Abstract
Providing safe and effective drug therapy to neonates requires knowledge of the impact of development on the pharmacokinetics and pharmacodynamics of drugs. Although maturational changes are observed throughout childhood, they are most prominent during the first year of life. Several of these processes overlap, making development an extremely dynamic system in the newborn compared with that in infants, children, or adults. Changes in body composition and porportions, liver mass, metabolic activity, and renal function collectively affect the pharmacokinetic behavior of medications. Instead of simply adapting doses by scaling adult or pediatric doses on the basis of a patient's weight and/or body surface area, integrated knowledge of clinical maturation and developmental pharmacology is critical to the safe and effective use of medications in neonates. Unfortunately, the effects of human ontogeny on both pharmacokinetics and pharmacodynamics have not been well established in these early stages of life, and information regarding the influence of developmental changes on the pharmacodynamics of medications is even more limited. Theoretically, age-dependent variations in receptor number and affinity for drugs have significant potential to influence an individual's response to drug therapy. In this review, some of the relevant covariates of pharmacokinetics and pharmacodynamics in neonates are reviewed and illustrated based on the published literature.
INDEX TERMS: developmental pharmacology, neonate, pharmacodynamic, pharmacokinetic
INTRODUCTION
By definition, neonates are children from birth to the age of 28 days of postnatal life, and this definition includes both preterm and term neonates. The combination of neonate-specific diseases (e.g., prematurity, respiratory distress syndrome, necrotizing enterocolitis, patent ductus arteriosus, meconium aspiration syndrome, and congenital malformations) and unique pharmacological therapies and surgical procedures has led to the worldwide creation of neonatal intensive care units (NICU). Pharmacologic therapies have dramatically improved the survival and quality of life of these neonates, despite the fact that up to 90% of the medications administered to neonates are either unauthorized, unapproved, or administered off-label.1–3
Off-label signifies the use of a compound or medication given for an unapproved indication or in a different age group with a different dosage, frequency, or route of administration, or based on extemporaneous formulations with untested bioavailability and stability.2–4 The fact that a neonate will receive a medication off-label has become a necessary and accepted part of daily medical practice.4 For this reason, all members of the healthcare team must be aware that such “daily practice” and off-label use of medications may be associated with potentially important negative consequences.
Not only does important maturation occur in perinatal life, there is potentially up to 1 log value of interindividual difference in weight (0.5 to 5 kg). These two factors result in extensive interindividual variability in both pharmacokinetics and pharmacodynamics (PD) within this population. For all of the above-mentioned reasons, neonates may have a subtherapeutic or supratherapeutic response to what should be an acceptable weight-based dosage. Either they receive ineffective dosages of potentially effective medicines, or they may be harmed by normal weight-adjusted dosages.
In neonates, the history of drug therapy is replete with examples of adverse reactions to drugs including, but not limited to, sulfonamides and ceftriaxone (e.g., kernicterus), chloramphenicol (gray syndrome), benzyl alcohol (gasping syndrome), promethazine (apnea), and propylene glycol (metabolic acidosis).4–8 When these undesired and unanticipated effects are considered, perinatologists, neonatologists, pediatricians, and clinical pediatric pharmacists need to recognize that rational evidence-based drug therapy is urgently needed for neonatal patients.4,9 Clinical pharmacology intends to predict drug-specific effects and adverse effects based on pharmacokinetics and PD.6,10 Pharmacokinetics describes the concentration/time profile, while PD predicts the concentration/effect profile. In this article, some of the relevant variables that regulate these processes in neonates are reviewed and illustrated using data published in the biomedical literature.
METHODS
All literature was identified using PubMed, CAS (Chemical Abstracts Service), and ISI Web of Science searches with the following key words: developmental pharmacology, pharmacokinetic, pharmacodynamic, neonate, fetus, drug, absorption, oral, intramuscular, subcutaneous, intrapulmonary, rectal, cutaneous, route, distribution, metabolism, excretion, body composition, intestine, renal function, hepatic metabolism, cytochrome, enzyme, phase I reactions, phase II reactions, and maturation. All relevant literature was allocated, and available data were retrieved to illustrate the developmental changes in neonates, including body composition, body porportions, hepatic and gastrointestinal metabolic activity, and renal function, which collectively affect the pharmacokinetic characteristics of drugs (i.e., absorption, distribution, metabolism, and excretion), and the effect of human ontogeny on the pharmacokinetics and PD of drugs.
