Abstract
The error-related negativity (ERN), an event-related potential component elicited by error responses in cognitive tasks, has been shown to be abnormal in most, but not all, studies of obsessive–compulsive disorder or obsessive–compulsive symptoms (OCD/S); these inconsistencies may be due to task selection, symptom subtype, or both. We used meta-analysis to further characterize the ERN in OCD/S, and pooled data across studies to examine the ERN in OCD/S with hoarding. We found an enhanced ERN in OCD/S relative to controls, as well as heterogeneity across tasks. When stratified, OCD/S showed a significantly enhanced ERN only in response conflict tasks. However, OCD/S + hoarding showed a marginally larger ERN than OCD/S–hoarding, but only for probabilistic learning tasks. These results suggest that abnormal ERN in OCD/S is task-dependent, and that OCD/S + hoarding show different ERN activity from OCD/S - hoarding perhaps suggesting different pathophysiological mechanisms of error monitoring.
Keywords: Error monitoring, ERN, Obsessive–compulsive disorder (OCD), Hoarding symptoms
1. Introduction
Obsessive–compulsive disorder (OCD) is a neuropsychiatric disorder that affects approximately 2% of the population world-wide and has strong biological underpinnings and a well-defined neurocircuitry (APA, 2000; Karno et al., 1988). Neuroimaging studies have implicated the ventromedial prefrontal cortex (VMPFC), the anterior cingulate cortex (ACC), the orbitofrontal cortex (OFC), the striatum (particularly the caudate nucleus), and the thalamus, as being involved in the pathophysiology of OCD (Grundler et al., 2009; Harrison et al., 2009; Saxena and Rauch, 2000). These brain regions are interconnected in multiple recurrent loops, making up the cortico-striatal-thalamic-cortical circuit, and are thought to be involved in action selection, performance monitoring, and goal directed behaviors (Gilbert et al., 2008; Harrison et al., 2009; Menzies et al., 2008; Nieuwenhuis et al., 2005). Hyperactivity of this circuit has been demonstrated in individuals with OCD, both at rest and following symptom provocation (Adler et al., 2000; Menzies et al., 2008; van den Heuvel et al., 2005). It has been proposed that, in this disorder, such cortico-striatal hyperactivity leads to a persistently high error signal, ultimately resulting in the psychopathology characteristic of OCD (Grundler et al., 2009; Remijnse et al., 2006). In this model, the brain’s error monitoring system compares intended responses (or “expected outcomes”) to actual responses (or “actual outcomes” in environmental stimuli, thoughts, feelings, and actions), and generates an error signal when a conflict is detected. It has been suggested that this error signal is amplified in individuals with OCD, leading to the feeling that something is “out of line” (thus, generating irrational fears or obsessions) or that an action was not completed correctly according to a set of internal unattainable rules, triggering repetitive, compensatory behaviors (i.e., compulsions) (Gehring et al., 2000; Pitman, 1987).
The hypothesis of a persistent and enhanced cortico-striatal error signal was first put forward by Pitman (1987), and since then, the enhanced error-signal hypothesis has been tested by multiple investigators using electrophysiological measures associated with performance monitoring and error detection, most specifically the error-related negativity or ERN (Endrass et al., 2008; Gehring et al., 2000; Hajcak et al., 2008; Hajcak and Simons, 2002; Johannes et al., 2001; Pitman, 1987; Santesso et al., 2006). The ERN (Gehring et al., 1993), or error negativity (Ne) (Falkenstein et al., 1991), component of the response-locked event-related potential (ERP) associated with performance errors in speeded choice-response tasks is evident following overt error responses (Gehring et al., 1995) and peaks 50–150 ms after the error is committed. Larger (i.e., more negative) ERNs are associated with instructions emphasizing accuracy over speed, faster errors, lower error rates, attempts to correct errors, greater post-error slowing, and greater error salience (Bernstein et al., 1995; Falkenstein et al., 2000; Gehring et al., 1995; Scheffers and Coles, 2000). Topographic scalp maps show the ERN to have a fronto-central maximum (Falkenstein et al., 1991; Gehring et al., 1995). Converging evidence from dipole modeling of the ERN (Dehaene et al., 1994), functional magnetic resonance imaging (fMRI), (Carter et al., 1998; Kiehl et al., 2000; Mathalon et al., 2003), and intracranial recordings from monkeys (Brooks, 1986; Gemba et al., 1986; Niki & Watanabe, 1979), suggests that the ACC is the principal generator of the ERN, which, as noted, has been implicated in OCD. The ERN has been suggested to reflect simple error detection (Falkenstein et al., 1991), high levels of response conflict (Botvinick et al., 2001; Carter et al., 1998; Danielmeier et al., 2009) but see (Carbonnell and Falkenstein, 2006; Masaki et al., 2007), and reward prediction errors in which outcomes are worse than expected (Holroyd and Coles, 2002). Interestingly, the ERN can be evoked by errors committed outside of conscious awareness (Nieuwenhuis et al., 2001; O’Connell et al., 2007).
To date, the published studies examining the ERN among individuals with OCD or high levels of OC symptoms (OCS) have been variable, with many (Endrass et al., 2008, 2010; Gehring et al., 2000; Hajcak et al., 2008; Johannes et al., 2001; Riesel et al., 2011; Ruchsow et al., 2005; Stern et al., 2010), but not all (Hammer et al., 2009; Nieuwenhuis et al., 2005), reporting an increased ERN amplitude in OCD/S subjects compared to controls. A recent hypothesis put forward by Grundler et al. (2009) suggested that some of the observed variability between studies could be due to differences in the task choice—i.e., that the relationship between ERN activation and error processing is task dependent, with hyperactivity seen in response conflict tasks, and hypoactivity seen in probabilistic or reinforcement learning tasks (Grundler et al., 2009). In response conflict tasks where the correct response is known to the subject, the ERN is generated from quick and impulsive errors known as “slips.” Slips are distinguished from “mistakes,” which reflect inaccurate intentions based on faulty knowledge (Reason, 1990). Under Grundler’s hypothesis, in an individual with OCD/S, protective measures against slips are added, thereby enhancing error signals and perpetuating maladaptive compensatory strategies. In contrast, probabilistic tasks enlist “NoGo learning,” (Frank et al., 2005; Holroyd and Coles, 2002) where the subject learns task rules from feedback on a trial-by-trial basis, and learns to inhibit responses that caused the error. If this system is under-activated, individuals with OCD/S may compulsively engage in repetitive behaviors, possibly due to attenuated error signals, leading to suboptimal performance. However, the hypothesis of task divergent ERN activation in OCD/S has not yet been tested (Grundler et al., 2009).
