harvard Apparatus) under a phase-contrast inverted microscope (Olympus, PA); the shear rate was set to 0.5 dynes/cm2. Video recordings were made using a Moticam camera (Motic) and adherent cells were counted; each cell that adhered for at least 1 second was deemed a firm adhesion/cell arrest event.
Microarray analysis
MVEC were treated for 4 hrs with recombinant asTF or LZ-TF added to the medium (final concentration – 50 nM); equal volumes of 50% glycerol in PBS served as the vehicle control. Total RNA was isolated using RNAeasy Kit (Qiagen), reverse transcribed, amplified, fragmented, and labeled for microarray analysis using the Nugen WT-Ovation FFPE V2 kit, Exon Module, and Encore biotin module, respectively (Nugen) according to the manufacturer’s instructions. Affymetrix Human Gene 1.ST microarray chips were used to assess the gene expression profile (Microarray Core Facility, Cincinnati Children’s Hospital and Medical Center). Transcripts that were differentially expressed as a result of either LZ-TF or asTF treatment were identified based on filtering for probesets with Robust Multichip Average-normalized raw expression of greater than 6.0 in either of the three pairs of replicates, that differed between treated and untreated MVEC by at least 1.5 fold with p<0.05 using a Welch t-test. Using this approach, we identified 223 genes that were upregulated in both MVEC subtypes by asTF,, and 63 by LZ-TF
Western blotting
Please refer to Online Supplement for the list of reagents employed for western blotting.
Transendothelial migration assay
MVEC were grown to confluence on 3.0 μm pore size 24-well inserts (Millipore) and treated with asTF (final concentration – 50 nM) for 4 hrs, following which the medium in the receiver plate was replaced with medium containing 50 μg/L recombinant MCP-1 (R&D Systems). 3×105 PBMC/THP-1 cells per insert were added to the upper chamber for 1 hr, following which the non-adherent cells were removed, fresh medium was added, and the adherent cells were left to migrate overnight in 5% CO2 at 37°C. The following day, cells at the luminal side of the insert were removed by a cotton swab, the insert was excised, fixed in methanol, and cells on the abluminal side of the insert were stained with DAPI. Cells were counted at three different randomly selected membrane locations, excluding the edge.
Statistical analysis
The comparisons of means were performed using Student t-test. Two-sided p-values less than 0.05 were considered statistically significant.
Results
flTF and asTF protein expression in cervical cancer tissue and atherosclerotic plaques
