Abstract
Background
Dysregulation of circadian rhythms can contribute to diseases of lipid metabolism. NAD-dependent deacetylase sirtuin-1(SIRT1) is an important hub which links lipid metabolism with circadian clock by its deacetylation activity depends on intracellular NAD+/NADH content ratio. Hydrogen sulfide (H2S) is an endogenous reductant which can affect the intracellular redox state. Therefore, we hypothesized that exogenous H2S can affect the expression of circadian clock genes mediated by sirt1 thereby affecting body's lipid metabolism. And also because the liver is a typical peripheral circadian clock oscillator that is intimately linked to lipid metabolism. Thus the effect of H2S were observed on 24-hour dynamic expression of 4 central circadian clock genes and sirt1gene in primary cultured hepatocytes.
Results
We established a hepatocyte model that showed a circadian rhythm by serum shock method. And detected that the expression level and the peak of circadian clock genes decreased gradually and H2S could maintain the expression and amplitude of circadian clock genes such as Clock, Per2, Bmal1 and Rev-erbαwithin a certain period time. Accordingly the expression level of sirt1 in H2S group was significantly higher than that in the control group.
Conclusion
Exogenous reductant H2S maintain the circadian rhythm of clock gene in isolated liver cells. We speculated that H2S has changed NAD+/NADH content ratio in hepatocytes and enhanced the activity of SIRT1 protein directly or indirectly, so as to maintain the rhythm of expression of circadian clock genes, they play a role in the prevention and treatment of lipid metabolism-related disease caused by the biological clock disorders.
Keywords: Hydrogen sulfide, sirt1, circadian clock genes, metabolism-related genes, lipid
Background
To adapt the changes of environment, all species on the earth have a life cycle synchronized approximately with the circadian rhythm of the planet. In mammals, the central circadian clock is located in hypothalamic supraoptic nucleus (SCN) [1] with a sensitivity of the outside light signal. Peripheral circadian clock is regulated by the central circadian clock. The negative feedback loop composed of circadian clock genes and their expression products oscillate autonomously. Circadian rhythm of life on earth is realized in this way [2]. Main circadian clock genes includes Clock, Bmal1, Per2, Rev-erbα, Cry1 etc. [3,4] Disorder of circadian rhythms can contribute to disease. Conversely, metabolic signals also feed back into the circadian system, modulating circadian gene expression and behavior [5]. Many peripheral tissues have a rhythmic expression of circadian genes as well as SCN. Especially products of circadian genes in fat, liver, heart, pancreatic β-cell are all directly or indirectly involved in energy metabolism [6,7]. Emerging evidence suggests that there are food-induced oscillators independent of the SCN [8-10]. The feedback loop formed by circadian clock genes and their products regulate the expression of different clock controlled genes, and then regulate the physiological, behavioral activities of the body [11]. Between the central and peripheral organs and between tissues of different peripheral organs, expression of clock controlled genes have significant specificity [12]. The liver is critical organ for lipid metabolism. It is very sensitive to changes in internal environment. Expression of many circadian genes and lipid metabolism-related genes shows a clear rhythm in hepatocytes. That is why we choose primary hepatocytes for our experiment.
Sirtuin-1 (NAD-dependent deacetylase sirtuin-1, SIRT1) is an NAD+-dependent deacetylase encoded by the sirt1gene. It is a key regulator of metabolic homeostasis [13] and can enhance gluconeogenesis and lipolysis, regulate differentiation of adipocyte, promote insulin secretion, and enhance tissue sensitivity to insulin [14]. In addition, SIRT1 exerts vasculoprotective effects by anti-inflammation, anti-apoptosis, blood vessel relaxation, inhibition of foam cell formation and etc [15]. Appealingly, SIRT1 is an integral part of the circadain clock operation. SIRT1 enhances the activation of ROR (RAR-related orphan receptor) on the transcription of mBmal1 by activating PGC-1α (peroxisome proliferator-activated receptor-γ coactivator-1α) [16,17]. CLOCK-BMAL1 heterodimer is the core components of circadian clock. It binds to the upstream E-BOX to regulate the expression of other clock genes. However, the acetylated dimer is not active. SIRT1 regulates the function of CLOCK-BMAL1 heterodimer through deacetylation to mediate energy metabolism and circadian clock [17].