1.1. ERN and OCD symptom subtype
OCD is a heterogeneous disorder, with multiple symptom subtypes, including contamination/cleaning, “taboo” symptoms (religious, sexual, and aggressive obsessions), doubts/fears of causing or incurring harm (often characterized by checking behaviors), rituals and superstitions, and hoarding (Katerberg et al., 2010; Pinto et al., 2008). Of these, the hoarding subtype appears to be the most distinct with regard to treatment response, and perhaps also with regard to neurocognitive and neuroanatomic patterns (An et al., 2008; Gilbert et al., 2008; Pertusa et al., 2008; Rachman et al., 2009; Saxena, 2008). In addition to the hallmark symptoms of compulsive collecting, poor organizational skills, and difficulty discarding, we, and others, have shown that many individuals with compulsive hoarding exhibit cognitive deficits across domains including information processing speed, decision-making, categorization, attention, and memory, and problematic behaviors including procrastination and slowed task completion (Grisham et al., 2007; Hartl et al., 2004; Lawrence et al., 2006; Luchian et al., 2007; Mackin et al., 2010; Mataix-Cols et al., 2004; Tolin et al., 2008).
Based on clinical observations, a recent model of the pathognomonic features of OCD (i.e., obsessions, compulsions, avoidance, and pathological doubt) reframes these symptoms as being secondary or observed manifestations of a more fundamental feature of this disorder, intolerance of uncertainty (Tolin et al., 2003). While the larger, more inclusive construct of intolerance of uncertainty may be a core feature of OCD, more recent theorists suggest that hoarding symptoms are part of a discrete clinical syndrome that also includes indecisiveness and poor organizational ability (Steketee and Frost, 2003). Compared to non-hoarding OCD patients, hoarders have less impulsivity, higher sensitivity to punishment (Fullana et al., 2004), more severe interpersonal disability (Steketee and Frost, 2003) and lower global functioning (Saxena et al., 2002). In addition, they often have less insight into their symptoms than non-hoarding OCD patients, making them less likely to seek treatment (Saxena, 2008). Thus, individuals with hoarding behaviors may have distinct neurocognitive abnormalities that lead to hoarding per se. Neuropsychological and neuroimaging examination of hoarding has implicated brain regions including ACC, dorsolateral prefrontal cortex, and orbitofrontal cortex, among others (Gilbert et al., 2008; Mataix-Cols et al., 2004; Saxena, 2008; Tolin et al., 2009) that are involved in response selection, decision-making, conflict monitoring, and error detection, further supporting the hypothesis that abnormalities in error monitoring may be seen in individuals with hoarding behaviors (An et al., 2008; Gilbert et al., 2008; Mataix-Cols et al., 2004). However, to date, no studies of the ERN to assess error monitoring in compulsive hoarding have been conducted.
1.2. Goals of the present study
The goals of this paper are to use currently published/available data to: (1) conduct meta-analyses to verify that the ERN deflection in OC-affected individuals is task-dependent, and to obtain a more precise estimate of the magnitude of the abnormality of the ERN in OCD/S compared to controls, and (2) conduct the first examination into the ERN among OCD/S with hoarding behaviors or symptoms (OCD/S + hoarding) compared to those without hoarding behaviors or symptoms (OCD/S–hoarding). As noted, although the available published studies have, for the most part, shown an association between OCD and an enhanced ERN, the relationship between task type and symptom subtype has not yet been fully examined. To our knowledge, there are no published analyses addressing potential differences in the ERN based on symptom profile. Although an analysis of all symptom subtypes would be of interest, we chose to focus on hoarding in this analysis in part because of the growing body of evidence that hoarding, although overlapping with other OCD symptoms, may have distinct etiologies and distinct neurocognitive profiles (An et al., 2008; Gilbert et al., 2008; Pertusa et al., 2008; Rachman et al., 2009; Saxena, 2008). We predicted that the meta-analysis would uphold the predominant findings in the individual studies, that is, that the ERN is larger (more negative) in OC-affected individuals when compared to healthy controls. We also predicted that this difference would be apparent primarily in those studies that used a response conflict task rather than a probabilistic learning task, as postulated by Grundler et al. (2009). Furthermore, we hypothesized that the phenotypic distinction between individuals with hoarding behaviors relative to those without hoarding behaviors would be reflected by differences in ERN amplitude dependent upon task type.
2. Methods
2.1. Meta analysis
2.1.1. Study selection
Published studies examining the ERN in individuals with OCD or OCS and in controls were initially identified using the MEDLINE-PubMed databases, using the terms OCD, obsessive–compulsive disorder, obsessive–compulsive, action monitoring, conflict monitoring, performance monitoring, ERN, and error-related negativity. Additional published studies were identified through examination of the reference lists of the studies included from MEDLINE-PubMed. The authors of the published studies were then contacted to identify additional unpublished datasets and to acquire symptom-level data on hoarding.
2.1.2. Inclusion/exclusion criteria
Studies or datasets were included in the study if they had an OCD group and a control group and if ERN data were reported, even if the ERN was not the main focus of the study. Studies examining OCS in non-clinical samples were also included if they used a standardized structured instrument (such as the Obsessive–Compulsive Inventory-Revised; OCI-R) (Foa et al., 2002; Hajcak et al., 2004), and if they compared individuals with high OCS scores to those with low OCS scores. Studies examining either adults or children as participants were included. Studies that examined only non-OCD anxiety disorders were excluded, as were studies that examined ERPs but did not report the ERN and those that did not report original data (e.g., review articles).