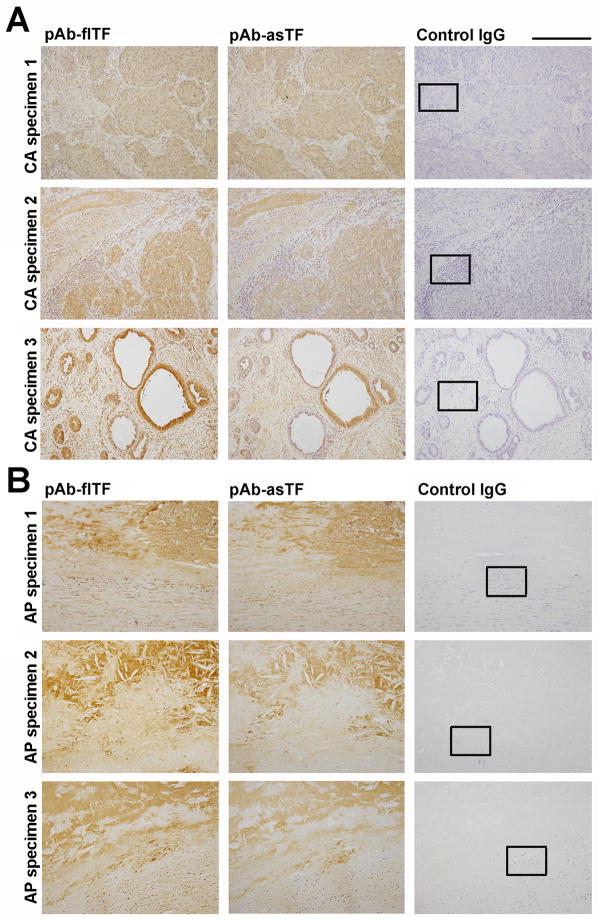
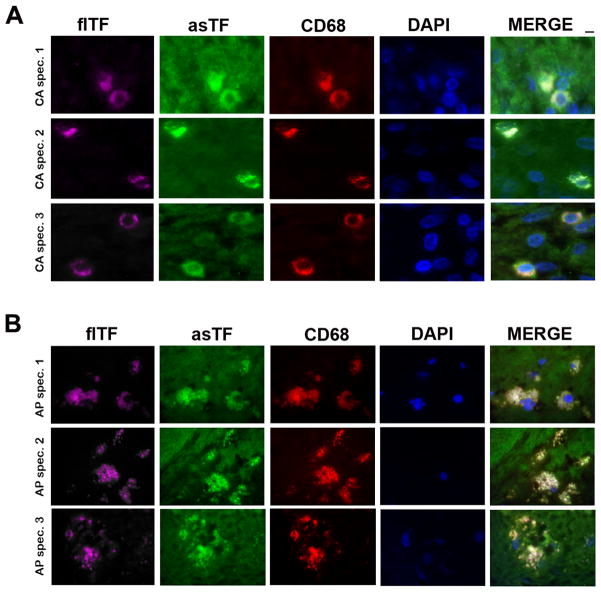
While it is known that asTF protein is present in cervical cancer tissue at concentrations in the 10–75 nM range, the cellular sources of asTF and/or the concomitance of asTF’s expression with that of flTF was not evaluated [2]; likewise, asTF was observed in organized spontaneous thrombi and atherosclerotic material, yet its cellular sources and/or co-localization with flTF was not examined in detail [1,10]. We analyzed the expression of flTF and asTF in sets of formalin-fixed, paraffin-embedded specimens of cervical adenocarcinoma (50%+ relative tumor mass, n=10) and lipid-rich aortic plaques (n=10). In cervical adenocarcinoma, flTF and asTF expression patterns appeared nearly identical, with extensive co-localization in most, yet not all, tumor lesions (Fig. 2A); in aortic plaques, flTF expression appeared more pronounced than that of asTF and again, overlapping patterns were observed for flTF and asTF (Fig. 2B). Aside from tumor lesions, stromal cells were also positive for flTF and asTF (Fig. 2A); we performed immunofluorescence studies using anti-CD68 antibodies and anti-fl/asTF specific antibodies (Fig. 1): asTF and flTF were found in abundance in CD68+ cells – monocytes/macrophages (Fig 3, S1). asTF staining in the extracellular space was clearly evident (Fig. 3A,B), in line with the fact that asTF can be secreted [14, 15]. CD68+ cells were also found to express flTF and asTF in aortic plaques (Fig. 3B).
Fig. 2.
Representative images, localization of flTF and asTF in paraffin-embedded specimens of cervical cancer tissue (A) and lipid-rich aortic plaques (B). CA, cervical adenocarcinoma; AP, aortic plaque. Final dilution for all primary antibodies – 2.0 μg/mL; original magnification – 20X; scale bar, 250 μm. In black boxes: the regions analyzed by immunofluorescence as shown in Fig. 3.
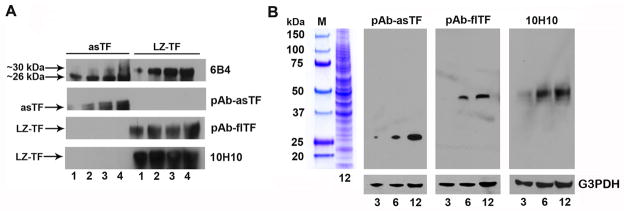
Fig. 1.
Characterization of the anti-human TF antibodies used in the study. (A) Reactivity of the four antibodies with recombinant asTF and LZ-TF: the indicated amounts of protein are in ng. (B) Specificity of the isoform-specific anti-TF antibodies: the indicated amounts of total protein (lysates of cervical adenocarcinoma) are in μg; blots we stripped and re-probed with anti-G3PDH antibody.
Fig 3.
Representative images, co-localization of CD68+cells (red), flTF (purple), and asTF (green) in cervical cancer tissue specimens (A) and lipid-rich aortic plaques (B). Final dilution for all primary antibodies – 2.0 μg/mL; original magnification – 100X; scale bar, 2 μm.