Deacetylation of SIRT1 depends on the NAD+/NADH content ratio in cytoplasm. While H2S is endogenous reductant and affects the intracellular redox state. H2S is one of degradation products of endogenous sulfur amino acid. H2S plays a vasculoprotective role in mammalian atherosclerosis. Firstly, H2S can inhibit the proliferation of vascular smooth muscle and the latter is considered to be an important part of the formation of atherosclerosis [18]. Secondly, metabolism of sulfur amino acids is closely related to lipid metabolism. Some patients with coronary artery disease have no traditional risk factors such as hypertension, hyperlipidemia, diabetes, smoking, etc. Hyperhomocysteinaemia induced by deposition of high homocysteine is now considered one of the independent risk factors of atherosclerosis [19]. Thirdly, H2S can inhibit the oxidative modification of low-density lipoprotein (LDL) in vitro [20]. In addition, of H2S restore vasodilation and inhibit angiogenesis via vasodilation [21,22]. H2S through these pathways involved in lipid metabolism-related diseases.
Then we hypothesized that H2S affect circadian clock genes expression by changing the activity of SIRT1 and thereby affecting the cellular lipid metabolism. So through the serum shock method we tried to establish a model of primary hepatocytes which has rhythmic expression of circadian clock genes and then detected the effects of H2S on the gene expression.
Results
Primary cultured hepatocytes
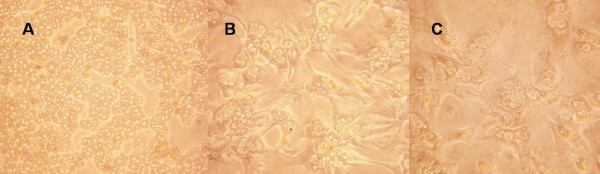
Newly isolated hepatocytes of mice were spherical with two striking nucleus. After cultured for 4 hours the hepatocytes adhered and formed into hepatic cord (Figure 1A). Hepatocytes were polygonal, tightly packed with clear nucleolus. After cultured by medium without serum for 24 hours, hepatocytes extend pseudopodium (Figure 1B). Cell condition has been restored to some extent after serum shock. After cultured by medium without serum for 4 days or after serum shock for 3 days, cell-fusion and fibroblastization could be observed. Cell condition decreased significantly (Figure 1C). Proliferation of hepatocytes was not observed in the culture process.
Figure 1.
Primary hepatocytes before and after serum shock: A After cultured for 4 hours, the hepatocytes adhered, and formed into hepatic cord; B After cultured by medium without serum for 24 hours, hepatocytes extend pseudopodia; C After cultured by medium without serum for 4 days or after serum shock for 3 days, cell-fusion and fibroblastization could be observed.(A × 200;B × 400;C × 400).
Diurnal expression patterns of circadian genes in control group
In the control group, genes of mBmal1, mPer2 and mRev-erbαhad shown a 24-hour cyclical rhythm. Expression of Bmal1gene from the ZT0 point (According Zeitgaber Time, the time adding 50% horse serum was denoted as ZT0) showed a rhythmicity. and the first expression peak occured at ZT8, the second peak occured at ZT32, the last peak occured at ZT56. Peaks decreased gradually and fluctuating rhythm was no longer obvious after ZT64(Figure 2); Expression of Per2 showed a rhythmicity from ZT4 point, and the first expression peak occured at ZT8, the second peak occurred at ZT32, the last peak occured at ZT56. Rhythm can be maintained more than three days(Figure 3); Expression of Rev-erbαgene showed a rhythmicity form ZT8 point and lasted three days. The first expression peak occured at ZT12, and the second peak occured at ZT36, the last peak occured at ZT60. Peaks decreased gradually (Figure 4).
Figure 2.
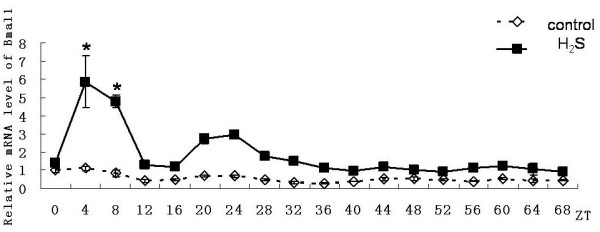
Effect on expression of Bmal1 gene in primary cultured hepatocytes. Horizontal axis shows the period and vertical axis shows the amplitude. The mRNA levels of Bmal1 gene were normalized to GAPDH mRNA. Each value represents the mean ± SD (n = 3). The expression differences were assessed by one-way ANOVA.