2.1.3. Data extraction
Mean ERN amplitude and standard deviations as reported by the authors, task type, electrode, study group (OCD/S vs. control), type of case group (OCD vs. OCS), age (adult vs. child), year of study, and sample size of each study group were extracted and entered into a database for analysis. ERN mean amplitudes and standard deviations for OCD/S and control groups were obtained from each study’s corresponding author or from published data. When raw data were not available, mean ERN amplitudes and, if available, standard deviations, were derived from published figures. If standard deviations were not available from the published data, pooled standard deviations for the combined case and control groups within each study were calculated using the reported t or F statistics. ERN amplitudes from the Cz electrode were used for all studies when available. If ERN amplitude from the Cz electrode was not available (as was the case for four studies), amplitude from the Fz or FCz electrode was used (Endrass et al., 2010; Hajcak et al., 2008; Riesel et al., 2011; Stern et al., 2010). For studies where several conditions or multiple study samples were reported, each condition/sample was considered to be a separate study and all were included in the meta-analysis.
2.1.4. Task type
Studies or conditions were categorized according to the task types utilized: (1) response conflict tasks (e.g., flanker-type tasks, go-no go tasks, reaction time tasks); (2) probabilistic learning tasks, and (3) other tasks (e.g., memory-based tasks, or flanker tasks with a punishment condition). When available, for the probabilistic learning tasks, ERN data were obtained for the final block of trials, to maximize the ERN deflection and to avoid confounding of the ERN by the feedback related negativity (FRN) that is produced during the learning phases of the task. This information was available only for the Nieuwenhuis et al. study (Nieuwenhuis et al., 2005); for the other studies using probabilistic learning tasks, published averaged ERN data were used (Endrass et al., 2010; Grundler et al., 2009).
2.1.5. Statistical approach
Random-effects meta-analysis methods were used to calculate pooled effect size estimates and their corresponding 95% confidence intervals based on study specific effect sizes and their standard deviations using the metan routine in STATA 11.0. A DerSimonian and Laird pooled standard mean deviation and associated 95% confidence interval was calculated for each meta-analysis, along with the z statistic and associated p-value (DerSimonian and Laird, 1995). Effects were expressed as the standardized mean differences (SMD), with the 95% confidence intervals, z-scores, and p-values. The standardized mean difference is the difference in the mean ERN between the OCD/S group and the control group, divided by the within-group standard deviation. A heterogeneity chi-squared statistic and associated p-value, an I2 statistic (the variation in the standard mean deviation attributable to heterogeneity), and the Tau-squared (an estimate of the between-study variance) were also calculated to assess for between-study heterogeneity. An I2 of ≥50% and/or a heterogeneity p-value of <0.05 was taken as evidence of significant heterogeneity.
Meta-analyses were initially conducted using all studies analyzed jointly, regardless of task type, and subsequently for studies separated by task type. Studies were divided into those that employed reinforcement learning tasks (probabilistic learning tasks), those that employed instructed response conflict tasks with speeded responses (flanker, Stroop, Simon, and choice reaction time tasks), and those that employed other tasks (e.g., memory-based tasks). Finally, meta-regression was performed to examine the effect of sample size, mean age of participants, electrode, year of study publication, and study population (i.e., OCD and healthy controls, vs. non-clinical samples with high and low OCS scores).
2.2. Hoarding analysis
2.2.1. Extraction of hoarding symptoms
None of the published studies examining ERN reported on OC symptom subtypes. Accordingly, we requested raw data on hoarding symptoms from the corresponding authors for each study, whether published or unpublished. We were able to obtain data on the presence or the absence of hoarding symptoms derived from the Yale-Brown Obsessive–Compulsive Scale (YBOCS) or the OCI-R for four of the studies included in the meta-analysis above (Grundler et al., 2009; Hajcak et al., 2008; Nieuwenhuis et al., 2005; Stern et al., 2010); for the other studies, individuals with hoarding symptoms were specifically excluded, the authors no longer had access to the original item-level datasets, symptom-level data were not available, or the authors either did not reply to or declined the request for the original data (Gehring et al., 2000; Hajcak and Simons, 2002; Hammer et al., 2009; Johannes et al., 2001; Ruchsow et al., 2005; Xiao et al., 2011). The four studies for which we did obtain primary data encompassed seven separate conditions, three using a response conflict task and four using a probabilistic learning task (Grundler et al., 2009; Hajcak et al., 2008; Nieuwenhuis et al., 2005; Stern et al., 2010). Based on these data, individuals with OCD were classified as having hoarding symptoms (OCD/S + hoarding) if they endorsed either hoarding obsessions or hoarding compulsions or both, or not having hoarding symptoms (OCD/S–hoarding) if they endorsed neither hoarding obsessions nor hoarding compulsions. Individuals with high levels of OCS (as defined by the original study authors) were classified as having hoarding symptoms (OCD/S + hoarding) if their score on the hoarding subscale of the OCI-R was >7. Individuals with scores of <5 on the hoarding subscale of the OCI-R were classified as not having hoarding symptoms (OCD/S–hoarding), while those with scores of 5–7 were classified as unknown and were not included in the present analysis. The mean hoarding subscale score for individuals classified as having hoarding symptoms was 9.1 (SD = 1.3), while for those classified as having no hoarding symptoms, the mean hoarding subscale score was 3.7 (SD = 2.3). Individuals in the current dataset either had OCD or high levels of OCS, and in all cases, hoarding symptoms occurred in the presence of other symptom subtypes as well; there were no individuals with only hoarding symptoms. Data on the severity and functional impact of hoarding obsessions and compulsions were not available.
2.2.2. Statistical approach
We examined the z-scores of the ERN for each individual in order to control for variation in ERN values due to technical or experimental differences between studies. z-Scores were created for each individual by subtracting the mean ERN for the control group of a given study from the individual’s ERN and dividing by the standard deviation of the control group. In this conversion, a lower (or more negative) z-score represents a more negative (or larger) ERN amplitude. To assess subgroup differences in error monitoring, the z-score of the ERN amplitude was analyzed in a 2 Hoard (OCD/S + hoarding vs. OCD/S–hoarding) × 2 Task (response conflict, probabilistic learning) analysis of variance (ANOVA). Interaction effects were parsed with pairwise comparisons.
3. Results
3.1. Search flow
The search strategy yielded 14 results from MEDLINE-PubMed, and one additional study identified through examination of the reference lists. Thirteen studies met our inclusion criteria out of this total of 15 initially identified (Table 1). One study was excluded because it did not contain original data and one because it examined only non-OCD anxiety disorders (Hajcak et al., 2003; van Veen and Carter, 2002). Ten studies examined individuals with OCD compared to healthy controls, and three studies examined individuals with high levels vs. low levels of OCS drawn from non-clinical samples. Eleven studies examined the ERN in adults, and two studies examined the ERN in children. Four studies had multiple conditions/arms; these are shown as separate studies in Table 1. In all, 18 separate samples were included in the meta-analyses.