flTF and asTF ligate β1 integrins on MVEC surfaces
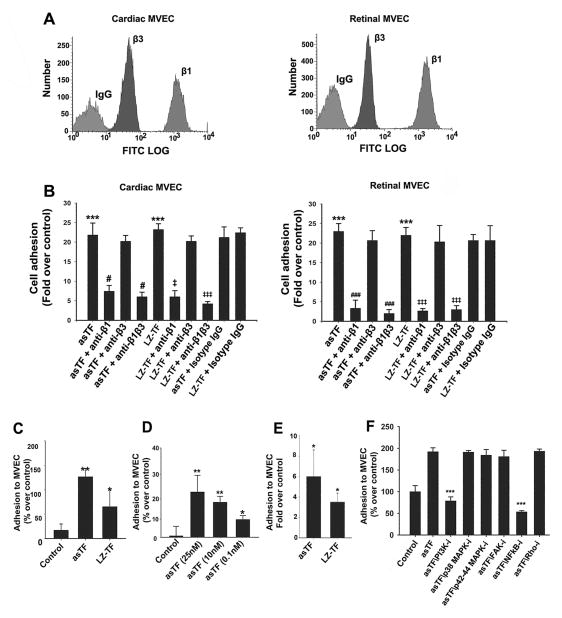
The αv integrin chain is expressed in human MVEC [16], yet it is not known whether MVEC express the β1 and β3 integrin chains that interacts with flTF as well as asTF [2]. β1 and β3 mRNA species were detected in cardiac and retinal MVEC; β3 mRNA expression appeared lower than that of β1 mRNA in both MVEC sub-types (not shown). Cell-surface expression ofβ1 and β3 integrins was analyzed by flow cytometry and again, β3 integrin levels were remarkably lower than those of β1 in both MVEC sub-types (Fig. 4A). We then performed cell adhesion assays to determine whether MVEC can bind TF isoforms. For flTF studies, we employed LZ-TF – the extracellular domain of human flTF able to form non-covalent homodimers and previously shown to effectively bind to integrins [13]. Approximately 20-fold increase in adhesion of cardiac and retinal MVEC was observed as early as 30 min after MVEC were placed in LZ-TF/asTF-coated wells (p<0.001, Fig. 4B). To determine the relative contribution of β1 and β3 integrins to LZ-TF/asTF binding by MVEC; we pre-incubated the cells with inhibitory antibodies to each specific integrin chain. β1 blockade effectively abolished MVEC adhesion to LZ-TF and asTF (p<0.05), whereas β3 blockade had no effect in either MVEC sub-type (Fig. 4B). We note that the markedly lower levels of β3 integrins were proposed to be a characteristic of microvascular, as opposed to macrovascular, endothelial cells [16].
Fig 4.
LZ-TF and asTF ligate β1 integrins on human MVEC and promote MVEC-monocyte interactions. (A). Flow cytometry, relative levels of β1/β3 integrins on the surfaces of cardiac and retinal MVEC. (B) MVEC adhesion to LZ-TF/asTF (n≥3). Values are mean ± SD; *** p<0.001 compared to BSA; # and ### p<0.05 and p<0.001, respectively, compared to asTF; ‡ and ‡‡‡ p<0.05 and p<0.001, respectively, compared to LZ-TF. (C) LZ-TF and asTF promote MVEC-PBMC interactions in the orbital shear assay. (D) Dose-response experiments, asTF in the orbital shear assays employing PBMC. (E) LZ-TF and asTF promote MVEC-PBMC interactions in the laminar flow assay – (F) Effects of kinase inhibition on asTF-triggered MVEC-monocyte interactions under orbital shear; MVEC were pre-incubated with PI3 kinase inhibitor LY294002 (10 μM), p38 MAPK inhibitor SB203580 (10 μM), p42/p44 MAPK inhibitor PD98059 (20 μM), FAK II inhibitor (10 μM), NFkB inhibitor BMS-345541 (10 μM), and Rho kinase inhibitor (10 μM). *p<0.01, **p<0.001, ***p<0.0001. n≥3 for each assay, the values are mean ± SD. # p<0.05 compared to asTF. ‡ and ‡‡ p<0.05 and p<0.01, respectively, compared to LZ-TF.
