Abstract
Enhanced spiral ganglion neuron (SGN) survival and regeneration of peripheral axons following deafness will likely enhance the efficacy of cochlear implants. Overexpression of Bcl-2 prevents SGN death, but inhibits neurite growth. Here we assessed the consequences of Bcl-2 targeted to either the mitochondria (GFP-Bcl-2-Maob) or endoplasmic reticulum (ER, GFP-Bcl-2-Cb5) on cultured SGN survival and neurite growth. Transfection of wild type GFP-Bcl-2, GFP-Bcl-2-Cb5, or GFP-Bcl-2-Maob increased SGN survival, with GFP-Bcl-2-Cb5 providing the most robust response. Paradoxically, expression of GFP-Bcl-2-Maob results in SGN death in the presence of neurotrophin-3 (NT-3) and brain derived neurotrophic factor (BDNF), neurotrophins that independently promote SGN survival via Trk receptors. This loss of SGNs is associated with cleavage of caspase 3 and appears specific for neurotrophin signaling, since co-expression of constitutively active mitogen activated kinase kinase (MEKΔEE) or phosphatidyl inositol-3 kinase (P110), but not other prosurvival stimuli (e.g. membrane depolarization), also results in the loss of SGNs expressing GFP-Bcl-2-Maob. MEKΔEE and P110 promote SGN survival while P110 promotes neurite growth to a greater extent than NT-3 or MEKΔEE. However wild-type GFP-Bcl-2, GFP-Bcl-2-Cb5 and GFP-Bcl-2-Maob inhibit neurite growth even in the presence of neurotrophins, MEKΔEE, or P110. Historically, Bcl-2 has been thought to act primarily at the mitochondria to prevent neuronal apoptosis. Nevertheless, our data show that Bcl-2 targeted to the ER is more effective at rescuing SGNs in the absence of trophic factors. Additionally, Bcl-2 targeted to the mitochondria results in SGN death in the presence of neurotrophins.
Keywords: auditory neuron, axon growth, phosphatidyl inositol-3 kinase, mitogen activated protein kinase kinase, nerve regeneration
INTRODUCTION
Spiral ganglion neurons (SGNs) provide the afferent fibers of the auditory nerve. They express TrkB and TrkC, but not Trk A, receptors and are thus supported by brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) along with other neurotrophic factors, which are produced in the cochlea, spiral ganglion Schwann cells, and the cochlear nucleus (Despres and Romand 1994; Farinas et al. 2001; Fritzsch et al. 1997; Hansen et al. 2001a; Pirvola et al. 1994; Schecterson and Bothwell 1994; Staecker et al. 1995; Wheeler et al. 1994; Ylikoski et al. 1993). Loss of hair cells leads to degeneration of the afferent fibers innervating the cochlea and ultimately death of the SGNs via apoptosis (Alam et al. 2007; Lee et al. 2003; Spoendlin 1975; Wang et al. 2001b). Strategies to prevent SGN death and regenerate the peripheral axons carries particular relevance for patients with profound hearing loss since most are candidates for cochlear implantation with an electrode to stimulate the residual SGNs (Bianchi and Raz 2004; Pettingill et al. 2007; Roehm and Hansen 2005). Disruption of the apoptotic mechanism represents one strategy to prevent SGN degeneration following deafening (Alam et al. 2007; Lee et al. 2003).
Members of the Bcl-2 family of proteins are critical regulators of apoptosis; some promote survival while others promote apoptosis (Rong and Distelhorst 2008). Prosurvival members of the Bcl-2 family (e.g. Bcl-2 and Bcl-XL) rescue SGNs deprived of trophic factor in vitro and in vivo (Hansen et al. 2007; Staecker et al. 2007). Bcl-2 expression decreases in the spiral ganglion following hair cell loss, correlated with SGN death, further supporting a role for Bcl-2 in SGN survival (Bae et al. 2008). In addition to their well-characterized effects on apoptosis, members of the Bcl-2 family of proteins also regulate neurite growth. For example, Bcl-2 enhances axon and neurite growth in retinal ganglion cells (RGCs), embryonic trigeminal neurons, and PC12 cells (Chen et al. 1997; Hilton et al. 1997; Jiao et al. 2005; Leaver et al. 2006). Conversely, overexpression of Bcl-2 and Bcl-XL, which promote SGN survival, strongly inhibit SGN neurite growth (Hansen et al. 2007).
Bcl-2 was initially described as localizing to the mitochondria where it disrupts the apoptotic machinery to promote cell survival (Gorman et al. 2000; Gross et al. 1999; Hockenbery et al. 1990; Kroemer et al. 2007; Reed et al. 1998; Shimizu et al. 1998; Tsujimoto et al. 2006). More recently, Bcl-2 function in the endoplasmic reticulum (ER) has been recognized, where it regulates ER Ca2+ stores and prevents apoptosis in many cells (Bassik et al. 2004; Rong and Distelhorst 2008; Thomenius and Distelhorst 2003). In PC12 cells, Bcl-2 targeted to either the mitochondria or ER promotes cell survival, whereas only Bcl-2 targeted to the ER promotes neurite growth, implying that the effects of Bcl-2 on neuronal differentiation depend on its subcellular localization (Jiao et al. 2005). Here we investigated the relative ability of Bcl-2 targeted to either the mitochondria or the ER to promote SGN survival. We also asked whether targeting Bcl-2 to the mitochondria or ER would differentially affect neurite growth. We find that Bcl-2 targeted to the mitochondria and ER both promote SGN survival, with the later being more effective. Further, overexpression of Bcl-2 inhibits SGN neurite growth regardless of its targeting to the mitochondria or ER. Finally, we found that targeting of Bcl-2 to the mitochondria paradoxically induces SGN death in the presence of neurotrophins, but not other pro-survival stimuli.
METHODS
Spiral ganglion culture and transfection
Dissociated spiral ganglion cultures were prepared from P5 rat pups, plated on polyornithine- and laminin-coated 8-well culture chambers (Nalge Nunc Intl., Naperville, IL), and maintained in high glucose Dulbecco’s Modified Eagle’s Medium with N2 supplement (Invitrogen, Carlsbad, CA) and fresh insulin (Sigma-Aldrich, St. Louis, 10 μg/ml) in a humidified incubator with 6.5% CO2 at 37°C as previously described (Hansen et al. 2001b; Hegarty et al. 1997). All cultures were initially plated in BDNF or NT-3 (R&D systems, Minneapolis, MN, 50 ng/ml) to support SGN survival during transfection. The cultures were then transfected with expression plasmids 3–4 hours following placement in culture using calcium-phosphate precipitation at 37°C as previously described (Hansen et al. 2003; Zha et al. 2001). Twelve to 14 hours after transfection the cultures were washed three times, the media was exchanged and the cultures were maintained in serum free DMEM with N2 supplements in the presence or absence of neurotrophic factors that promote SGN survival for an additional 48 hours.
Wild-type, ER and mitochondrially targeted GFP tagged Bcl-2 constructs, and corresponding constructs lacking Bcl-2 were kindly provided by Clark Distelhorst (Case Western Univ. Cleveland, OH). pGFP-Bcl-2-Cb5 and pGFP-Cb5 encode amino acids 100–134 of human cytochrome b5 to target expression to the ER (Wang et al. 2001a). pGFP-Bcl-2-Maob and pGFP-Maob encode amino acids 492–520 of human monoamine oxidase B to target expression to the mitochondria (Wang et al. 2001a). Constitutively active MEK1 (MEKΔEE) and P110 expression plasmids were kindly provided by Dr. Steven Green (University of Iowa, Iowa City, IA) (Cowley et al. 1994; Hu et al. 1995; Klippel et al. 1996).