Figure 3.
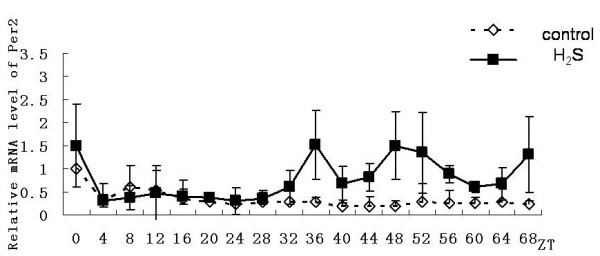
Effect on expression of Per2 gene in primary cultured hepatocytes. Horizontal axis shows the period and vertical axis shows the amplitude. The mRNA levels of Per2 gene were normalized to GAPDH mRNA. Each value represents the mean ± SD (n = 3). The expression differences were assessed by one-way ANOVA.
Figure 4.
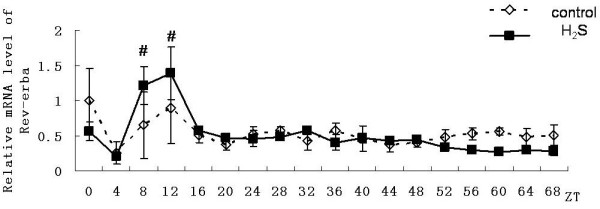
Effect on expression of Rev-erbα gene in primary cultured hepatocytes. Horizontal axis shows the period and vertical axis shows the amplitude. The mRNA levels of Rev-erbα gene were normalized to GAPDH mRNA. Each value represents the mean ± SD (n = 3). The expression differences were assessed by one-way ANOVA.
Diurnal expression patterns of circadian clock genes in H2S group
In the hydrogen sulfide group, same with the control group genes of Bmal1, Per2 and Rev-erbαhad also shown a 24-hour cyclical rhythm. Although still no significant rhythmicity was shown in the expression of Clock gene, the expression level Clock gene was significantly higher than in control group (n = 3, p < 0.05). However it also reduced gradually (Figure 5), the expression level of Per2 gene was significantly higher than that in control group from the ZT36 point (n = 3, p < 0.05). Until to the ZT40 point it showed rhythmicity and the amplitude was significantly higher than control group (Figure 3). The emergence time of rhythm and peak of the Bmal1gene and Rev-erbαgene expression were both synchronized with the control group. And peaks of the genes both reduced gradually. For mBmal1gene, not only the expression level but also the volatility were significantly increased (n = 3,*p < 0.01). However the maintain time was not more than 48 hours (Figure 2). At ZT8 and ZT12 time point the expression level of Rev-erbαgene was significantly higher than that in control group(n = 3,#p < 0.05). However subsequently the amplitude decreased rapidly and the expression level decreased under the control group from the third day (Figure 4).
Figure 5.
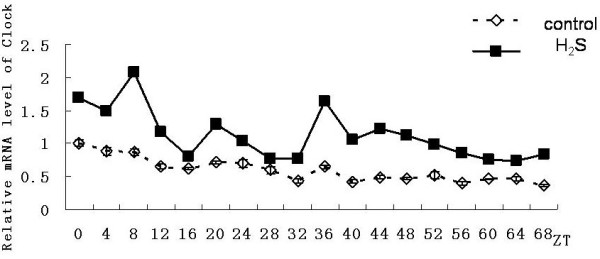
Effect on expression of Clock gene in primary cultured hepatocytes. Horizontal axis shows the period and vertical axis shows the amplitude. The mRNA levels of Clock gene were normalized to GAPDH mRNA. Each value represents the mean ± SD (n = 3). The expression differences were assessed by one-way ANOVA.
Diurnal expression patterns of sirt1gene
In addition to circadian clock genes' expression, we also detected the diurnal expression patterns of sirt1 gene. The results showed that in the control group there was a small peak of expression of sirt1 gene at ZT8 point. And then, gradually, the expression level decreased. No significant circadian rhythm was seen throughout the experiment. While in the H2S group, in the first 32 hours after serum shock, the expression level of sirt1gene was significantly higher than that in the control group (Figure 6).
Figure 6.