flTF and asTF promote MVEC-monocyte interactions
The ligation of β1 integrins on MVEC surfaces by both TF variants prompted us to investigate whether LZ-TF and/or asTF promote MVEC-monocyte interactions – a crucial hallmark in the progression of cancer and atherosclerosis. We employed two assays designed to recapitulate the conditions within the (micro)circulatory tree in distinct settings, i.e., the orbital shear assay simulating the conditions in recirculation zones of partially occluded vessels where cyclic shear stress predominates, and the laminar flow assay. LZ-TF and asTF-treated MVEC revealed increased interaction with PBMC: in the orbital shear assay, a ~130% increase in PBMC adhesion was observed in response to asTF and ~65% in response to LZ-TF (p<0.01, Fig. 4C); of note, asTF potentiated PBMC adhesion to MVEC at 0.1 nM in this assay (Fig. 4D). The monoclonal antibody 6B4 that binds to the integrin-binding domain of TF [2] potently inhibited the effects of LZ-TF as well as asTF in enhancing the monocyte-MVEC interactions (Fig. S2). To rule out the possibility that the effects of asTF were elicited by traces of endotoxin in protein preparations, we performed a series of control experiments: addition of LPS inhibitor polymyxin B to MVEC cultures as well as asTF preparations and/or incubation with non-charged agarose beads did not affect the experimental outcome, whereas the depletion of asTF with Ni-charged beads and heat denaturation eliminated the observed effects, confirming that the observed phenomena are asTF-specific (Fig S3 and data not shown). In the laminar flow assay, asTF again elicited a more pronounced effect compared to LZ-TF: ~6 fold increase in PBMC adhesion was observed for asTF-stimulated MVEC (p<0.001) compared to ~3 fold increase for LZ-TF-stimulated MVEC (p<0.05, Fig. 4E). To identify the intracellular pathways employed by asTF to potentiate monocyte adhesion to MVEC, we performed orbital shear assays in which MVEC were pre-treated with a panel of integrin-linked kinase inhibitors. asTF-triggered enhancement of the interactions between cardiac MVEC and monocytes was completely suppressed by PI3K/Akt inhibitor LY294002 and by NFkB inhibitor BMS345541, whereas inhibition of p38 MAPK, p42/p44 MAPK, FAK and/or ROCK had no effect (Fig. 4F); the results obtained with retinal MVEC were analogous (not shown).
Ligation of β1 integrins by asTF elicits global changes in gene expression in MVEC
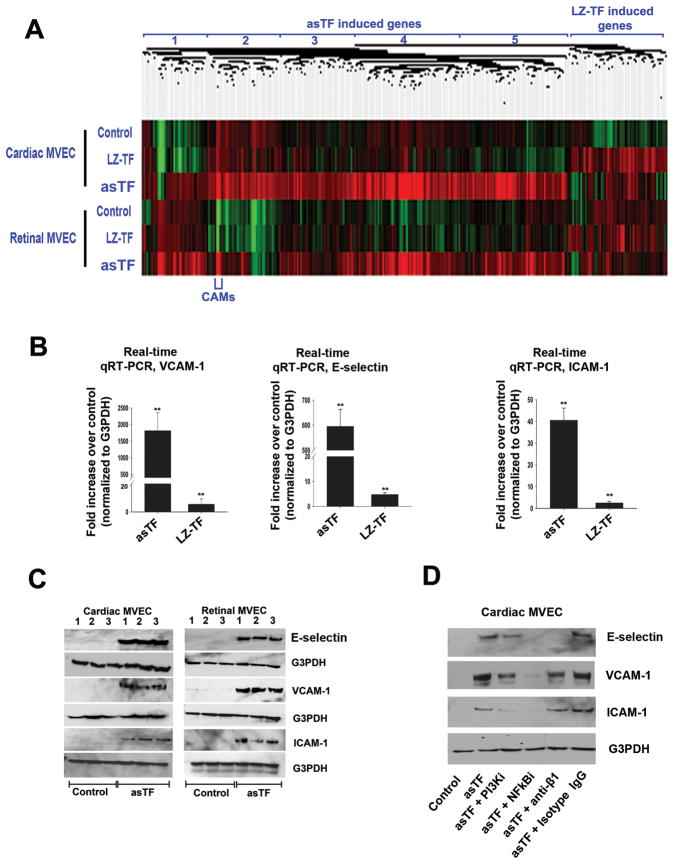
Of the two forms of human TF, the minimally coagulant asTF is a more potent inducer of integrin-mediated angiogenesis [2]. Multiple kinases are engaged in response to asTF ligation in murine and human macrovascular cells [2], the combined effect of which results in a remarkable potentiation of monocyte adhesion (Fig. 4C-F). We evaluated the nature of the changes in global gene expression elicited by LZ-TF and asTF in MVEC and the extent to which these changes were independent of the MVEC sub-type, using microarrays. The concordance among the genes whose expression was upregulated by LZ-TF and asTF in cardiac or retinal MVEC was high, yet the nature of the response to asTF and LZ-TF at the ≥1.5 fold cutoff was remarkably different (Fig. 5A). Of the 813 probesets that exhibited a significant up or down-regulation elicited by asTF in either MVEC sub-type (FDR<0.05, Benjamini Hochberg), 332 probesets corresponding to 223 genes were upregulated ≥1.5 fold by asTF in both MVEC sub-types. The major categories of their function were identified using ToppGene and are listed in Table 1; they include a broad range of defense response, cytokine signaling, and NFkB-driven programs/pathways associated with cell adhesion, cell