Immunolabeling and microscopy
Following completion of each experiment, cultures were fixed with 4% paraformaldehyde for 10 minutes and permeabilized with 0.1% Triton-X in phosphate buffered saline (PBS). Non-specific antibody was blocked with blocking buffer (5% goat serum, 2% bovine serum albumin (BSA) and 0.1% Triton-X in PBS) and immunolabeled with anti-neurofilament 200 (NF200) monoclonal antibody (N52, Sigma-Aldrich, St. Louis, MO) that recognizes phosphorylated and unphosphorylated NF200 (1:1000, Sigma-Aldrich; St. Louis, MO). Subsets of cultures were simultaneously immunostained with rabbit anti-heat shock protein 60 (1:100, Cell Signaling, Boston, MA), rabbit anti-calnexin (1:100, SPA-860, Stressgen Biotechnologies, San Diego, CA), rabbit anti-Bcl-2 (1:1,1000, ab18210, Abcam, Cambridge, MA) or rabbit anti-cleaved caspase-3 (Active caspase, 1:400, 5A1E, Cell Signaling, Boston, MA). Alexa 350, Alexa 546, Alexa 568, and/or Alexa 647 conjugated secondary antibodies (1:1000, Invitrogen, Carlsbad, CA) were used to detect primary antibody labeling as indicated.
Following fixation and immunostaining, apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the In Situ Cell Death Detection Kit, TMR red kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions as previously described (Hansen et al. 2008). Nuclei were labeled with Hoechst 3342 (10 μg/ml).
Digital images of transfected SGN were captured on a Leica DMRIII microscope equipped with epifluorescent filters and a cooled CCD camera using Leica FW4000 software (Leica Microsystems, Bannockburn, IL). For detection of anti-Hsp60, anti-calnexin, anti-Bcl-2, or anti-Active caspase immunolabeling, MitoTracker Red labeling, and TUNEL the cultures were imaged on a Leica SP5 laser scanning confocal microscope equipped with 405 nm, 488 nm, 543 nm, and 633 nm laser lines (Leica Microsystems, Bannockburn, IL)
Neuron survival and neurite length determination
Survival of transfected SGNs was determined as previously described (Hansen et al. 2003; Hansen et al. 2007). Briefly, every transfected, surviving neuron was counted for each condition. Criteria for scoring neuronal survival were: (1) GFP positive, (2) NF200 immunoreactive, and (3) and an intact, non-pyknotic nucleus. Each condition was performed in duplicate or triplicate and repeated at least three times. For each repetition, the control condition consisted of the number of surviving SGNs transfected with GFP and maintained in NT-3 (50 ng/ml). SGN survival was calculated as percentage of surviving transfected SGNs relative to the number of transfected SGNs present in control conditions for each repetition. We typically obtained ~1,000 surviving SGNs/well in cultures maintained in NT3 (50 ng/ml) corresponding to ~2,000 surviving SGNs/cochlea in culture, similar to the plating efficiency of other methods of culturing rat SGNs (Hansen et al. 2007; Ripoll and Rebillard 1997). Typically 10–15% of the SGNs were transfected yielding approximately 130 transfected SGNs per well (Hansen et al. 2007). Significance for difference in means among groups for cell survival was tested by one way ANOVA followed by a post hoc Tukey test using SigmaStat (Systat Software, Inc., Point Richmond, CA).
Neurite length was determined by measuring the entire length of the longest process extending from transfected SGNs (GFP- and NF200-positive) using the measurement tool in ImageJ (NIH; Bethesda, MD). Ten to thirty randomly selected SGNs were scored for each experimental repetition. Length is defined as the maximal possible distance along a neurite, i.e., the distance from the soma to the end of the longest neurite (if more than one neurite was present) and to the end of the longest branch at each branchpoint for branched neurites. If the longest neurite/branch of the chosen cell extended beyond the image border, additional images were acquired to include and measure the entire length of the process (Hansen et al. 2007; Roehm et al. 2008). Cumulative histograms based on neurite length with 100 μm bins were constructed for all transfected SGNs in each condition using Excel software (Microsoft, Seattle, WA). Significance for differences in neurite length among treatment groups was determined by Kruskal-Wallis ANOVA on ranks followed by a post hoc Dunn’s method using SigmaStat.
MitoTracker Red labeling
SG cultures, transfected with GFP-Maob or GFP-Bcl-2-GFP and maintained in the absence of trophic factors, were loaded for 15 min with 100 nM MitoTracker® Red CMXRos (Invitrogen, Carlsbad, CA) at 37 °C 24 h after transgene expression according to the manufacturer’s protocol. Following 3 rinses with media, the cultures were fixed with 4% paraformaldehyde and immunolabeled with anti-NF200 antibody followed by an Alexa 350 conjugated secondary antibody. Cells were imaged with a Leica SP5 laser scanning confocal microscope.
RESULTS
Bcl-2 promotes SGN survival, but inhibits neurite growth (Hansen et al. 2007; Staecker et al. 2007). We sought to determine if the ability of Bcl-2 to promote cell survival or inhibit neurite growth depends on its subcellular localization. Primary spiral ganglion cultures were prepared from p5rat pups and were transiently transfected with plasmids encoding Bcl-2 tagged with green fluorescent protein (GFP) and targeted to either the mitochondria or ER. GFP-Bcl-2-Maob, which bears the mitochondria targeting region of monoamine oxidase b (Maob) (Wang et al. 2001a), localizes to the mitochondria in SGNs, verified by colocalization of GFP-Bcl-2-Maob with heat shock protein (Hsp) 60 immunoreactivity (Fig. 1). GFP-Bcl-2-Cb5, bearing the ER targeting sequence of cytochrome b5 (Cb5) (Wang et al. 2001a), localizes to the ER in SGNs, verified by colocalization with calnexin immunoreactivity (Fig. 1).
Figure 1.
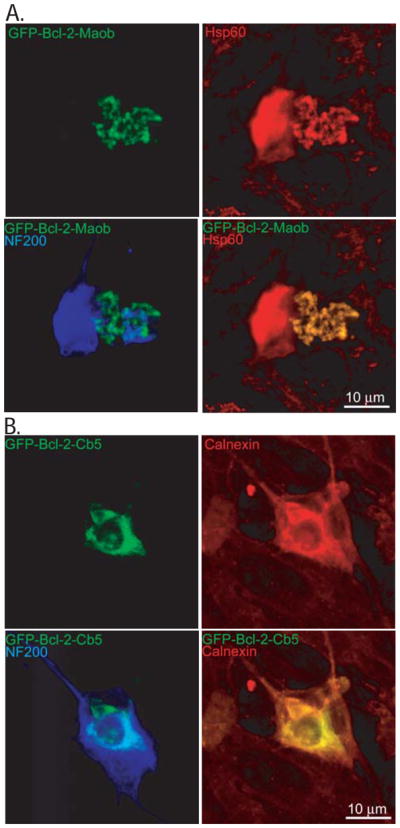
Targeting of Bcl-2 to the mitochondria and endoplasmic reticulum in spiral ganglion neurons. Spiral ganglion neurons were transfected with GFP-Bcl-2-Maob (A) or GFP-Bcl-2-Cb5 (B). Cultures were immunostained with anti-heat shock protein (Hsp) 60 (A, red) or anti-calnexin (B, red) and anti-neurofilament 200 (NF200, blue) antibodies and imaged with laser confocal microscopy. GFP-Bcl-2-GFP-Maob colocalizes with Hsp60 immunoreactivity and GFP-Bcl-2-GFP-Cb5 colocalizes with calnexin immunoreactivity.
For survival assays, SG cultures were transfected with wild-type GFP-Bcl-2, GFP-Bcl-2-Maob, or GFP-Bcl-2-Cb5 or with the control plasmids, GFP, GFP-Maob, or GFP-Cb5. Immunostaining with anti-Bcl-2 antibodies confirmed increased Bcl-2 expression in neurons transfected with wild-type GFP-Bcl-2, GFP-Bcl-2-Moab, or GFP-Bcl-2-Moab compared with nontransfected neurons or neurons transfected with control plasmids (Fig. 2). Forty eight hours after transgene expression the cultures were fixed and immunolabeled with anti-neurofilament 200 (NF200) antibody to identify SGNs and their neurites. The number of surviving SGNs was determined in each condition and expressed as a percent of SGNs transfected with GFP and maintained in neurotrophin-3 (NT-3, 50 ng/ml), which promotes SGN survival (Hegarty et al. 1997). The number of GFP-positive surviving SGNs in NT-3 across all repetitions was 127±24 (mean±standard deviation).
Figure 2.