Table 1.
Characteristics of the studies included in the meta-analyses.
| Study | Year | Study sample | Adults vs. children | Electrode | Task | Sample size | Hoarding data available |
|---|---|---|---|---|---|---|---|
| Gehring et al. | 2000 | OCD | Adults | Cz | Speeded reaction time | 9 per group | No |
| Johannes et al. | 2001 | OCD | Adults | Cz | Reaction time | 10 per group | No |
| Hajcak & Simons | 2002 | OCS | Adults | Cz | Stroop | 17 per group | No |
| Nieuwenhuis et al. (Study 1) | 2005 | OCD | Adults | Cz | Probabilistic learning (80% valid feedback) | 16 per group | Yes |
| Nieuwenhuis et al. (Study 2) | 2005 | OCD | Adults | Cz | Probabilistic learning (100% valid feedback) | 16 per group | Yes |
| Ruchsow et al. | 2005 | OCD | Adults | Cz | Go/no go | 11 per group | No |
| Santesso et al. | 2006 | OCS | Children | Cz | Visual flanker | 16 high OCS; 21 low OCS | No |
| Endrass et al. | 2008 | OCD | Adults | Cz | Modified flanker | 20 per group | No |
| Hajcak et al. | 2008 | OCD | Children | Fz | Modified Simon | 18 per group | Yes |
| Hammer et al. (Study 1) | 2009 | OCD | Adults | Fz | Errorful learning | 16 per group | No |
| Hammer et al. (Study 2) | 2009 | OCD | Adults | Fz | Response locked errorless | 16 per group | No |
| Grundler et al. (Study 1) | 2009 | OCS | Adults | Cz | Probabilistic learning (variable feedback values) | 10 high OCS; 30 low OCS | Yes |
| Grundler et al. (Study 2) | 2009 | OCS | Adults | Cz | Probabilistic learning (variable feedback values) | 14 high OCS; 16 low OCS | Yes |
| Grundler et al. (Study 3) | 2009 | OCS | Adults | Cz | Eriksen flanker | 18 high OCS; 18 low OCS | Yes |
| Endrass et al. (Study 1) | 2010 | OCD | Adults | Fz | Modified Eriksen flanker | 22 per group | No |
| Endrass et al. (Study 2) | 2010 | OCD | Adults | Fz | Flanker with punishment condition | 22 per group | No |
| Stern et al. | 2010 | OCD | Adults | Fz | Modified flanker | 38 OCD; 40 controls | Yes |
| Xiao et al. | 2010 | OCD | Adults | Cz | Modified flanker | 25 OCD; 27 controls | No |
| Riesel et al. | 2011 | OCD | Adults | Fcz | Modified flanker | 30 per group | No |
3.2. Meta-analyses of OCD/S
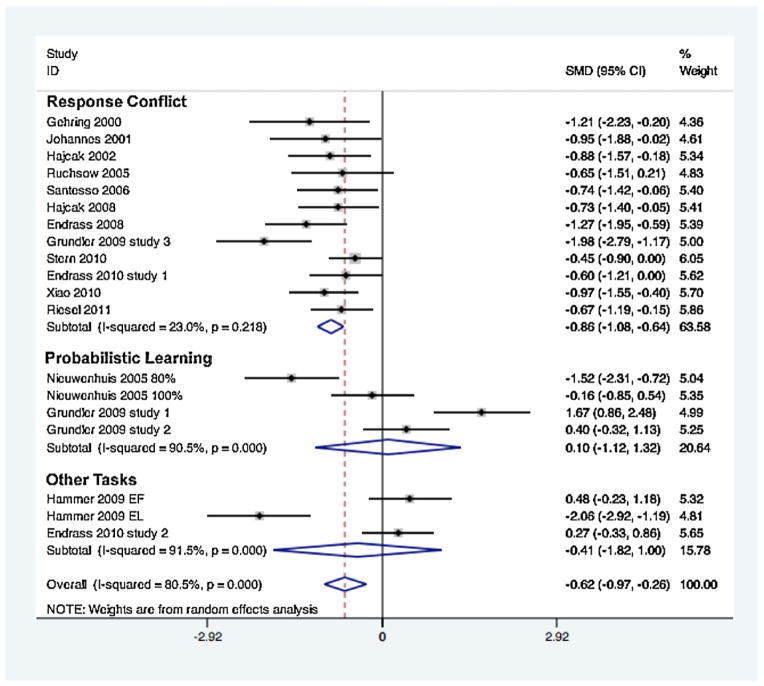
When all studies were meta-analyzed jointly, without regard to task type, the result suggested that there were substantially increased mean ERN amplitudes in OCD/S participants compared to healthy controls (DerSimion and Laird pooled standardized mean difference or SMD = −0.616; z = 3.37; p = 0.001) (Table 2). However, the studies had a high degree of heterogeneity, with a heterogeneity chi-square of 92.28 (df = 18, p < 0.0001), I2 = 80.5%, and Tau-squared = 0.50.
Table 2.
Standardized mean differences (SMD) and 95% CI estimates for available ERN studies of OCD or high OCS compared to healthy matched controls, separated by task type. CI = confidence interval.
| Task type | Pooled SMD (95% CI) | z (p value) | Tau-squared | I2 | Heterogeneity statistic (df, p-value) |
|---|---|---|---|---|---|
| Response conflict | −0.86 (−1.08 to −0.64) | 7.62 (<0.0001) | 0.03 | 23.0% | 14.28 (11, 0.22) |
| Probabilistic learning | 0.10 (−1.13 to 1.32) | 0.16 (0.87) | 1.41 | 90.5% | 31.7 (3, <0.0001) |
| Other | −0.78 (−3.3 to 1.7) | 0.57 (0.57) | 1.42 | 91.5% | 23.5 (1, <0.0001) |
| Overall (all studies) | −0.62 (−0.98 to −0.26) | 3.37 (0.001) | 0.50 | 80.5% | 92.3 (18, <0.0001) |
When the studies were analyzed separately by task type, only those studies that used a response conflict task remained significant, with a pooled SMD of −0.855 (z = 7.62, p < 0.0001) (Fig. 1 and Table 2). The heterogeneity statistics for the response conflict task studies were not significant, suggesting no substantial between-study heterogeneity. In contrast, the heterogeneity statistics for the probabilistic learning task studies and for the studies using other types of tasks, such as memory-based tasks, showed a high degree of between-study heterogeneity (Table 2).