Pharmacokinetics
Pharmacokinetics is a quantitative analysis of how living systems handle xenobiotics, thereby characterizing the time course of drug absorption, distribution, metabolism, and excretion.11 Although there is extensive interindividual variability, most of the processes involved have been described recently.
Absorption
Absorption refers to the translocation of a drug from the site of administration into the blood stream. Drugs administered extravascularly (e.g., by oral, intramuscular, subcutaneous, intrapulmonary, rectal or topical, or cutaneous route) must overcome chemical, physical, mechanical, and biological barriers to cross multiple membranes and reach the systemic circulation where they ultimately distribute to the sites of action (i.e., the effect compartment). Developmental changes in the absorptive surfaces (e.g., gastrointestinal tract, skin, pulmonary tree, muscles) can be determinants of bioavailability. Developmental differences in the physiologic composition and function of these barriers can alter the rate and/or extent of drug absorption.4,9
Enteral route
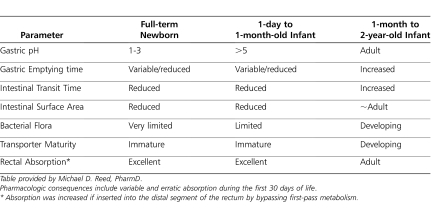
Absorption from the gastrointestinal tract is affected by several factors. Various regional intraluminal pH levels in different segments of the gastrointestinal tract can directly affect both the stability and the degree of ionization of a drug, thus influencing the relative amount available for absorption. At birth, gastric pH usually ranges between 6 and 8 but rapidly falls within 3 to 5 hours to adult values (i.e., pH 1–5).12–14 Both the volume of gastric juice and the acid concentration are age-dependent factors (Table 1). Gastric acid secretions generally approach adult values by 2 years of age.4,14 Extrauterine factors (e.g., nutrition) are most likely responsible for initiating acid production, as basal acid secretion correlates with postnatal but not postconceptional age. Other external factors, like frequent milk intake, will also affect stomach acidity in the newborn. If the pH of the stomach is high, drugs that are weak acids are absorbed more slowly than drugs that are weak bases.9,15,16
Table 1.
Comparative Intestinal Variables Affecting Gastrointestinal Drug Absorption
Because most orally administered drugs are absorbed in the small intestine, the rate of gastric emptying is an important determinant of the rate and extent of drug absorption. During the neonatal period, gastric emptying rate is variable and characterized by irregular and unpredictable peristaltic activity.17,18 At birth, the coordination of antral contractions improves, resulting in a marked increase in gastric emptying during the first week of life.13,14 The rate of gastric emptying appears to be directly affected by gestational and postnatal age as well as the type of feeding (Table 1).12,17,18 Gastric emptying is also affected by the composition of the meal and is faster in neonates after they are fed an extensively hydrolyzed formula than an intact protein or partial hydrolyzed formula.19 In contrast, slower gastric emptying times have been reported with increasing caloric density and medium-chain triglycerides in premature infants.17 Gastric emptying time appears to approach adult values within the first 6 to 8 months of life. Intestinal motor activity matures throughout early infancy with increases in the frequency, amplitude, and duration of propagating contractions and is also influenced by food.17,20,21
In general, the rate at which most drugs are absorbed is slower in neonates and young infants than in older children.22 Additionally, it is thought that intestinal surface area is reduced in early life; villous formation begins at 8 weeks of gestation and matures by week 20 (Table 1).23 This leads to reductions in the surface areas of the small intestine, which reduces absorption. Furthermore, age-associated changes in splanchnic blood flow during the first 2 to 3 weeks of life may influence absorption rates by altering the concentration gradient across the intestinal mucosa.9,24–26