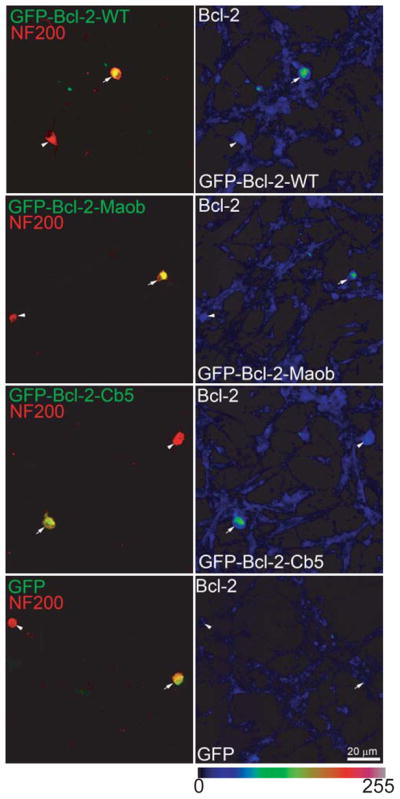
Transfection of GFP-Bcl-2-WT, GFP-Bcl-2-Maob or GFP-Bcl-2-Cb5 results in increased Bcl-2 expression. Spiral ganglion cultures were transfected with GFP-Bcl-2-WT, GFP-Bcl-2-Maob or GFP-Bcl-2-Cb5, or GFP as indicated. Twenty four hrs after transfection, the cultures were immunostained with anti-NF200 (red) and anti-Bcl-2 (scaled, right panels) antibodies and imaged with laser confocal microscopy. The intensity of Bcl-2 immunostaining is scaled (0-255) in the right panels. Images were obtained a lower magnification to allow demonstration of a transfected (GFP-positive, green, arrow) and untransfected neuron (GFP-negative, arrowhead) in each condition. SGNs expressing GFP-Bcl-2-WT, GFP-Bcl-2-Maob or GFP-Bcl-2-Cb5 demonstrate increased Bcl-2 immunoreactivity compared with SGNs expressing GFP, GFP-Maob (not shown), or GFP-Cb5 (not shown). Scale bar=20 μm.
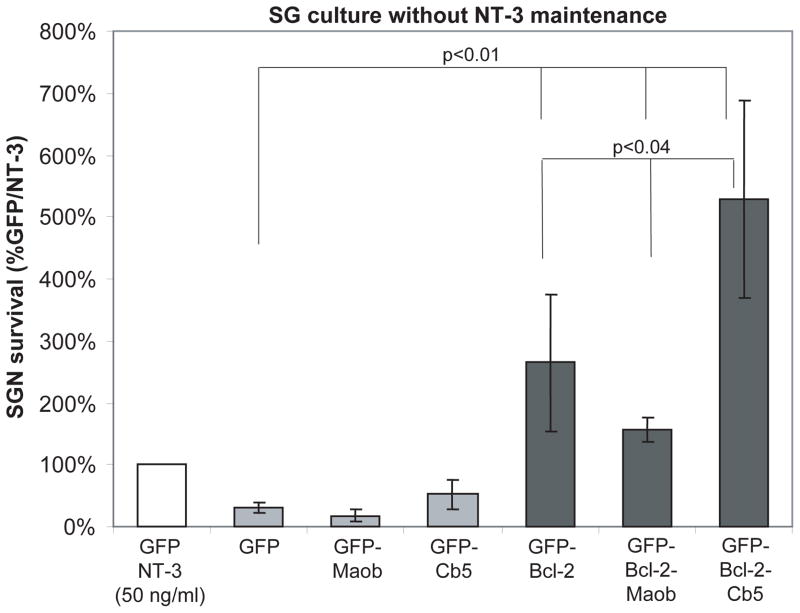
Similar to wild-type GFP-Bcl-2 (Hansen et al. 2007), both GFP-Bcl-2-Maob and GFP-Bcl-2-Cb5 promote SGN survival compared with GFP-Maob or GFP-Cb5, respectively (p<0.01) (Fig. 3). Survival of SGNs transfected with GFP-Bcl-2-Cb5 was significantly greater than survival of SGNs transfected with either GFP-Bcl-2 or GFP- Bcl-2-Maob (p<0.04). On the other hand, survival of SGNs transfected with GFP-Maob or GFP-Cb5 and maintained in NT-3 was comparable to survival of SGNs transfected with GFP and maintained in NT-3 (Fig. 5).
Figure 3.
Expression of Bcl-2 targeted to the mitochondrial or endoplasmic reticulum promotes spiral ganglion neuron (SGN) survival. Mean SGN survival in cultures transfected with GFP and maintained in neurotrophin-3 (NT-3, 50 ng/ml) is defined as 100%. The number of surviving SGNs in control conditions was 127±24 (mean±standard deviation) across all repetitions. Transfection of GFP-Bcl-2-Cb5 or GFP-Bcl-2-Maob increases mean SGN survival 5 and 1.5 times, respectively, compared with transfection of GFP and maintenance of cultures in NT-3 (control). Each condition was performed in duplicate or triplicate and repeated at least three times. Error bars present standard deviation from each repetition. Significance of difference among means was determined by one-way ANOVA followed by a post-hoc Tukey analysis.
Figure 5.
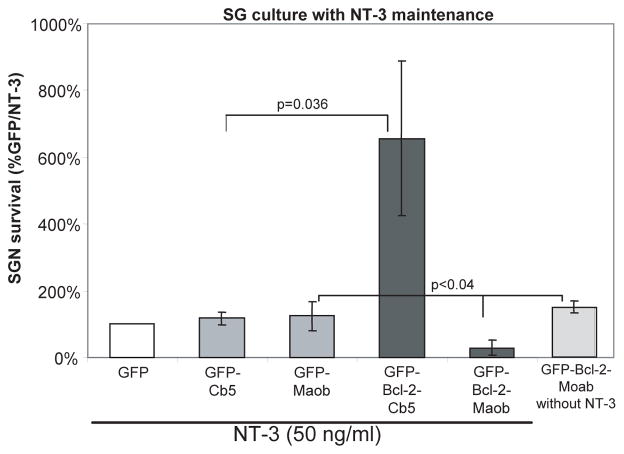
Mitochondrially targeted Bcl-2 decreases SGN survival in the presence of NT-3. Mean SGN survival in cultures transfected with GFP and maintained in neurotrophin-3 (NT-3, 50 ng/ml) is defined as 100%. Expression of GFP-Bcl-2-Maob in the presence of NT-3 decreased mean SGN survival by 81% relative to cultures expressing GFP-Bcl-2-Maob in the absence of NT-3. Each condition was performed in duplicate or triplicate and repeated at least three times. Error bars present standard deviation from each repetition. Significance of difference among means was determined by one-way ANOVA followed by a post-hoc Tukey analysis.
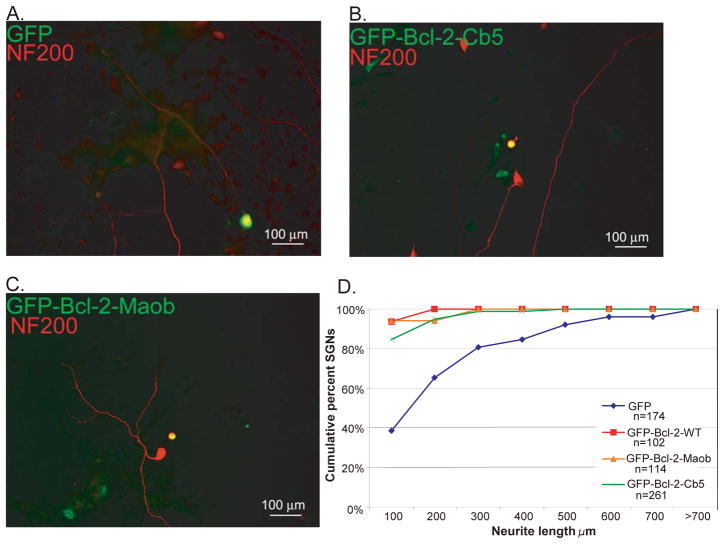
Overexpression of wild-type Bcl-2 or Bcl-XL inhibits SGN neurite growth, even in the presence of neurotrophic factors (Hansen et al. 2007). We next asked whether the ability of Bcl-2 to inhibit neurite depends on its subcellular targeting. We compared neurite length of SGNs transfected with wild-type GFP-Bcl-2, GFP-Bcl-2-Maob, or GFP-Bcl-2-Cb5 with SGNs transfected with GFP. Neurite lengths are presented as a cumulative histogram so that conditions with longer neurites are shifted to the right compared with conditions with shorter neurites (Fig. 4). Both GFP-Bcl-2-Maob and GFP-Bcl-2-Cb5 significantly reduced SGN neurite growth relative to control, (p<0.05, Kruskal-Wallis ANOVA on ranks and post hoc Dunn’s method), even in the presence of NT-3, a neurotrophin that promotes SGN neurite growth (Aletsee et al. 2001). Thus, similar to wild-type Bcl-2 and Bcl-XL, Bcl-2 targeted to the mitochondria or ER inhibits SGN neurite growth.