Fig. 1.
Standardized mean differences (SMD) and 95% confidence interval (CI) estimates of all available ERN studies of OCD or high OCS compared to healthy matched controls, separated by task type. EF = effortful learning. EL = effortless learning. OCD = obsessive–compulsive disorder. OCD = obsessive–compulsive symptoms.
Separate meta-regressions were then conducted to examine the effects of sample size, mean age of participants, electrode used, and case (OCD vs. high OCS). Task type was included in each meta-regression as a covariate. While task type continued to be highly significant, no other variable significantly contributed to the variance of the model. This was also true when all of the variables were included in the model simultaneously. Similarly, when meta-regression was performed for only those studies that used a response conflict task, there was no significant effect of any of the additional predictor variables. These covariates accounted for 43.9% of the between-study variance (adjusted R2); the overall model was not significant (F(4,5) = 1.16, p = 0.43).
3.3. Hoarding vs. non-hoarding OCD/S subtype
We examined the ERN in individuals with hoarding symptoms compared to those without hoarding symptoms using raw data from the subset of studies for which we had symptom-level data. Data on the presence or the absence of hoarding symptoms were available for four of the studies (equivalent to seven separate study populations) (Grundler et al., 2009; Hajcak et al., 2008; Nieuwenhuis et al., 2005; Stern et al., 2010). Three of the samples used a response conflict task, and four used a probabilistic learning task (Table 3). In some cases, the same individuals within a study were included in multiple tasks or conditions; for the purposes of these analyses, they are considered to be separate events.
Table 3.
Characteristics of studies for which hoarding data were available.
| Study | Task | OCD vs. OCS | Number (n) with hoarding symptoms | Number (n) without hoarding symptoms |
|---|---|---|---|---|
| Nieuwenhuis et al. (Study 1)a | Probabilistic learning (100% valid feedback) | OCD | 1 | 15 |
| Nieuwenhuis et al. (Study 2)a | Probabilistic learning (80% valid feedback) | OCD | 1 | 14 |
| Hajcak et al. (2008) | Response conflict (modified flanker) | OCD | 7 | 10 |
| Grundler et al. (Study 1) | Probabilistic learning (variable feedback) | OCS | 9 | 8 |
| Grundler et al. (Study 2)a | Probabilistic learning (variable feedback) | OCS | 9 | 5 |
| Grundler et al. (Study 3)a | Response conflict (modified flanker) | OCS | 9 | 14 |
| Stern et al. (2010) | Response conflict (modified flanker) | OCD | 11 | 27 |
| Total | 47 | 93 |
Overlapping samples, as some participants were tested under more than one condition or using more than one task.
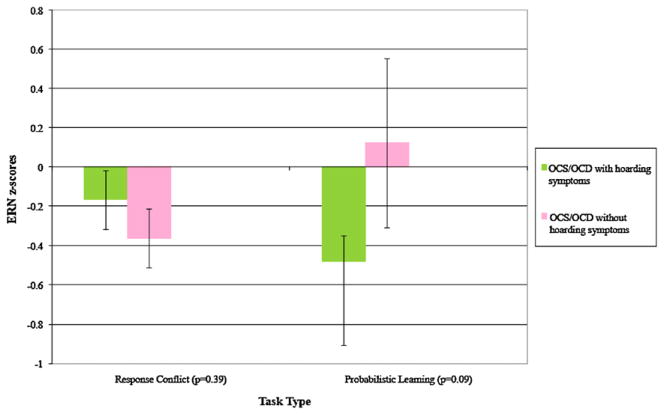
We generated the z-scored ERN values for the entire sample, and compared OCD/S + hoarding to OCD/S–hoarding, and subsequently parsed analyses by task type. Results of the ANOVA are presented in Table 4, and group means (±SD) are shown in Fig. 2. While we did not observe significant main effects for Hoard or Task, the Hoard × Task interaction was marginally significant (p = 0.05). As Fig. 2 indicates, there were some apparent differences between OCD/S + hoarding and OCD/S–hoarding in ERN response by task type. Relative to OCD/S–hoarding, the ERN z-score was marginally more negative for OCD/S + hoarding in the probabilistic learning tasks (OCD/S + hoarding: M = −0.48, SE = 0.43, n = 18; OCD/S–hoarding: M = 0.12, SE = 0.14, n = 47; t(63) = 1.74, p = .09) but not significantly different in the response conflict tasks (OCD/S + hoarding: M = 0.17, SE = 0.15, n = 26; OCD/S–hoarding: M = −0.36, SE = 0.15, n = 41; t(65) = −0.86, p = .39).
Table 4.
ANOVA examining the relationship between hoarding, task and ERN. RC = response conflict. PL = probabilistic learning. OCD/S + hoarding = obsessive–compulsive disorder or obsessive–compulsive symptoms with hoarding. OCD/S–hoarding = obsessive–compulsive disorder or obsessive–compulsive symptoms without hoarding.
| Source | df | F | p-Value |
|---|---|---|---|
| Model | 3 | 2.04 | 0.112 |
| Hoarding (OCD/S + hoarding vs. OCD/S–hoarding) | 1 | 0.99 | 0.321 |
| Task (RC vs. PL) | 1 | 0.18 | 0.669 |
| Hoarding × Task | 1 | 3.82 | 0.053 |
Fig. 2.
Overall mean ERN z-scores by the presence or the absence of hoarding symptoms. Response conflict task: OCD/S + hoarding n = 26, OCD/S–hoarding n = 41. Probabilistic learning task: OCD/S + hoarding n = 18, OCD/S–hoarding n = 47.