Absorption of some medications requires pancreatic exocrine and biliary function. At birth, pancreatic and biliary functions are immature in premature and in full-term neonates.27 The rates of synthesis, pool size, and intestinal transport of bile acids are reduced in neonates. Lipase activity is present by 34 to 36 weeks of gestation and increases 5-fold during the first week and 20-fold during the first 9 months of postnatal life.28 Amylase activity has been detected as early as 23 weeks of gestation but remains very low even after birth (approximately 10% of adults values). Trypsin secretion in response to pancreozymin and secretin administration is blunted in term neonates but develops during the first year of life.12 The net result of bile salt and pancreatic enzyme deficiency is a reduction in the bioavailability of drugs that requires solubulization or intraluminal hydrolysis.10
Developmental differences in the activity of intestinal drug-metabolizing enzymes and efflux transporters can markedly alter the bioavailability of drugs.29 The efflux transporter P-glycoprotein (P-gp), also known as multidrug resistance protein-1 (MDR1), is a member of the adenosine-5′-triphosphate binding cassette family of proteins and is responsible for cellular drug efflux, translocating substances from the intracellular to the extracellular compartment.30,31 P-gp is normally found within the cellular membranes of the gastrointestinal tract, apical membranes of hepatocytes, and renal proximal tubular cells and on the luminal side of the capillary endothelial cells that make up the blood-brain barrier.30–32 The variability in intestinal absorption characteristics for many drugs is likely a direct result of the variability of P-gp expression within the intestinal tract as well as the presence or abscence of P-gp modulators (Table 1).30,31 Unfortunately, the ontogeny of P-gp in any human organ is not described, and thus, any influence P-gp ontogeny has on drug absorption and distribution throughout childhood remains to be elucidated.
Colonization of the gastrointestinal tract by bacteria, a process that influences the metabolism of bile salts and drugs as well as intestinal motility, varies with age (Table 1), route of delivery, type of feeding, and concurrent drug therapy.33–35 All full-term, formula-fed, vaginally delivered newborns are colonized with anaerobic bacteria by 4 to 6 days of postnatal life. By 5 to 12 months of age, an adult pattern of microbial reduction products is established. Despite the descriptions of these maturational changes, there are limited data for drug metabolic activity of the gut flora in neonates.34,35
Besides maturational issues, the presence of a given disease will most likely have significant impact on oral drug absorption. For example, patients with short bowel syndrome will have a lower intestinal resorption surface.36 Similarly, protein caloric malnutrition37 will affect the total surface area available for absorption through villous atrophy and will also result in delayed gastric emptying and increased intestinal transit time.13 Associated intestinal, congenital, or acquired diseases affecting the esophagus (e.g., atresia and esophagitis), duodenum (e.g., atresia), biliary tract, intestines (e.g., atresia, necrotizing enterocolitis), or colon (e.g., atresia, Hirsprung disease) will possibly affect intestinal functions. Furthermore, multiple systemic diseases such as hypo- or hyperthyroidism may affect absorption (by prolonging or reducing intestinal transit time). Congestive heart failure, congenital heart disease (e.g., coarctation of the aorta), and patent ductus arteriosus (by shunting blood flow from the systemic to the lung circulation, called “steal phenomenon”) may also affect gastrointestial drug absorption.