Figure 4.
Expression of Bcl-2 to the mitochondrial or endoplasmic reticulum inhibits SGN neurite growth. A, B, and C. Representative epifluorescence images of spiral ganglion cultures transfected with GFP, GFP-Bcl-2-Cb5, or GFP-Bcl-2-Maob and maintained in the presence of NT-3 (50 ng/ml) for 48 h following transgene expression. Cultures were immunolabeled with anti-NF200 antibodies (red). SGNs transfected with GFP-Bcl-2-Cb5 (B) or GFP-Bcl-2-Maob (C) extend short neurites relative to SGNs transfected with GFP (A). D. Neurite length was determined by measuring the longest process extending from each transfected SGN. Results are presented as cumulative percent histograms where conditions with shorter neurites are shifted to the left compared with conditions with longer neurites. Each condition was performed in duplicate or triplicate and repeated at least three times. n=total cumulative number of SGNs scored for each condition for all repetitions. Neurite length in cultures transfected with wild-type GFP-Bcl-2, GFP-Bcl-2-Cb5, or GFP-Bcl-2-Maob was significantly different from cultures transfected with GFP (p<0.05 by Kruskal-Wallis ANOVA on ranks and post hoc Dunn’s method).
To further characterize the pro-survival effects of Bcl-2, we asked whether the ability of Bcl-2 targeted to either the mitochondria or ER to promote SGN survival was additive with neurotrophins. SG cultures transfected with GFP-Bcl-2-Maob, GFP-Bcl2-Cb5, GFP-Maob, or GFP-Cb5 were maintained in the presence of absence of NT-3 (50 ng/ml) and SGN survival was determined as above. There was no difference in SGN survival in cultures transfected with GFP-Bcl-2-Cb5 in the presence of absence of NT-3, indicating that the prosurvival effects of neurotrophins are not additive with Bcl-2 localized to the ER (Figs. 3 and 5). By contrast, treatment with NT-3 significantly reduced the survival of SGNs transfected with GFP-Bcl-2-Moab relative to those maintained in the absence of NT-3 or to SGNs transfected with GFP-Maob and maintained in NT-3 (p<0.04). The increase in cell death in SGNs expressing GFP-Bcl-2-Maob in the presence of NT-3 was not simply due to increased levels of proteins localized to the outer mitochondrial membrane since NT-3 did not increase death of SGNs expressing GFP-Maob. Also, it is not due to a generalized overexpression of Bcl-2 since NT-3 does not induce cell death in SGN transfected with wild-type GFP-Bcl-2 or GFP-Bcl-2-Cb5.
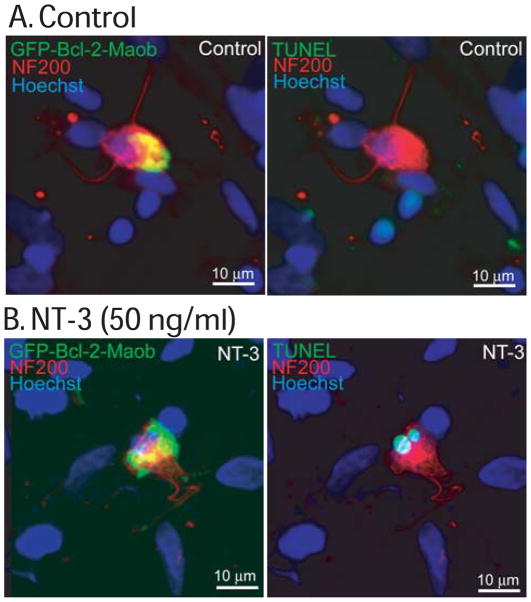
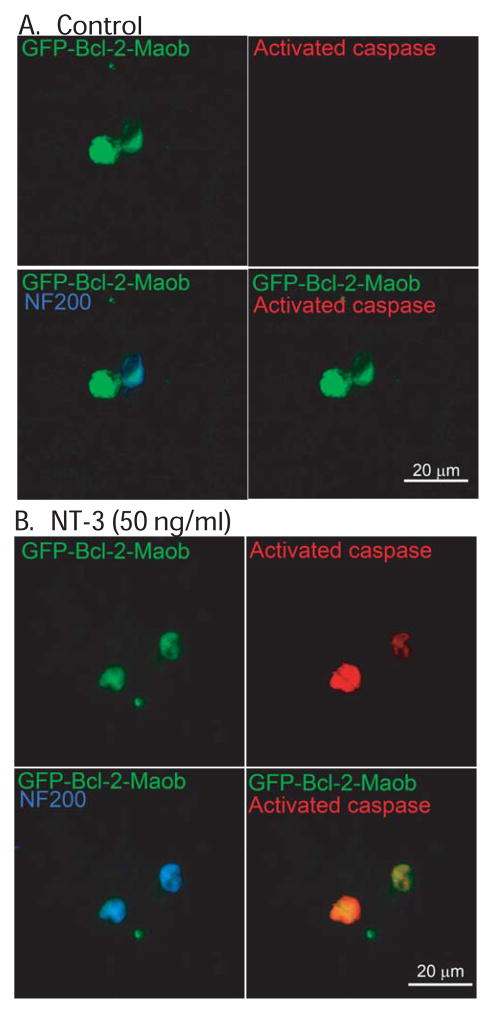
To determine the type of cell death induced by NT-3 in SGNs expressing GFP-Bcl-2-Maob, we labeled a subset of cultures with TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling), which specifically labels nuclei of apoptotic cells (Gavrieli et al. 1992). SGNs expressing GFP-Bcl-2-Maob and maintained in NT-3 demonstrated condensed and/or fragmented nuclei that labeled with TUNEL suggesting that these neurons die by apoptosis (Fig. 6). To confirm apoptosis in SGNs expressing GFP-Bcl-2-Maob and treated with NT-3, we immunostained a subset of cultures with an anti-active caspase-3 antibody which specifically detects cleaved caspase-3, a hallmark of apoptosis (Rong and Distelhorst 2008). Activated caspase 3 was detected in the cytoplasm of SGNs expressing GFP-Bcl-2-Maob in the presence, but not the absence, of NT-3 (Fig. 7). Thus, NT-3 induces apoptosis in SGNs expressing Bcl-2 localized to the mitochondria.
Figure 6.
Treatment with NT-3 leads to apoptosis of SGNs expressing mitochondrially targeted Bcl-2. Spiral ganglion cultures were transfected with GFP-Bcl-2-Maob and maintained in the absence (A) or presence (B) of NT-3 for 24 h after transgene expression. Cultures were immunostained with anti-NF200 (red) antibodies and labeled with TUNEL (green, right panels) and imaged with laser confocal microscopy. SGNs transfected with GFP-Bcl-2-Maob and treated with NT-3 (B), but not control SGNs (A) demonstrate TUNEL-positive condensed and fragmented nuclei.
Figure 7.
Treatment with NT-3 leads to activation of caspase 3 in SGNs expressing mitochondrially targeted Bcl-2. Spiral ganglion cultures were transfected with GFP-Bcl-2-Maob and maintained in the absence (A) or presence (B) of NT-3 for 24 h after transgene expression. Cultures were immunostained with anti-NF200 (blue) and anti-activated caspase-3 (red) antibodies and imaged with laser confocal microscopy. SGNs transfected with GFP-Bcl-2-Maob and treated with NT-3 (B) demonstrate activated caspase-3 immunoreactivity. Scale bar=10 μm.