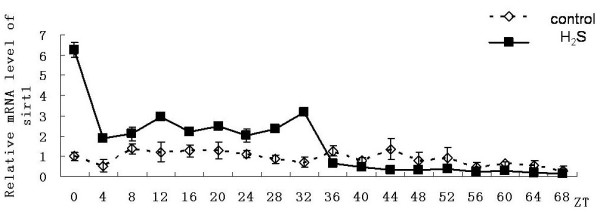
Effect on expression of sirt1 gene in primary cultured hepatocytes. Horizontal axis shows the period and vertical axis shows the amplitude. The mRNA levels of sirt1 gene were normalized to GAPDH mRNA. Each value represents the mean ± SD (n = 3). The expression differences were assessed by one-way ANOVA.
Discussion
Oscillators independent of the SCN exist in peripheral tissues [10]. They regulate various physiological and biochemical activities specifically. Such as mouse embryonic fibroblasts (MEFs) show circadian rhythm after serum shock [23]. We also found mouse myocardial cells cultured in vitro in pre-trial were able to maintain the circadian rhythm at 2 ~ 3d. This indicates that expression of circadian clock genes in peripheral tissue generates independent rhythmic vibration. The liver is a key organ about lipid metabolism. It receive signals of energy and hormone fome internal environment, regulate the β-oxidation of fatty acid and lipoprotein uptake and release [24]. The expression of many genes in liver cells shows a significant circadian rhythm. In our study, H2S in the form of NaHS [25] was prepared prior to use temporarily. Two-thirds of the ions exist in the form of hydrogen sulfide in the NaHS solution, one-third of the sulfur in the form of hydrogen ions. As the H2S is a gas, the stability of H2S in the solution varied depending on the initial H2S concentrations. At the highest concentration tested (1 mM), a drop of the H2S concentration around 15% within 30 min was observed [26]. Of note, at 37°C, the concentration of H2S in the solution is very stable below 1 mM [27,28] and does not affect the PH value of culture medium [29]. So we tested at low dose as much as possible. However, we found that the experimental group showed unstable rhythms at the concentration of 2.5 × 10-4 mol/L, and even showed no rhythm sometimes. Then we increased the dose. However, the concentration of freshly preparad NaHS was far below 1 mM. And determined the final concentration of H2S to use is 5 × 10-4mol/L, and the solution is added every 24 hours after the serum shock.
The expression of Clock gene in liver in vivo were constant, therefore we found that in isolated hepatocytes, expression level decreased gradually, but no noticeable circadian changes were observed in 24-hour period(table 1). In the experimental group Clock gene expression was significantly higher than the control group, Clock expression was gradually reduced over time, the two groups expressed almost the same level after ZT48. Accordingly, we concluded that H2S can maintain the expression levels of Clock gene in isolated hepatocytes within a certain period of time or H2S alleviated the attenuation of Clock gene expression in hepatocytes in vitro.
Table 1.
Diurnal expression patterns of circadian clock genes and sirt1 gene
| Genes | CRc | CRt | RZTc | RZTt | MAc | MAt | MAZTc | MAZTt |
|---|---|---|---|---|---|---|---|---|
| Clock | - | - | - | - | - | - | - | - |
| Bmal1 | + | + | ZT0 | ZT0 | 1 | 5.29↑ | ZT4 | ZT4 |
| Per2 | + | + | ZT4 | ZT44 | 1 | 2.53↑ | ZT8 | ZT48 |
| Rev-erbα | + | + | ZT8 | ZT8 | 1 | 1.56↑ | ZT12 | ZT12 |
| Sirt1 | - | - | - | - | - | - | - | - |
Notation: CR, Circadian rhythm; RZT, Appearance of rhythm(ZT); MA, Max Amplitude(relative value); MAZT, Appearance of Max Amplitude(ZT).
Lower case letters in the first line: c:control; t: test
As can be seen from the Table 1, the Bmal1 gene, Per2 gene and Rev-erbα gene have shown a rhythm in the control group. In the H2S groups, the expression level and amplitude of three genes were significantly increased. The expression of Bmal1 and Rev-erbα showed a circadian rhythm, peak time of which was synchronized with the control group. However the maintenance of H2S on rhythmic expression of Bmal1 gene and Rev-erbα gene did not lasted a longer time than the control group. This showed that H2S improved and maintained their volatility of expression transiently. The impaction of H2S appeared in a short time and the sensitivity decreased rapidly. We speculated that high expression of Rev-erbα gene at ZT8, ZT12-point activated mCry1 which inhibit the expression of Rev-erbα gene. Rhythmic of Per2 gene expression delayed. So we speculated that H2S affect mPer2 gene expression indirectly and promote transcription until more than 36 hours.