4. Discussion
The aims of this study were twofold: (1) to use meta-analysis to characterize the ERN in OC-affected individuals delineated by ERN reflections of task type, and (2) to use pooled analyses of original raw data to examine if individuals with a specific OCD/S subtype, i.e., OCD/S with hoarding, exhibit specific ERN abnormalities relative to those without hoarding. We hypothesized that the ERN would be larger (i.e., more negative) in OC-affected participants than in controls, and further, that this response would be specific to those studies that used response conflict tasks. This hypothesis was based in part on the findings reported by Grundler et al., who found a hyperactive ERN in a response conflict task and a hypoactive ERN in a probabilistic learning task. Specifically, they predicted that hyperactive error monitoring circuits would lead to repetition and over-learning in OC-affected individuals (to avoid making choices that lead to errors) in response conflict tasks. It was further predicted by Grundler et al. that in uncertain environments requiring adaptive responses, such as in reinforcement or probabilistic learning tasks, OC-affected individuals would show enhanced ERN mechanisms as a reflection of rigidity and avoidance learning (learning to avoid maladaptive choices). However, Grundler et al. reported that in his study, OCD patients ultimately demonstrated hypoactive ERN during reinforcement learning tasks.
The present findings from our meta-analysis confirm the prediction of hyperactive ERN responses for those studies using a response conflict task, and in fact show that there is a large effect size (−0.86). All of the studies showed effects in the same direction (larger ERN for OC-affected individuals) and there was no significant evidence of between-study heterogeneity, suggesting that the results of the meta-analysis are quite robust. In contrast, the meta-analysis did not support the presence of ERN abnormalities when tested with probabilistic learning tasks. Due to the high level of heterogeneity between studies for this task type, the failure to find ERN abnormalities in OCD/S may be due to specific weaknesses of study design in one or more of the studies, or greater differences between these studies due to subject recruitment, task type, or the point in which the ERN, rather than the FRN, is elicited over the course of the task. Alternatively, it may be that probabilistic learning tasks are not an appropriate tool to detect ERN alterations in OCD/S if the source of the ERN abnormality cannot be defined; that is, group differences in ERN responses that arise in probabilistic learning tasks may be due to differences in the underlying physiology, but may also be due to differences in how well each group learned the task. In any case, the result from probabilistic learning tasks was not statistically significant, and the high level of heterogeneity between studies suggests that the results of the meta-analysis for this condition are not entirely reliable. While it is possible that variability in task design across studies have led to inconsistencies in the literature, or that the inclusion of OC-affected individuals who do not necessarily meet clinical criteria for OCD biases the heterogeneity among probabilistic learning tasks, these task and sample properties were present in the response conflict condition as well, but still led to conclusive findings. Therefore, at the least, evidence for enhanced error signals in OCD/S appears to be convergent across response conflict studies, and is suggestive of a neural basis for the pathological repetitious behavior observed in these individuals.
In addition to the meta-analysis of ERN differences across studies examining error-monitoring dysfunction in OCD/S, we conducted a critical analysis of the ERN in OC-affected individuals with hoarding symptoms vs. OC-affected individuals without hoarding in a subgroup of studies (N = 4, total of 7 independent conditions) for which we had symptom-level data. In contrast to our hypothesis, we found no significant differences between those with hoarding and those without hoarding in the ERN for the overall sample (not separated by task type), or for the response conflict task. However, we did find a trend for an enhancement in the ERN for those with hoarding symptoms compared to those without among the OCD/S participants in the probabilistic learning task (Table 4). As suggested previously (Frank et al., 2005; Holroyd and Coles, 2002), a larger ERN in the probabilistic learning task has been associated with a greater tendency to avoid an error, i.e., the “NoGo bias.” As evidenced by individuals exhibiting hoarding behaviors, and considering the strong reluctance with which OCD patients part with belongings, even if dangerous, non-sentimental, or unhygienic, avoidance behaviors are necessarily the rule, and not the exception, in defining hoarding. Accordingly, our findings suggest that OC-affected individuals with hoarding symptoms may express neural circuitry consistent with a tendency toward an avoidance bias.
Interestingly, our ERN results in individuals who hoard are consistent with Grundler’s original hypothesis predicting an enhanced ERN in reinforcement or probabilistic learning tasks, as a reflection of avoidance learning (learning to avoid maladaptive choices) (Grundler et al., 2009). While there was no evidence of a hypoactive ERN for OCD/S participants compared to healthy controls in probabilistic learning tasks in the overall meta-analysis, the results of the analysis of the original pooled symptom data suggest that OC-affected individuals with hoarding behaviors have a hyperactive rather than a hypoactive ERN when compared to OCD/S individuals without hoarding behaviors. This result was unexpected, and the results merely trending toward significance, may be due to stochastic variation within the sample. Alternatively, individuals with hoarding behaviors may have more difficulty with the probabilistic learning task, have slower learning rates, or may take longer to complete the task. Nevertheless, despite the heterogeneity across these scenarios, OC-affected individuals who hoard appear to have distinctive pathophysiology underlying error monitoring compared to OC-affected individuals without hoarding behaviors. Under this hypothesis, individuals who hoard may generate an enhanced ERN in the context of a reinforcement environment, such that the NoGo bias would result in an over-activation of avoidance and rigidity.
Compulsive hoarding is currently characterized as a symptom subtype of OCD in the DSM-IV-TR (APA, 2000). However, as mentioned previously, several researchers have suggested that hoarding behaviors are part of a discrete clinical syndrome comprising additional symptoms and abnormalities suggestive of frontally mediated neurocognitive dysfunction (Grisham et al., 2007; Hartl et al., 2004; Lawrence et al., 2006; Mataix-Cols et al., 2010; Pertusa et al., 2008; Saxena, 2007; Steketee and Frost, 2003). Our studies of the neurocognitive function of older individuals with and without hoarding behaviors support this hypothesis (Mackin et al., 2010), and suggest that those with hoarding behaviors have several abnormalities of executive function, most prominently, impairments in speed of information processing, as well as deficits in categorization and attention/working memory. Consistent with this hypothesis and with the results from imaging and other studies, the results of the current study suggest that individuals with compulsive hoarding may have an overlapping, yet distinct, neural circuitry from that of individuals with OCD/S but without hoarding. The putative neurocognitive profile of individuals with compulsive hoarding, which awaits confirmation in larger samples, consists of (1) abnormalities in speed of information processing, categorization, attention and working memory, and (2) a hyperactive ERN in probabilistic learning tasks but not in response conflict tasks. As more information is acquired on the task-related pathophysiology underlying the discrepant symptom clusters of OCD, clarification of subtype-specific cognitive impairments may subserve targeted interventions.