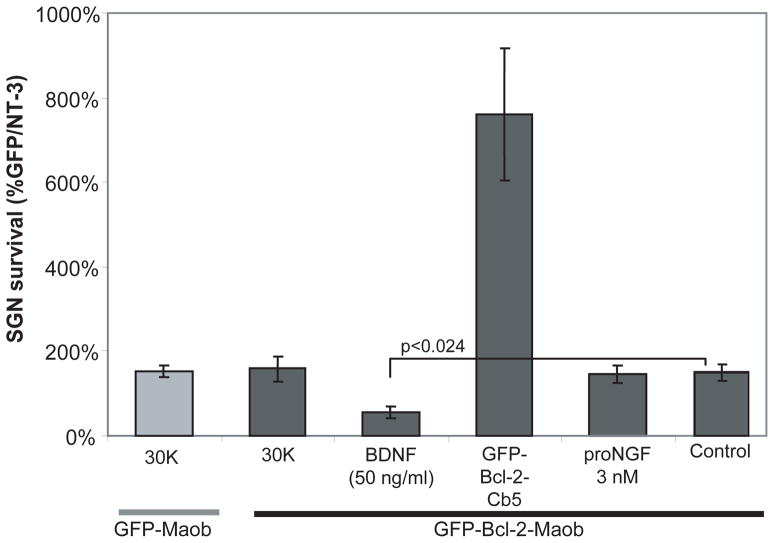
The paradoxical increase in apoptosis of SGNs expressing GFP-Bcl-2-Maob treated with NT-3 prompted us to ask whether other pro-survival stimuli also increase apoptosis in SGNs expressing GFP-Bcl-2-Maob. Membrane depolarization with 30 mM extracellular potassium (30K) or cotransfection of GFP-Bcl-2-Cb5, both potent pro-survival stimuli (Figs. 3 and 8) (Hansen et al. 2007; Hegarty et al. 1997), did not increase cell death in SGNs transfected with GFP-Bcl-2-Maob (Fig. 8). Conversely, BDNF, which promotes SGN neurite growth and survival (Hegarty et al. 1997), increased cell death in SGNs transfected with GFP-Bcl-2-Moab (p=0.024), similar to the effect of NT-3. SGNs express TrkB and TrkC suggesting that the increase in SGN death by BDNF and NT-3 results from activation of Trk signaling. Another possibility is that NT-3 and BDNF promote apoptosis by activating p75NTR, a low affinity receptor for neurotrophins that induces apoptosis in some neurons (Lee et al. 2001; Linggi et al. 2005). To test this possibility, we treated SG cultures transfected with GFP-Bcl-2-Maob with proNGF (3 nM), a high affinity ligand for p75NTR. Additionally, SGNs lack TrkA so that proNGF cannot activate Trk in SGNs. Contrary to the effect of mature neurotrophins, proNGF did not induce cell death in SGNs expressing GFP-Bcl-2-Maob (Fig. 8). Taken together these results demonstrate that neurotrophins induce cell death in SGNs expressing GFP-Bcl-2-Maob via activation of Trk.
Figure 8.
Mitochondrially targeted Bcl-2 decreases SGN survival specifically in the presence of neurotrophins. Mean SGN survival in cultures transfected with GFP and maintained in neurotrophin-3 (NT-3, 50 ng/ml) is defined as 100%. Maintenance of cultures expressing GFP-Bcl-2-Maob in brain derived neurotrophic factor (BDNF, 50 ng/ml) decreased mean SGN survival by 64% relative to cultures expressing GFP-Bcl-2-Maob in the absence of neurotrophic factors. Conversely, maintenance of cultures expressing GFP-Bcl-2-Maob in 30 mM extracellular K+ (30K) or pro-nerve growth factor (proNGF, 3 nM) did not reduce SGN survival. Error bars present standard deviation from each repetition. Each condition was performed in duplicate or triplicate and repeated at least three times. Significance of difference among means was determined by one-way ANOVA followed by a post-hoc Tukey analysis.
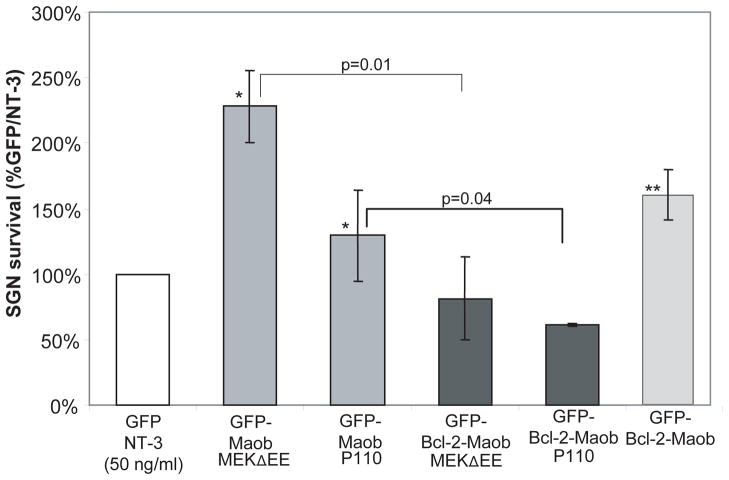
Neurotrophins activate mitogen activated protein kinase kinase/extracellular regulated kinase (MEK/Erk) and phosphatidyl inositol-3 kinase/Akt (PI3-K/Akt) signaling to promote SGN survival; inhibition of MEK or PI3-K reduces survival of SGNs treated with neurotrophins (Hansen et al. 2001b). We asked which of these pathways contributes to the death of SGNs expressing GFP-Bcl-2-Moab in the presence of neurotrophins. To address this question, we transfected SGNs with plasmids encoding P110, the catalytic domain of PI3-K which is constitutively active, or a constitutively active mutant of MEK (MEKΔEE) (Cowley et al. 1994; Hu et al. 1995; Klippel et al. 1996). Both P110 and MEKΔEE maintained SGN survival in the absence of other trophic stimuli, indicating that each is sufficient for SGN survival (Fig. 9).
Figure 9.
Activation of PI3-K or MEK1 promotes SGN survival and decreases survival of SGNs expressing mitochondrially targeted Bcl-2. Spiral ganglion cultures were cotransfected with plasmids expressing the catalytic domain (P110) of PI3-K or constitutively active MEK1 (MEKΔEE) and GFP or GFP-Bcl-2-Maob. Mean SGN survival is expressed as relative to survival in cultures transfected with GFP and maintained in NT-3. Error bars present standard deviation from each repetition. Each condition was performed in duplicate or triplicate and repeated at least three times. Significance of difference among means was determined by one-way ANOVA followed by a post-hoc Tukey analysis. * p<0.05 compared with SGNs transfected with GFP-Maob or GFP-Cb5 (data shown in Fig. 2). ** p<0.05 compared with SGNs cotransfected with GFP-Bcl-2-Maob and P110 and SGNs cotransfected with GFP-Bcl-2-Maob and MEKΔEE.
Next, SGNs were co-transfected with GFP-Bcl-2-Moab and either P110 or MEKΔEE and SGN survival was determined. Overexpression of either P110 (p=0.04) or MEKΔEE (p=0.01) significantly reduced the survival SGNs co-expressing GFP-Bcl-2- Maob (Fig. 9). Thus, activation of PI3-K or MEK induces cell death in SGNs expressing Bcl-2 localized to the mitochondria similar to the effect of neurotrophins.