migration, innate immunity, and wound healing. Specifically, transcription of several classes of genes was upregulated by asTF in both MVEC sub-types:1) growth factor genes such as VEGF-A and FGF, 2) genes encoding cell adhesion molecules (CAMs) E-selectin, VCAM-1, and cytokines including CCL2, CCL20, CCL5, CXCL3 and IL-1A – the molecules implicated in attracting peripheral blood monocytes [17]; 3) genes governing apoptosis, of which ~70% were anti-apoptotic, e.g. NFKB1 and SERPINB2, as well as genes promoting EC migration and proliferation, e.g., RELB; 4) genes encoding various transcription factors governing cytokine expression, e.g., CEBPD, KLF-7, and NRIP1; 5) genes involved in metabolic processes (e.g., APOL3, PISD, COX6A1), iron transport (e.g., SLC7A11, SLC39A6, SLC7A5), and structural proteins (e.g., FNDC3B, COLA6, PSEN1) (Fig. 5A, Supplementary Table, and data not shown). In contrast to asTF, LZ-TF potentiated ≥1.5 fold upregulation of 63 genes in MVEC, most notable of which were seven genes encoding snoRNA (Fig. 5A). E-selectin, VCAM-1, and ICAM-1 were markedly upregulated on mRNA and protein levels in asTF-treated cardiac and retinal MVEC (Fig. 5B,C). The upregulation of these three CAMs was blocked by PI3K- and NFkB inhibitors as well as anti-β1 antibody, which had a particularly striking effect on the levels of E-selectin (Fig. 5D); the effect of antiβ3 antibody did not differ from that of isotype control IgG (not shown). Although we previously demonstrated that asTF-triggered, integrin-mediated signaling elicits angiogenesis [2], this is the first report on the ability of the (as)TF/integrin axis to significantly upregulate the expression of CAMs in human EC.
Fig 5.
(A) Heat map, asTF- and LZ-TF-stimulated cardiac and retinal MVEC; see text for details on gene clusters 1–5. (B) Real-time quantitative RT-PCR for VCAM-1, ICAM-1, and E-selectin (n=3). (C) Western blotting for E-selectin, VCAM-1, and ICAM-1 (samples from three independent experiments are shown). (D) Effects of pharmacologic inhibitors and anti-β1 antibody on asTF-triggered expression of CAMs in MVEC; blots were stripped and re-probed with anti-G3PDH antibody.
Table 1.
Summary of the pathways and genes maximally activated by asTF in cardiac and retinal MVEC.
| Category type | Category name | p-value |
|---|---|---|
| Biological process | Cell adhesion | 5.58E-03 |
| Biological process | Locomotion | 1.04E-04 |
| Biological process | Leukocyte migration | 8.79E-04 |
| Molecular function | Receptor binding | 1.50E-04 |
| Molecular function | Cytokine activity | 2.56E-04 |
| Pathway | Cytokine-receptor interaction | 8.65E-06 |
| Pathway | TNF receptor signaling | 6.13E-04 |
| Co-expression | Genes up-regulated by TNF | 8.57E-16 |
| Genes with transcription factor binding site | GATTGGY (NFkB) | 1.79E-04 |
| Genes with transcription factor binding site | TTGCWCAAY (CEBP) | 9.76E-04 |
Transendothelial migration of monocytes adhered to MVEC in response to asTF stimulation
Monocyte egress from the lumen is highly significant in the macrovascular as well as microvascular contexts: monocytes act as a rich source of TF leading to a procoagulant environment in the arterial wall. We sought to examine whether asTF is able to promote monocyte transmigration in the presence of CCL2/MCP-1, whose transcription was induced by asTF. In a transwell assay, the migration of PBMC as well as THP-1 cells across asTF-stimulated MVEC monolayers was enhanced >3 fold when MCP-1 was present on the abluminal side of the insert; this effect was fully inhibited by anti-β1 antibody whereas the effect of antiβ3 antibody did not differ from that of isotype control IgG (p<0.001, Fig. 6, S4 and data not shown).