In addition, H2S did not impact a long-lasting maintenance on the rhythm of the isolated hepatocytes. That might be resulted from the long time for serum-free culture. H2S has a protective effect on the maintenance of circadian rhythm of isolated hepatocytes. Then what is the role of the relatively high expression of Sirt1 gene in this process? Our results showed that in the first 32 hours after serum shock, the expression level of sirt1 gene in hydrogen sulfide group were significantly higher than that in the control group. There are two possibilities: (1) H2S directly stimulated the expression of sirt1gene by changing the intracellular redox status, thus enhancing the total intracellular biological activity of SIRT1 protein. (2) The change of intracellular ratio of NAD+/NADH speed up the activation and inactivation of SIRT1 protein which resulted in the half-life of SIRT1 protein were shortened, and then led to sirt1 gene highly expressed through feedback ways. Finally, a large number of activated SIRT1 protein played a protective role in the rhythmic maintenance of circadian clock genes' expression in isolated hepatocytes. Of course, the mystery revealed awaits further experimental results.
In summary, we established hepatocytes model with circadian rhythm by serum shock method. And found that H2S could maintain the expression and the vibration amplitude of Clock, mPer2, mBmal1 and mRev-erbαwithin 48 hours genes. However, there are differences in the time point of occurrence and its duration between different genes. In this process, sirt1 gene sustained high expression. Accordingly, we concluded that exogenous reductant H2S has a protective effect on the maintenance of circadian rhythm of circadian clock genes in isolated hepatocytes. The effect is achieved through changing the intracellular ratio of NAD+/NADH and enhancing the activity of SIRT1 protein. This conclusion may find a new focus on the prevention and treatment of lipid metabolism related diseases caused by circadian clock disorders.
Materials and methods
Cell culture
Selected 8-week-old C57BL/6J mice, and then separated hepatocytes by liver perfusion of collagenase IV(Sigma). After centrifugation, were grown in Williams' Medium E supplemented with 10% FBS(GIBCO), ITS(Sigma)and sodium pyruvate(Gibeco). Four hours after inoculation hepatocytes completely adherent. Cultured for 24 hours with serum free Williams' Medium E medium containing ITS and sodium pyruvate. Subsequently cells were treated with Williams' Medium E containing 50% horse serum for two hours (this process is called serum shock). And then continued to culture with serum free Williams' Medium E medium containing ITS and sodium pyruvate until the end of the experiment. At the same time the experimental group was given NaHS. Every four hours after serum shock cells were harvested. There were 18 time points. All animal experiments were performed according to the criteria of the Medical Laboratory Animal administrative Committee of Shanghai.
Total RNA extraction and reverse transcription
Total RNA from hepatocytes were isolated with Trizol Reagent (Invitrogen), according to the manufacturer's instructions. 2 μg of total RNA were reversely transcribed and amplified using the ReverTra Ace qPCR RT Kit (TOYOBO).
Real-time PCR
The real-time PCR was carried out using SYBR Green Real-time PCR Master Mix(Bio-Rad) in a total volume of 20 μl. PCR amplifications were performed in a real-time PCR system (Bio-Rad) in duplicate. The relative quantification of gene expression was analyzed from the measured threshold cycles (CT) by using the 2-ΔΔCt method in the experiment. The data were normalized by determination of the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in each sample. In our experience, the target genes names and database names are as follows: Clock[GenBank:NM_007715], Bmal1[GenBank:NM_007489], Per2[GenBank:NM_011066], Rev-erbα[GenBank:NM_145434], Sirt1[GenBank:NM_019812], GAPDH[GenBank:BC_083149]. Primer sequences of the target genes in the present study were found in Genbank as shown in Table 2.
Table 2.