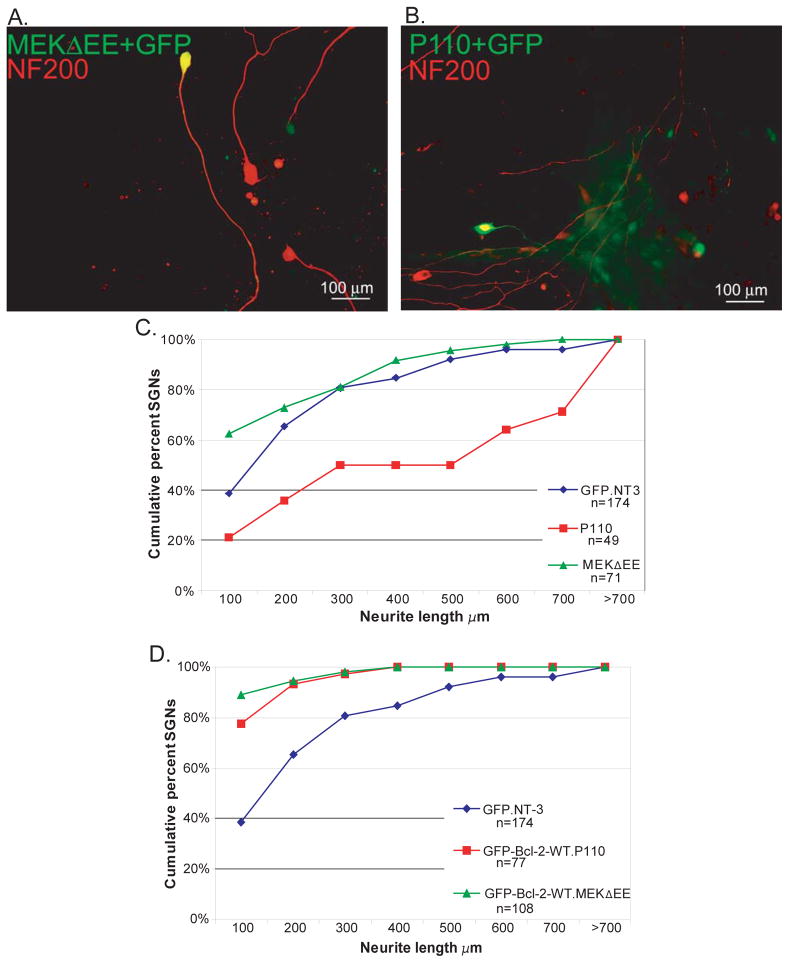
Similarly, P110 and MEKΔEE promote SGN neurite growth with the former being more effective than MEKΔEE or NT-3 (p<0.05) (Fig 10). These data indicate that PI3-K or MEKΔEE is sufficient to promote SGN survival and neurite growth in the absence of other trophic stimuli. Neither however was able to overcome the inhibition of neurite growth by GFP-Bcl-2 (Fig. 10).
Figure 10.
Activation of PI3-K promotes SGN neurite growth but fails to rescue neurites in SGNs expressing Bcl-2. A and B. Representative epifluorescence images of spiral ganglion cultures cotransfected with GFP and MEKΔEE (A) or P110 (B). Cultures were immunolabeled with anti-NF200 antibodies (red). C. Neurite length was determined as above for spiral ganglion cultures cotransfected with empty vector, P110, or MEKΔEE and GFP in the presence (empty vector) or absence (P110 and MEKΔEE) of NT-3. n=total cumulative number of SGNs scored for each condition for all repetitions. Neurite length in cultures cotransfected with P110 and GFP was significantly different from cultures cotransfected with MEKΔEE and GFP or transfected with GFP (p<0.05 by Kruskal-Wallis ANOVA on ranks and post hoc Dunn’s method). D. Neurite length was determined as above for spiral ganglion cultures cotransfected with wild-type GFP-Bcl-2 and P110 or MEKΔEE. Neurite length in cultures cotransfected with wild-type GFP-Bcl-2 and P110 or MEKΔEE was significantly different from cultures transfected with GFP (p<0.05 by Kruskal-Wallis ANOVA on ranks and post hoc Dunn’s method).
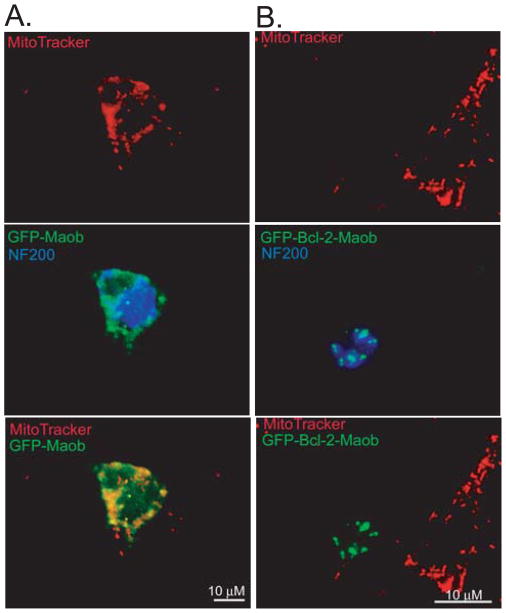
Given the ability of GFP-Bcl-2-Maob to induce apoptosis in SGNs maintained in neurotrophins, we considered the possibility that GFP-Bcl-2-Maob compromised mitochondrial function. To probe mitochondrial fitness, we labeled SGN transfected with GFP-Bcl-2-Maob or GFP-Maob with MitoTrackerR Red CMXRos, a cationic fluorophore that is concentrated in the cytosol of mitochondria with an intact mitochondrial transmembrane potential (ΔΨm) (Duchen et al. 2003; Jayaraman 2005). SGNs expressing GFP-Bcl-2-Maob, but not GFP-Maob or untransfected cells, did not label with MitoTracker Red indicating that GFP-Bcl-2-Maob disrupts the ΔΨm (Fig. 11). A subset of cultures were cultured on glass coverslips and imaged live after MitoTracker Red labeling and yielded similar results as the fixed cultures (data not shown).
Figure 11.
Bcl-2 targeted to the mitochondria disrupts the mitochondrial transmembrane potential. Spiral ganglion cultures were transfected with GFP-Maob or GFP-Bcl-2-Maob and loaded for 15 min with MitoTracker® Red CMXRos 24 h after transgene expression. Cultures were subsequently immunostained with anti-NF200 antibodies (blue) and imaged with laser confocal microscopy. SGNs expressing GFP-Bcl-2-Maob, but not GFP-Maob, fail to label with MitoTracker Red indicating disruption of the mitochondrial transmembrane potential.
DISCUSSION
Many neurons, including SGNs, die in the absence of neurotrophic factors (Bok et al. 2003; Hegarty et al. 1997; Lefebvre et al. 1991; Scarpidis et al. 2003). Here we show that overexpression of wild-type, mitochondrially-targeted, or ER-targeted Bcl-2 promotes SGN survival with ER-targeted Bcl-2 providing the most robust response. Thus, inhibition of the apoptotic pathway is sufficient for SGN cell survival. The survival of SGN transfected with wild-type or ER targeted Bcl-2 exceeds that of SGNs treated with neurotrophins and neurotrophins provide no additional survival advantage for SGNs expressing wild-type or ER-targeted Bcl-2 (Figs 2 and 4) (Hansen et al. 2007), implying that Bcl-2 does not rescue a distinct subpopulation of SGNs compared to those supported by neurotrophins.
Effect of subcellular localization of Bcl-2 on cell survival
Wild-type Bcl-2 localizes to mitochondrial, ER, and nuclear membranes (Akao et al. 1994; Krajewski et al. 1993). Bcl-2 prevents apoptosis, at least in part, by protecting the mitochondria. For example, prosurvival members of the Bcl-2 family prevent apoptosis in response to trophic factor withdrawal by inhibiting Bax-induced opening of the mitochondrial permeabilization pore, cytochrome c release, and subsequent caspase activation (Green and Reed 1998; Gross et al. 1999; Kluck et al. 1997; Polster and Fiskum 2004; Yang et al. 1997; Yuan and Yankner 2000). Bcl-2 localized to the ER inhibits of the disruption of the ΔΨm, cytochrome c release, and Bax activation suggesting that Bcl-2 localized to the ER indirectly protects the mitochondria (Annis et al. 2001; Hacki et al. 2000; Thomenius et al. 2003). The mechanisms by which ER-targeted Bcl-2 protects the mitochondria and prevents apoptosis are still being elucidated although, at least in part, ER-targeted Bcl-2 interacts with inositol 1,4,5, triphosphate (IP3) receptor Ca2+ channels to prevent IP3-mediated Ca2+release and redistribution to the mitochondria (Rong and Distelhorst 2008).
In addition to its well-characterized ability to prevent apoptosis, Bcl-2 can induce apoptosis in certain circumstances (Uhlmann et al. 1998; Wang et al. 2001a); an effect that has been attributed to cleavage by caspase 3 with consequent truncation of the BH4 domain (Cheng et al. 1997; Clem et al. 1998; Kirsch et al. 1999). In HEK293 cells, transient expression of wild-type and mitochondrially targeted Bcl-2 induces apoptosis while stable transfection results in cell survival (Wang et al. 2001a). However, mitochondrially targeted Bcl-2 is not cleaved and mutation of the caspase 3 cleavage site in wild-type Bcl-2 fails to prevent apoptosis indicating that in these cells the pro-apoptotic effect does not depend on Bcl-2 cleavage by caspases (Wang et al. 2001a). Our data demonstrate that, in the absence of trophic support, overexpression of Bcl-2 supports SGN survival regardless of its subcellular targeting.