Fig. 6.
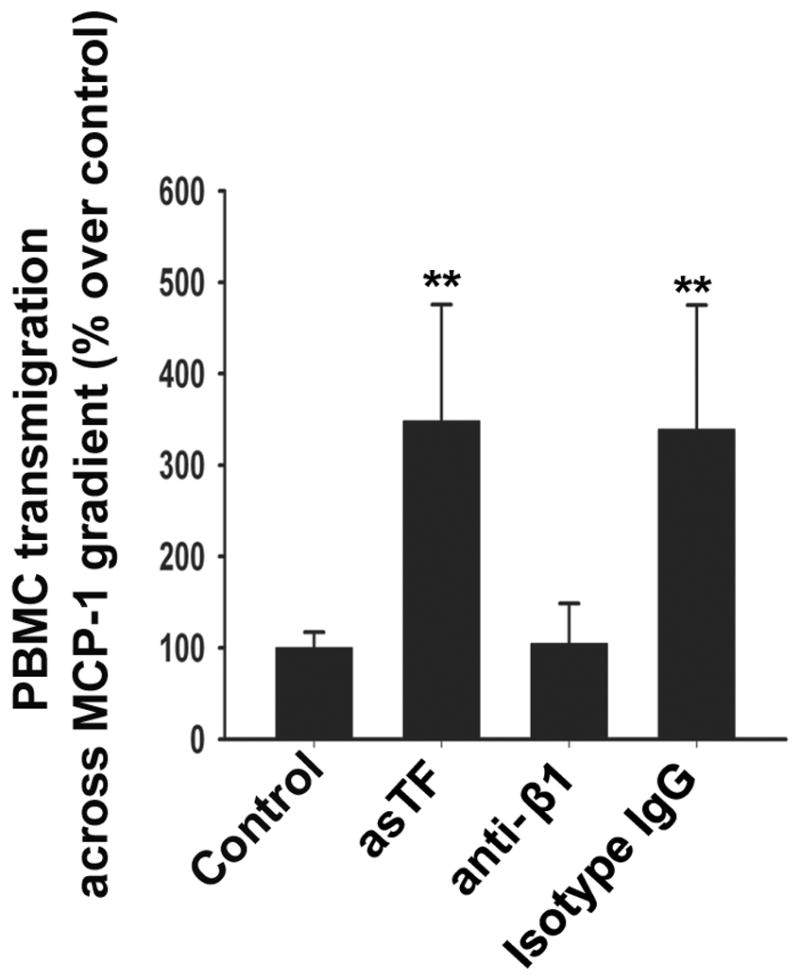
Transwell assay under MCP-1 gradient (50 μg/L): PBMC on the abluminal surface of the inserts chamber were stained with DAPI and counted using Image J (n=3, mean ± SD, **p<0.001).
Discussion
This is the first report on the ability of the TF-integrin axis to upregulate CAMs in human EC as a result of integrin ligation, leading to the increased EC-monocyte interactions. Monocyte recruitment contributes to the progression of atherosclerosis, diabetic retinopathy, and many forms of neoplasia [4,5,9]. We here demonstrate that flTF and asTF co-localize with CD68+ cells in cervical cancer and aortic plaque specimens; thus, tumor cells as well as stromal cells likely contribute to the high concentrations of asTF protein found in cervical cancer [2]. Atherosclerotic plaques display a uniform pattern of flTF/asTF co-localization with CD68+ monocytes/macrophages, suggesting a possible feedback loop whereby the monocytes recruited to the developing plaque synthesize TF variants that further augment monocyte/macrophage accumulation by promoting their adhesion to MVEC via ligation of β1 integrins. We note that flTF was recently reported to upregulate the expression of mRNA encoding E-selectin and various chemokines in human macrovascular cells – HUVEC [18]. The remarkable potency of asTF under laminar flow, and asTF-elicited upregulation of CXC and CC chemokines support the notion that asTF, alongside flTF, may play a major role in atherogenesis [14]. Of note, it was reported that a systemic 50% reduction in total TF and selective elimination of total TF expression in hematopoietic cells does not affect atherosclerotic phenotype in the genetically modified mouse models [19]; thus, asTF effects are likely to be mostly relevant to solid cancer tissues where asTF protein levels are relatively high [2]. However, many differences exist between humans and mice, particularly with regard to monocyte sub-populations and their relative importance in disease progression [20,21]; thus, new mouse models featuring splice isoform-specific TF phenotypes are needed to assess the significance in, and the relative contributions of the TF splice variants to the pathobiology of cancer and/or atherosclerosis. We here demonstrate that compared to recombinant truncated flTF (LZ-TF), asTF is a more potent inducer of the three essential CAMs – ICAM-1, VCAM-1, and E-selectin – required for the firm adhesion of leukocytes to the endothelium [21]. We found a highly significant increase in monocyte adhesion to LZ-TF/asTF-exposed MVEC in two distinct assays simulating various flow conditions, performed in the defined medium free of FVII(a). Orbital shear and laminar flow assays revealed that while both forms of TF increase monocyte adhesion to MVEC; asTF is much more potent when freshly isolated blood PBMC are employed, and it markedly potentiates monocyte transmigration across MVEC monolayers in the presence of a chemokine gradient. While the PI3K/Akt-NFkB pathway appears to play a critical role in TF-triggered upregulation of CAMs in human MVEC, it cannot be excluded that flTF/asTF might also recruit other integrin-binding proteins, such as uPAR, to differentially modulate signaling in specific EC subtypes [22].