The primary limitation of this study is that it uses previously collected data from a variety of research groups, and thus, data on specific severity of symptoms, and clinical or functional impact of symptoms are not available. This is particularly relevant for the hoarding analysis; in this analysis, we were only able to examine the relationship of the ERN in the context of the presence or the absence of hoarding symptoms among individuals with OCD or high OCS. All individuals with hoarding symptoms also had additional obsessive–compulsive symptoms, and we do not have information about whether the hoarding symptoms were the primary symptoms, nor about whether they were clinically impairing. Future work with individuals currently diagnosed with OCD, yet primarily defined by their hoarding behaviors, is important to reduce sample heterogeneity, and to gain further understanding of the distinct neurocognitive and neurophysiological abnormalities associated with the hoarding subtype; examination of the ERN within participants with primary hoarding is ongoing in our laboratory.
The use of secondary data leads to variance caused by differences in task type, phenotype definition, and measurement of the ERN, among other factors. We were able to measure the degree of heterogeneity between studies in the meta-analysis, and in fact, for the response conflict task, where there was little evidence of heterogeneity, the results were quite robust. To control for cross-study variance in the hoarding analysis, we used within-study z-scores rather than raw scores to capture the degree of difference from each study’s healthy control sample means; nevertheless, the remaining heterogeneity, particularly for the probabilistic learning tasks, may have contributed either to an under-estimate of the ERN differences in those with and without hoarding symptoms, or alternatively, an over-estimate. Neurophysiological and neuroimaging data contributing to the clarification of hoarding behaviors are currently underway.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV TR. 4. American Psychiatric Association; Washington, DC: 2000. Text Revision ed. [Google Scholar]
- Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. Journal of Psychiatric Research. 2000;34 (4–5):317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. To discard or not to discard: the neural basis of hoarding symptoms in obsessive–compulsive disorder. Molecular Psychiatry. 2008 doi: 10.1038/sj.mp.4002129. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Scheffers MK, Coles MG. Where did I go wrong? A psychophysiological analysis of error detection. Journal of Experimental Psychology: Human Perception and Performance. 1995;21 (6):1312–1322. doi: 10.1037//0096-1523.21.6.1312. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108 (3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brooks VB. How does the limbic system assist motor learning? A limbic comparator hypothesis. Brain, Behavior and Evolution. 1986;29 (1–2):29–53. doi: 10.1159/000118670. [DOI] [PubMed] [Google Scholar]
- Carbonnell L, Falkenstein M. Does the error negativity reflect the degree of response conflict? Brain Research. 2006;1095 (1):124–130. doi: 10.1016/j.brainres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280 (5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Wessel JR, Steinhauser M, Ullsperger M. Modulation of the error-related negativity by response conflict. Psychophysiology. 2009;46 (6):1288–1298. doi: 10.1111/j.1469-8986.2009.00860.x. pii:PSYP860. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- DerSimonian R, Laird N. Random effects regression model for meta-analysis. Statistics in Medicine. 1995;14:395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive–compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46 (7):1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N. Performance monitoring and error significance in patients with obsessive–compulsive disorder. Biological Psychology. 2010;84 (2):257–263. doi: 10.1016/j.biopsycho.2010.02.002. pii:S0301-0511(10)00041-4. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78 (6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51 (2–3):87–107. doi: 10.1016/s0301-0511(99)00031-9. pii:S0301051199000319. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, et al. The Obsessive–Compulsive Inventory: development and validation of a short version. Psychological Assessment. 2002;14 (4):485–496. [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47 (4):495–501. doi: 10.1016/j.neuron.2005.06.020. pii:S0896-6273(05)00526-X. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Mataix-Cols D, Caseras X, Alonso P, Manuel Menchon J, Vallejo J, et al. High sensitivity to punishment and low impulsivity in obsessive–compulsive patients with hoarding symptoms. Psychiatry Research. 2004;129 (1):21–27. doi: 10.1016/j.psychres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Coles MG, Meyer DE, Donchin E. A brain potential manifestation of error-related processing. Electroencephalography and Clinical Neurophysiology Supplement. 1995;44:261–272. [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive–compulsive disorder. Psychological Science. 2000;11 (1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. ‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neuroscience Letters. 1986;70 (2):223–227. doi: 10.1016/0304-3940(86)90467-2. pii:0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Mataix-Cols D, Almeida JR, Lawrence N, Nutche J, Diwadkar V, et al. Brain structure and symptom dimension relationships in obsessive–compulsive disorder: a voxel-based morphometry study. Journal of Affective Disorders. 2008;109 (1–2):117–126. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Brown TA, Savage CR, Steketee G, Barlow DH. Neuropsychological impairment associated with compulsive hoarding. Behaviour Research and Therapy. 2007;45 (7):1471–1483. doi: 10.1016/j.brat.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Grundler TO, Cavanagh JF, Figueroa CM, Frank MJ, Allen JJ. Task-related dissociation in ERN amplitude as a function of obsessive–compulsive symptoms. Neuropsychologia. 2009;47 (8–9):1978–1987. doi: 10.1016/j.neuropsychologia.2009.03.010. pii:S0028-3932(09)00129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive–compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165 (1):116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Huppert JD, Simons RF, Foa EB. Psychometric properties of the OCI-R in a college sample. Behaviour Research and Therapy. 2004;42 (1):115–123. doi: 10.1016/j.brat.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64 (1–2):77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive–compulsive undergraduates. Psychiatry Research. 2002;110 (1):63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hammer A, Kordon A, Heldmann M, Zurowski B, Munte TF. Brain potentials of conflict and error-likelihood following errorful and errorless learning in obsessive–compulsive disorder. PLoS One. 2009;4 (8):e6553. doi: 10.1371/journal.pone.0006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive–compulsive disorder. Archives of General Psychiatry. 2009;66 (11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. pii:66/11/1189. [DOI] [PubMed] [Google Scholar]