Interaction of mitochondrially targeted Bcl-2 with neurotrophic signaling
GFP-Bcl-2-Maob and neurotrophins both independently promote SGN survival; however overexpression of GFP-Bcl-2-Maob increases apoptosis of SGNs maintained in neurotrophins. Several observations suggest that the prodeath effect of GFP-Bcl-2-Maob in the presence of neurotrophins results from Trk activation. First, the prodeath response occurs in cultures maintained in NT-3 and BDNF, but not proNGF. SGNs express p75NTR, TrkB and TrkC, but not TrkA. Thus, NT-3 and BDNF, but not proNGF, activate Trk signaling in SGNs.(Despres and Romand 1994; Farinas et al. 2001; Fritzsch et al. 1997; Pirvola et al. 1994; Schecterson and Bothwell 1994; Staecker et al. 1995; Wheeler et al. 1994; Ylikoski et al. 1993) The fact that proNGF fails to increase cell death in SGNs expressing GFP-Bcl-2-Maob excludes the possibility that the prodeath response is mediated by p75NTR, a high-affinity receptor for proneurotrophins capable of promoting cell death in neurons and Schwann cells (Lee et al. 2001; Linggi et al. 2005; Provenzano et al. 2008; Syroid et al. 2000). Second, membrane depolarization, which promotes SGN survival independent of neurotrophin/Trk signaling (Bok et al. 2007; Bok et al. 2003; Hansen et al. 2001b), does not increase death in SGNs expressing GFP-Bcl-2-Maob. Finally, transfection of constitutively active MEK or PI3-K constructs both increase cell death in SGNs co-transfected with GFP-Bcl-2-Moab. Both MEK and PI3-K are required for neurotrophin/Trk-mediated SGN survival (Hansen et al. 2001b) and we show here that both independently are sufficient to promote SGN survival and neurite growth.
SGNs expressing mitochondrially targeted Bcl-2 fail to label with MitoTracker Red indicating loss of ΔΨm. Loss of ΔΨm is felt to be an early event in the apoptotic cascade;(Castedo et al. 1996; Macho et al. 1996) nevertheless, SGNs expressing mitochondrially targeted Bcl-2 survive in the absence of other trophic stimuli and in the presence of elevated extracellular potassium or Bcl-2 targeted to the ER. However, when these neurons are treated with neurotrophins, they undergo apoptosis. These observations suggest that SGNs with compromised ΔΨm are susceptible to death in the presence of neurotrophins, perhaps due to increased bioenergetic demands (Kroemer et al. 2007).
Effect of subcellularly targeted Bcl-2 on neurite growth
We found that overexpression of Bcl-2 strongly inhibits SGN neurite growth regardless of its subcellular localization. These results are consistent with our previous observation that overexpression of wild-type Bcl-2 or Bcl-XL, another prosurvival member of the Bcl-2 family, inhibits SGN neurite growth (Hansen et al. 2007). In contrast to our observations in SGNs, Bcl-2 and Bcl-XL targeted to the ER, but not wild- type Bcl-XL, promote neurite growth in CNS and embryonic sensory neurons and in PC12 cells (Chen et al. 1997; Hilton et al. 1997; Jiao et al. 2005). In the case of PC12 cells, Bcl-2 appears to promote neurite growth by decreasing Ca2+ uptake by the ER, thereby increasing [Ca2+]i which promotes PC12 neuritogenesis.(Jiao et al. 2005) Conversely increases in [Ca2+]i above basal levels activates calpain and inhibits SGN neurite growth, even at [Ca2+]i optimal for cell survival (Hegarty et al. 1997; Roehm et al. 2008).
Implications
Disruption of the apoptotic mechanism represents a prevalent strategy to prevent the neuronal loss in neurodegenerative diseases. In the cochlea, SGN degenerate following hair cell loss in a pattern characterized initially by loss of the peripheral process followed by apoptosis of the neurons (Alam et al. 2007; Dodson and Mohuiddin 2000; Ladrech et al. 2004; Lee et al. 2003; Spoendlin 1971). Cochlear implant prostheses restore auditory perception to the majority of patients with extensive hair cell loss and optimal performance with a cochlear implant requires the long-term survival of deafferented SGNs (Gillespie and Shepherd 2005; Hillman et al. 2003; Roehm and Hansen 2005; Rubinstein 2004). Thus, prevention of SGN apoptosis and regeneration of the peripheral processes following deafness holds critical implications for optimal cochlear implant performance as well as future strategies aimed restoring hearing by regenerating lost hair cells (Izumikawa et al. 2005; Leake et al. 1999; Roehm and Hansen 2005; Shinohara et al. 2002). Overexpression of Bcl-2 rescues neurons, including SGNs, from cell death and targeting of Bcl-2 to specific subcellular compartments may optimize axon regenerative responses (Jiao et al. 2005; Staecker et al. 2007). However, our data suggest that overexpression of Bcl-2, regardless of its subcellular targeting, inhibits SGN neurite regeneration, thus potentially limiting the effectiveness of this strategy as a means of restoring neural function. Further, the observation that mitochondrially targeted Bcl-2 results in SGN apoptosis in the presence of neurotrophins highlights the importance of careful evaluation of strategies that combine multiple prosurvival stimuli to prevent neuronal loss (Leake et al. 2007; Leake et al. 2008; Shepherd et al. 2005).
Acknowledgments
Support: Support for this study was provided by the American Otological Society and NIDCD KO8 DC006211
References
- Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y. Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 1994;54(9):2468–2471. [PubMed] [Google Scholar]
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503(6):832–852. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Aletsee C, Beros A, Mullen L, Palacios S, Pak K, Dazert S, Ryan AF. Ras/MEK but not p38 signaling mediates NT-3-induced neurite extension from spiral ganglion neurons. J Assoc Res Otolaryngol. 2001;2(4):377–387. doi: 10.1007/s10162001000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B, Andrews DW. Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene. 2001;20(16):1939–1952. doi: 10.1038/sj.onc.1204288. [DOI] [PubMed] [Google Scholar]
- Bae WY, Kim LS, Hur DY, Jeong SW, Kim JR. Secondary apoptosis of spiral ganglion cells induced by aminoglycoside: Fas-Fas ligand signaling pathway. Laryngoscope. 2008;118(9):1659–1668. doi: 10.1097/MLG.0b013e31817c1303. [DOI] [PubMed] [Google Scholar]
- Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. Embo J. 2004;23(5):1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi LM, Raz Y. Methods for providing therapeutic agents to treat damaged spiral ganglion neurons. Curr Drug Targets CNS Neurol Disord. 2004;3(3):195–199. doi: 10.2174/1568007043337454. [DOI] [PubMed] [Google Scholar]
- Bok J, Wang Q, Huang J, Green SH. CaMKII and CaMKIV mediate distinct prosurvival signaling pathways in response to depolarization in neurons. Mol Cell Neurosci. 2007;36(1):13–26. doi: 10.1016/j.mcn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Zha XM, Cho YS, Green SH. An extranuclear locus of cAMP-dependent protein kinase action is necessary and sufficient for promotion of spiral ganglion neuronal survival by cAMP. J Neurosci. 2003;23(3):777–787. doi: 10.1523/JNEUROSCI.23-03-00777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Hirsch T, Susin SA, Zamzami N, Marchetti P, Macho A, Kroemer G. Sequential acquisition of mitochondrial and plasma membrane alterations during early lymphocyte apoptosis. J Immunol. 1996;157(2):512–521. [PubMed] [Google Scholar]
- Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385(6615):434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278(5345):1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95(2):554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Despres G, Romand R. Neurotrophins and the development of cochlear innervation. Life Sci. 1994;54(18):1291–1297. doi: 10.1016/0024-3205(94)00506-0. [DOI] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol. 2000;29(7):525–537. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–389. doi: 10.1016/s0076-6879(03)61019-0. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21(16):6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Fariñas I. Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation. Semin Cell Dev Biol. 1997;8(3):277–284. doi: 10.1006/scdb.1997.0144. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragments. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22(9):2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman AM, Ceccatelli S, Orrenius S. Role of mitochondria in neuronal apoptosis. Dev Neurosci. 2000;22(5–6):348–358. doi: 10.1159/000017460. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1311. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hacki J, Egger L, Monney L, Conus S, Rosse T, Fellay I, Borner C. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene. 2000;19(19):2286–2295. doi: 10.1038/sj.onc.1203592. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Bok J, Devaiah AK, Zha XM, Green SH. Ca2+/calmodulin-dependent protein kinases II and IV both promote survival but differ in their effects on axon growth in spiral ganglion neurons. J Neurosci Res. 2003;72(2):169–184. doi: 10.1002/jnr.10551. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Clark JJ, Gantz BJ, Goswami PC. Effects of ErbB2 signaling on the response of vestibular schwannoma cells to gamma-irradiation. Laryngoscope. 2008;118(6):1023–1030. doi: 10.1097/MLG.0b013e318163f920. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Roehm PC, Xu N, Green SH. Overexpression of Bcl-2 or Bcl-xL prevents spiral ganglion neuron death and inhibits neurite growth. Dev Neurobiol. 2007;67(3):316–325. doi: 10.1002/dneu.20346. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001a;161(1–2):87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001b;21(7):2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cyclic AMP, and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17(6):1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman T, Badi AN, Normann RA, Kertesz T, Shelton C. Cochlear nerve stimulation with a 3-dimensional penetrating electrode array. Otol Neurotol. 2003;24(5):764–768. doi: 10.1097/00129492-200309000-00013. [DOI] [PubMed] [Google Scholar]
- Hilton M, Middleton G, Davies AM. Bcl-2 influences axonal growth rate in embryonic sensory neurons. Curr Biol. 1997;7(10):798–800. doi: 10.1016/s0960-9822(06)00339-3. [DOI] [PubMed] [Google Scholar]
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jayaraman S. Flow cytometric determination of mitochondrial membrane potential changes during apoptosis of T lymphocytic and pancreatic beta cell lines: comparison of tetramethylrhodamineethylester (TMRE), chloromethyl-X-rosamine (H2-CMX-Ros) and MitoTracker Red 580 (MTR580) J Immunol Methods. 2005;306(1–2):68–79. doi: 10.1016/j.jim.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Jiao J, Huang X, Feit-Leithman RA, Neve RL, Snider W, Dartt DA, Chen DF. Bcl-2 enhances Ca(2+) signaling to support the intrinsic regenerative capacity of CNS axons. Embo J. 2005;24(5):1068–1078. doi: 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274(30):21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16(8):4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53(19):4701–4714. [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Ladrech S, Guitton M, Saido T, Lenoir M. Calpain activity in the amikacin-damaged rat cochlea. J Comp Neurol. 2004;477(2):149–160. doi: 10.1002/cne.20252. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412(4):543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Vollmer M, Rebscher SJ. Neurotrophic effects of GM1 ganglioside and electrical stimulation on cochlear spiral ganglion neurons in cats deafened as neonates. J Comp Neurol. 2007;501(6):837–853. doi: 10.1002/cne.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hradek GT, Hetherington AM. Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hear Res. 2008;242(1–2):86–99. doi: 10.1016/j.heares.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Bernard O, Harvey AR. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur J Neurosci. 2006;24(12):3323–3332. doi: 10.1111/j.1460-9568.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Dong Y, Yuki K, Naito Y, Lee SH, Ito J. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003;113(6):994–999. doi: 10.1097/00005537-200306000-00015. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Van de Water TR, Weber T, Rogister B, Moonen G. Growth factor interactions in cultures of dissociated adult acoustic ganglia: neuronotrophic effects. Brain Res. 1991;567:306–312. doi: 10.1016/0006-8993(91)90809-a. [DOI] [PubMed] [Google Scholar]
- Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, Carter BD. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280(14):13801–13808. doi: 10.1074/jbc.M410435200. [DOI] [PubMed] [Google Scholar]
- Macho A, Decaudin D, Castedo M, Hirsch T, Susin SA, Zamzami N, Kroemer G. Chloromethyl-X-Rosamine is an aldehyde-fixable potential-sensitive fluorochrome for the detection of early apoptosis. Cytometry. 1996;25(4):333–340. doi: 10.1002/(SICI)1097-0320(19961201)25:4<333::AID-CYTO4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Richardson RT, Wise AK, O'Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54(6 Pt 1):1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hearing Res. 1994;75(1–2):131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90(6):1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Provenzano MJ, Xu N, Ver Meer MR, Clark JJ, Hansen MR. p75NTR and Sortilin Increase After Facial Nerve Injury. Laryngoscope. 2008;118(1):87–93. doi: 10.1097/MLG.0b013e31814b8d9f. [DOI] [PubMed] [Google Scholar]
- Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366(1–2):127–137. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- Ripoll C, Rebillard G. A simple technique to efficiently dissociate primary auditory neurons from 5 day-old rat cochleas. J Neurosci Methods. 1997;73(2):123–128. doi: 10.1016/s0165-0270(96)02217-0. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13(5):294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Xu N, Woodson EA, Green SH, Hansen MR. Membrane depolarization inhibits spiral ganglion neurite growth via activation of multiple types of voltage sensitive calcium channels and calpain. Mol Cell Neurosci. 2008;37(2):376–387. doi: 10.1016/j.mcn.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):444–448. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- Scarpidis U, Madnani D, Shoemaker C, Fletcher CH, Kojima K, Eshraghi AA, Staecker H, Lefebvre P, Malgrange B, Balkany TJ, Van De Water TR. Arrest of apoptosis in auditory neurons: implications for sensorineural preservation in cochlear implantation. Otol Neurotol. 2003;24(3):409–417. doi: 10.1097/00129492-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hearing Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486(2):145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci U S A. 1998;95(4):1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99(3):1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Degeneration behavior of the cochlear nerve. Archiv fur Klinische und Experimentelle Ohren-, Nasen- und Kehlkopfheilkunde. 1971;200:275–291. doi: 10.1007/BF00373310. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Staecker H, Liu W, Hartnick C, Lefebvre P, Malgrange B, Moonen G, Van de Water TR. NT-3 combined with CNTF promotes survival of neurons in modiolus-spiral ganglion explants. Neuroreport. 1995;6(11):1533–1537. doi: 10.1097/00001756-199507310-00017. [DOI] [PubMed] [Google Scholar]
- Staecker H, Liu W, Malgrange B, Lefebvre PP, Van De Water TR. Vector-mediated delivery of bcl-2 prevents degeneration of auditory hair cells and neurons after injury. ORL J Otorhinolaryngol Relat Spec. 2007;69(1):43–50. doi: 10.1159/000096716. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Induction of postnatal Schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J Neurosci. 2000;20(15):5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomenius MJ, Distelhorst CW. Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J Cell Sci. 2003;116(Pt 22):4493–4499. doi: 10.1242/jcs.00829. [DOI] [PubMed] [Google Scholar]
- Thomenius MJ, Wang NS, Reineks EZ, Wang Z, Distelhorst CW. Bcl-2 on the endoplasmic reticulum regulates Bax activity by binding to BH3-only proteins. J Biol Chem. 2003;278(8):6243–6250. doi: 10.1074/jbc.M208878200. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757(9–10):1297–1300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Subramanian T, Vater CA, Lutz R, Chinnadurai G. A potent cell death activity associated with transient high level expression of BCL-2. J Biol Chem. 1998;273(28):17926–17932. doi: 10.1074/jbc.273.28.17926. [DOI] [PubMed] [Google Scholar]
- Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001a;276(47):44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li H, Chi F, Shen Y. Transient Bax-protein immunoreactivity prior to apoptosis of spiral ganglion neurons in the postnatal rat. Acta Otolaryngol. 2001b;121(7):777–780. doi: 10.1080/00016480152602195. [DOI] [PubMed] [Google Scholar]
- Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73(1):46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumäe U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hearing Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zha XM, Bishop JF, Hansen MR, Victoria L, Abbas PJ, Mouradian MM, Green SH. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hear Res. 2001;156(1–2):53–68. doi: 10.1016/s0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]