Our data indicate that cells of monocytic origin appear to be the major source of flTF/asTF within the plaque, which is in agreement with the earlier findings that CD14+ cells are the major source of flTF and asTF in systemic circulation [1]. In an elegant study by Borisoff et al, early and late atherosclerotic lesions were found to be the sites of active local synthesis of several coagulation proteins, including TF, with the early lesions exhibiting a more procoagulant phenotype [23]. As the monocytes recruited to the plaque greatly contribute to its instability characterized by the graduate change of the plaque phenotype from proliferative to inflammatory/pro-angiogenic in the course of atherosclerotic progression [24], it is reasonable to propose that the “TF isoform profile” of the intraplaque monocytes/macrophages may also evolve: the biosynthesis of asTF mRNA is largely dependent on the activity of the splicing regulator (SR) proteins ASF/SF2, SRp55, SRp40, SC35, and SRp75 whose functional interplay in the course of TF pre-mRNA processing is controlled by several kinases sensitive to the extracellular environment [12,25,26]. While it has now been established that TF is locally synthesized within the plaque during its progression from early-procoagulant to advanced-imflammatory/pro-angiogenic [23], it is yet to be examined whether a decrease in flTF biosynthesis with a concomitant increase in asTF biosynthesis may contribute to the change of the plaque phenotype. Monocyte transmigration across the EC monolayer is a hallmark event in atherogenesis [5,6,24]; we stress that leukocyte infiltration of the plaque occurs chiefly through microvascular EC [3]. We propose that monocyte/foam cell-derived flTF and asTF may stimulate MVEC to produce adhesion molecules and chemokines to recruit additional monocytes, which can in turn support flTF/asTF accumulation: the expression of both forms of TF is markedly increased when human monocytes come in contact with fibronectin [27].
In sum, our results expand the scope of the TF system’s non-proteolytic, integrin-mediated effects, underscoring the significance of high flTF/asTF expression for tumor progression and, possibly, atherogenesis. asTF, while being minimally coagulant, is likely to promote the recruitment of i) potentially pro-angiogenic monocytes to cancer lesions and ii) potentially pro-coagulant monocytes to the developing plaque; thus, asTF may represent a novel therapeutic target: the impairment of asTF’s function may significantly slow tumor growth as well as the progression of vascular lesions, while having only minimal effect on the maintenance of normal hemostasis.
Supplementary Material
Acknowledgments
This study was partially supported by NIH grant HL094891 (V.Y. B.). The authors are grateful to Prof. Dr. Ursula Rauch (Charite-Berlin) for providing the specimens of aortic plaques.
Footnotes
Addendum
Author contribution: R. Srinivasan*, E. Ozhegov* and M.B. Palascak* performed experiments, analyzed data, and wrote the paper; Y.W. van den Berg†, B.J. Aronow‡, R.S. Franco*, J.T. Fallon§, W. Ruf¶, and H.H. Versteeg† analyzed data, provided key reagents, and contributed to writing the paper; V.Y. Bogdanov* conceived the research, analyzed data, and wrote the paper.