- Hartl TL, Frost RO, Allen GJ, Deckersbach T, Steketee G, Duffany SR, et al. Actual and perceived memory deficits in individuals with compulsive hoarding. Depression and Anxiety. 2004;20 (2):59–69. doi: 10.1002/da.20010. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109 (4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, et al. Discrepant target detection and action monitoring in obsessive–compulsive disorder. Psychiatry Research. 2001;108 (2):101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Karno M, Golding JM, Sorenson SB, Burnam A. The epidemiology of obsessive–compulsive disorder in five US communities. Archives of General Psychiatry. 1988:45. doi: 10.1001/archpsyc.1988.01800360042006. [DOI] [PubMed] [Google Scholar]
- Katerberg H, Delucchi KL, Stewart SE, Lochner C, Denys DA, Stack DE, et al. Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behavior Genetics. 2010;40 (4):505–517. doi: 10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37 (2):216–223. [PubMed] [Google Scholar]
- Lawrence NS, Wooderson S, Mataix-Cols D, David R, Speckens A, Phillips ML. Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive–compulsive disorder. Neuropsychology. 2006;20 (4):409–419. doi: 10.1037/0894-4105.20.4.409. [DOI] [PubMed] [Google Scholar]
- Luchian SA, McNally RJ, Hooley JM. Cognitive aspects of nonclinical obsessive–compulsive hoarding. Behaviour Research and Therapy. 2007;45 (7):1657–1662. doi: 10.1016/j.brat.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Arean PA, Delucchi KL, Mathews CA. Cognitive functioning in individuals with severe compulsive hoarding behaviors and late life depression. International Journal of Geriatric Psychiatry. 2010 doi: 10.1002/gps.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H, Falkenstein M, Sturmer B, Pinkpank T, Sommer W. Does the error negativity reflect response conflict strength? Evidence from a Simon task. Psychophysiology. 2007;44 (4):579–585. doi: 10.1111/j.1469-8986.2007.00522.x. pii:PSYP522. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Frost RO, Pertusa A, Clark LA, Saxena S, Leckman JF, et al. Hoarding disorder: a new diagnosis for DSM-V? Depression and Anxiety. 2010;27 (6):556–572. doi: 10.1002/da.20693. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive–compulsive disorder. Archives of General Psychiatry. 2004;61 (6):564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biological Psychology. 2003;64 (1–2):119–141. doi: 10.1016/s0301-0511(03)00105-4. pii:S0301051103001054. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive–compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32 (3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. pii:S0149-7634(07)00114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Nielen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive–compulsive disorder. Psychiatry Research. 2005;134 (2):111–122. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38 (5):752–760. [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Research. 1979;171 (2):213–224. doi: 10.1016/0006-8993(79)90328-7. pii:0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, et al. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. European Journal of Neuroscience. 2007;25 (8):2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. pii:EJN5477. [DOI] [PubMed] [Google Scholar]
- Pertusa A, Fullana MA, Singh S, Alonso P, Menchon JM, Mataix-Cols D. Compulsive hoarding: OCD symptom, distinct clinical syndrome, or both? American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07111730. [DOI] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, 3rd, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics Study: symptoms vs. categories. Psychiatry Research. 2008;160 (1):83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive–compulsive psychopathology. Comprehensive Psychiatry. 1987;28 (4):334–343. doi: 10.1016/0010-440x(87)90070-8. pii:0010-440X(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Rachman S, Elliott CM, Shafran R, Radomsky AS. Separating hoarding from OCD. Behaviour Research and Therapy. 2009;47 (6):520–522. doi: 10.1016/j.brat.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Reason J. Human Error. Cambridge University Press; Cambridge, England: 1990. [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive–compulsive disorder. Archives of General Psychiatry. 2006;63 (11):1225–1236. doi: 10.1001/archpsyc.63.11.1225. pii:63/11/1225. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive–compulsive disorder: evidence from unaffected first-degree relatives. American Journal of Psychiatry. 2011;168 (3):317–324. doi: 10.1176/appi.ajp.2010.10030416. pii:appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Gron G, Reuter K, Spitzer M, Hermle L, Kiefer M. Error-related brain activity in patients with obsessive–compulsive disorder and in healthy controls. Journal of Psychophysiology. 2005;19 (4):298–304. [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive–compulsive behaviors. Developmental Neuropsychology. 2006;29 (3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Saxena S. Is compulsive hoarding a genetically and neurobiologically discrete syndrome? Implications for diagnostic classification. American Journal of Psychiatry. 2007;164 (3):380–384. doi: 10.1176/ajp.2007.164.3.380. [DOI] [PubMed] [Google Scholar]
- Saxena S. Neurobiology and treatment of compulsive hoarding. CNS Spectrums. 2008;13 (9 Suppl 14):29–36. doi: 10.1017/s1092852900026912. [DOI] [PubMed] [Google Scholar]
- Saxena S, Maidment KM, Vapnik T, Golden G, Rishwain T, Rosen RM, et al. Obsessive–compulsive hoarding: symptom severity and response to multimodal treatment. Journal of Clinical Psychiatry. 2002;63 (1):21–27. [PubMed] [Google Scholar]
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive–compulsive disorder. Psychiatric Clinics of North America. 2000;23 (3):563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000;26 (1):141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Steketee G, Frost R. Compulsive hoarding: current status of the research. Clinical Psychology Review. 2003;23 (7):905–927. doi: 10.1016/j.cpr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, et al. Chronic medication does not affect hyperactive error responses in obsessive–compulsive disorder. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.00988.x. pii:PSYP988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive–compulsive disorder. Journal of Anxiety Disorders. 2003;17 (2):233–242. doi: 10.1016/s0887-6185(02)00182-2. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Kiehl KA, Worhunsky P, Book GA, Maltby N. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychological Medicine. 2008:1–12. doi: 10.1017/S0033291708003371. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Kiehl KA, Worhunsky P, Book GA, Maltby N. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychological Medicine. 2009;39 (2):325–336. doi: 10.1017/S0033291708003371. pii:S0033291708003371. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive–compulsive disorder. Archives of General Psychiatry. 2005;62 (3):301–309. doi: 10.1001/archpsyc.62.3.301. pii:62/3/301. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77 (4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. pii:S0031938402009307. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, et al. Error-related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35 (1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. pii:S0278-5846(10)00439-2. [DOI] [PubMed] [Google Scholar]