The primer Sequences Used for PCR Amplification
| Gene | Genebank Accession No. | Annealing temperature | Primer sequence 5' to 3' |
|---|---|---|---|
| Clock | NM_007715 | 58°C | Forward:CTTCCTGGTAACGCGAGAAAG Reverse:TCGAATCTCACTAGCATCTGACT |
| Bmal1 | NM_007489 | 60°C | Forward:CACTGACTACCAAGAAAGTATG Reverse:ATCCATCTGCTGCCCTGAGA |
| Per2 | NM_011066 | 58°C | Forward:CAGACTCATGATGACAGAGG Reverse:GAGATGTACAGGATCTTCCC |
| Rev-erbα | NM_145434 | 60°C | Forward:TACATTGGCTCTAGTGGCTCC Reverse:CAGTAGGTGATGGTGGGAAGTA |
| Sirt1 | NM_019812 | 58°C | Forward:GAACAGGTTGCGGGAATC Reverse:AACATGAAGAGGTGTGGGTG |
| GAPDH | BC_083149 | 60°C | Forward:ACAGCCGCATCTTCTTGTGCAGTA Reverse:GGCCTTGACTGTGCCGTGAATTT |
Statistical analysis
Each value represents the mean ± SD. The values for mRNA levels are presented as relative values in all experiments. The oscillation of each gene expression was evaluated by one-way analysis of variance (ANOVA) by SPSS 16.0 software and the post hoc Student's t-test was used to compare the values between the groups at the same CT point. A probability value < 0.05 was considered statistically significant.
List of abbreviations
SIRT1: NAD-dependent deacetylase sirtuin-1; Clock: circadian l Locomotor output cycles kaput; Per: Period; Bmal1: brain and muscle Arnt-like protein 1; Rev-erbα: NR1D1 (nuclear receptor subfamily 1, group D, member 1) a member of the nuclear receptor family of intracellular transcription factors; SCN: supraoptic nucleus; ROR: RAR-related orphan receptor; PGC-1α: peroxisome proliferator-activated receptor-γcoactivator-1α; LDL: low-density lipoprotein; ZT: Zeitgeber time(Zeitgeber a German word that literally means 'time giver', it refers to external cues that entrain the endogenous clock); MEFs: mouse embryonic fibroblasts; ITS: Insulin-Transferrin-Selenium-X Supplement; CT: threshold cycles; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ZS carried out all aspects of experiments and data analysis, and drafted the manuscript. CL and SC participated in the design of experiments. LH participated in the design of study and proofread manuscript. RQ conceived of the study and performed the experimental instruction. All authors read and approved the final manuscript.
Contributor Information
Zhanxian Shang, Email: shangzhanxian@gmail.com.
Chao Lu, Email: luchao@shmu.edu.cn.
Sifeng Chen, Email: chen1216@fudan.edu.cn.
Luchun Hua, Email: drhua@126.com.
Ruizhe Qian, Email: rzqian@shmu.edu.cn.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81070234 and 81000355).
References
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons form diverse organisms. Nature Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Young MW. Circadian rhythms. Marking time for a kingdom. Science. 2000;288:451–453. doi: 10.1126/science.288.5465.451. [DOI] [PubMed] [Google Scholar]
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controllled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Leid M. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis Marcia C, Guarente1 Leonard P. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS Axis as a Potential Target against Vascular Senescence, Dysfunction and Atherosclerosis. J Atheroscler Thromb. 2010;17:431–435. doi: 10.5551/jat.3525. [DOI] [PubMed] [Google Scholar]
- Liu C, Li SM, Liu TH, Borjigin J, Lin JD. Transcriptional coactivatorPGC-1a integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–4U4. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- Zhou J, Møller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, Falk E. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]
- Laggner H, Muellner MK, Schreier S, Sturm B, Hermann M, Exner M, Gmeiner BM, Kapiotis S. Hydrogen sulphide: a novel physiological inhibitor of LDL atherogenic modification by HOCl. Free Radic Res. 2007;41:741–747. doi: 10.1080/10715760701263265. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2Sasa novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QH, Yang G, Yang W, Jiang B, Wu L, Wang R. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol. 2007;170(4):1406–14. doi: 10.2353/ajpath.2007.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G. The role of the liver inmetabolic homeostasis: implications for inborn errors of metabolism. Inherit Metab Dis. 1991;14:407–420. doi: 10.1007/BF01797914. [DOI] [PubMed] [Google Scholar]
- Abe k, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, Tang C. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels. 2004;19:75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li F, Tong W, Zhang A, He Y, Fu T, Liu B. Hydrogen Sulfide, a Gaseous Transmitter, Stimulates Proliferation of Interstial Cells of Cajal via Phosphorylation of AKT Protein Kinase. Tohoku J Exp Med. 2010;221(2):125–32. doi: 10.1620/tjem.221.125. [DOI] [PubMed] [Google Scholar]