References
- 1.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova K, Ruf W, Spek CA, Reitsma PH, Bogdanov VY, Versteeg HH. Alternatively spliced Tissue Factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci USA. 2009;106:19497–19502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, Duimel H, Frederik PM, van Hinsbergh VW, Virmani R, Daemen MJ. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–1527. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 7.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NFkappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto T, Ito S, Yoshikawa H, Hata Y, Ishibashi T, Sueishi K, Inomata H. Tissue factor increases in the aqueous humor of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2001;239:865–871. doi: 10.1007/s004170100373. [DOI] [PubMed] [Google Scholar]
- 9.Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg YW, Versteeg HH. Alternatively spliced tissue factor. A crippled protein in coagulation or a key player in non-haemostatic processes? Hämostaseologie. 2010;30:144–149. [PubMed] [Google Scholar]
- 11.Sovershaev MA, Egorina EM, Bogdanov VY, Seredkina N, Fallon JT, Valkov AY, Østerud B, Hansen JB. Bone morphogenetic protein -7 increases thrombogenicity of lipid-rich atherosclerotic plaques via activation of tissue factor. Thromb Res. 2010;126:306–310. doi: 10.1016/j.thromres.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Tardos JG, Eisenreich A, Deikus G, Bechhofer DH, Chandradas S, Zafar U, Rauch U, Bogdanov VY. SR proteins ASF/SF2 and SRp55 participate in tissue factor biosynthesis in human monocytic cells. J Thromb Haemost. 2008;6:877–884. doi: 10.1111/j.1538-7836.2008.02946.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W. Cross-talk of integrin alpha3beta1 and tissue factor in cell migration. Mol Biol Cell. 2004;15:4416–4425. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs JE, Zakarija A, Cundiff DL, Doll JA, Hymen E, Cornwell M, Crawford SE, Liu N, Signaevsky M, Soff GA. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res. 2007;120 (Suppl 2):S13–21. doi: 10.1016/S0049-3848(07)70126-3. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SH, Ljubimov AV, Morla AO, Caballero S, Shaw LC, Spoerri PE, Tarnuzzer RW, Grant MB. Fibronectin fragments promote human retinal endothelial cell adhesion and proliferation and ERK activation through alpha5beta1 integrin and PI3-kinase. Invest Ophthalmol Vis Sci. 2003;44:1704–1715. doi: 10.1167/iovs.02-0773. [DOI] [PubMed] [Google Scholar]
- 17.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 18.Grosser M, Magdolen V, Baretton G, Luther T, Albrecht S. Gene expression analysis of HUVEC in response to TF-binding. Thromb Res. 2010;127:259–263. doi: 10.1016/j.thromres.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Tilley RE, Pedersen B, Pawlinski R, Sato Y, Erlich JH, Shen Y, Day S, Huang Y, Eitzman DT, Boisvert WA, Curtiss LK, Fay WP, Mackman N. Atherosclerosis in mice is not affected by a reduction in tissue factor expression. Arterioscler Thromb Vasc Biol. 2006;26:555–562. doi: 10.1161/01.ATV.0000202028.62414.3c. [DOI] [PubMed] [Google Scholar]
- 20.Wilson HM. Macrophages heterogeneity in atherosclerosis - implications for therapy. J Cell Mol Med. 2010;14:2055–2065. doi: 10.1111/j.1582-4934.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 23.Borissoff JI, Heeneman S, Kilinç E, Kassák P, Van Oerle R, Winckers K, Govers-Riemslag JW, Hamulyák K, Hackeng TM, Daemen MJ, ten Cate H, Spronk HM. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation. 2010;122:821–830. doi: 10.1161/CIRCULATIONAHA.109.907121. [DOI] [PubMed] [Google Scholar]
- 24.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 25.Chandradas S, Deikus G, Tardos JG, Bogdanov VY. Antagonistic roles of four SR proteins in the biosynthesis of alternatively spliced tissue factor transcripts in monocytic cells. J Leukoc Biol. 2010;87:147–152. doi: 10.1189/jlb.0409252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenreich A, Bogdanov VY, Zakrzewicz A, Pries A, Antoniak S, Poller W, Schultheiss HP, Rauch U. Cdc2-like kinases and DNA topoisomerase I regulate alternative splicing of tissue factor in human endothelial cells. Circ Res. 2009;104:589–599. doi: 10.1161/CIRCRESAHA.108.183905. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj MS, Ghosh M, Bajaj SP. Fibronectin-adherent monocytes express tissue factor and tissue factor pathway inhibitor whereas endotoxin-stimulated monocytes primarily express tissue factor: physiologic and pathologic implications. J Thromb Haemost. 2007;5:1493–1499. doi: 10.1111/j.1538-7836.2007.02604.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.