Percutaneous route
Percutaneous absorption of a drug is directly related to the degree of skin hydration and relative absorptive surface area and inversely related to the thickness of the stratum corneum.38 Full-term neonates possess an intact skin barrier function39 that is similar to that of an older child or adult. However, the ratio of surface area to body weight is much higher in the full-term neonate than in an adult. Thus, the newborn will be exposed to a relatively greater amount (approximately 2.7 times) of drug topically than an older infant or adult.38 If the integrity of the skin is compromised (e.g., denuded, burned, inflamed), percutaneous translocation of compounds into the blood will be enhanced. Classic examples are systemic adverse effects following local desinfection with iodide-containing compounds or local corticoid application.39
Intramuscular route
Both physicochemical (lipophilicity, a degree of water solubility at physiologic pH) and physiologic factors (blood flow to the injected site) affect the rate of drug absorption from the intramuscular injected site. Absorption from the site is also influenced by the total surface area of muscle coming into contact with the injected solution. The ratio of muscle mass to body mass is lower in neonates than in adults. Reduced skeletal muscle blood flow and inefficient muscular contractions, which are responsible for drug dispersion, may reduce the rate of intramuscular absorption of drugs in neonates.40 Sick, immobile neonates or those receiving a paralyzing agent may have slower absorption rates after intramuscular administration. However, the influence of these factors on bioavailability may be offset by the relatively higher density of skeletal muscle capillaries in infants than that in older children.41 Accordingly, evidence supports the concept that intramuscular absorption of specific agents (e.g., amikacin and cephalothin) is relatively more efficient in neonates and infants than in older children.42,43
Intrapulmonary route
Intrapulmonary administration via drug inhalation is increasingly being used to achieve a predominantly local effect.44 As with other organ systems, development of the lung influences this effectiveness of drugs delivered by this route. From 16–26 weeks gestation, the lung periphery and air-blood barrier is formed to allow for gas exchange, and from 26–36 weeks gestation, expansion of air spaces occurs, and surfactant is detected. Between 36 weeks gestation and 2 years of age, the alveoli form from terminal endings of the alveolar sacculi. Over time, the alveoli increase in diameter. Following premature birth, the final stages of normal lung development are interrupted, causing the neonate to have reduced capacity for gas exchange due to decreased lung volumes and capillary surface area. These developmental changes in the architecture of the lung and its ventilatory capacity (e.g., minute ventilation, vital capacity, and respiratory rate) most likely alter the patterns of drug disposition and subsequent systemic absorption after intrapulmonary administration.45
Rectal route
The rectal route serves as an important alternative site for systemic drug administration when nausea, vomiting, seizures, and/or preparation for surgery preclude the use of oral dosage formulations.46 Both the physicochemical properties of a medication and host factors influence the rectal absorption of drugs (Table 1). Important factors such as the specific fomulation used (e.g., solid suppository, or liquid), retention time within the rectal vault, and venous drainage system of the upper and lower parts of the rectum affect drug absorption.47 In general, absorption from aqueous or alcoholic solutions is more rapid than from suppositories. Liphophilic and un-ionized drugs (e.g., barbiturates and benzodiazepines) are readily absorbed and attain systemic drug concentrations sooner.48
Distribution
After gaining access to the systemic circulation, drugs and other compounds distribute into various body compartments, tissues, and cells. The distribution of most medications in the body can be influenced by a variety of age-dependent factors, including extent of protein binding (Table 2), body compartment sizes and composition (Table 3), hemodynamic factors (e.g., cardiac output and regional blood flow), membrane permeability, and physiochemical properties (fat or water solubility) of drugs.4,9,49
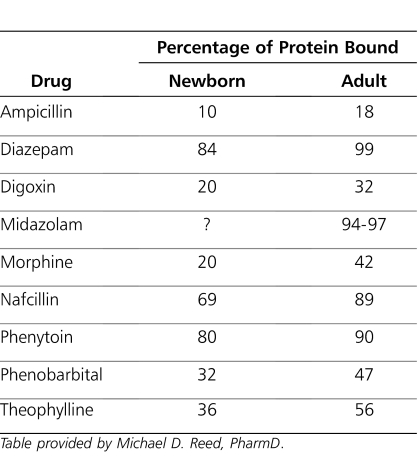
Table 2.
Comparative Percentages of Protein Binding of Selected Representative Drugs
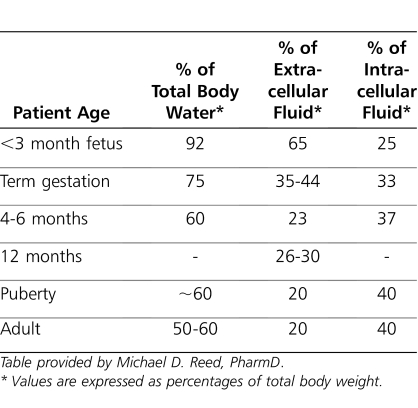
Table 3.
Developmental Aspects of Fluid Compartment Sizes
Plasma protein binding may affect clearance, volume of distribution, and half-life of a medication (Tables 2, 4, and 5). Drug-protein binding is influenced by physicochemical properties of drug, concentration of drug, and concentrations of protein present in the body. Other factors that affect protein binding are the affinity between the drug and protein, the number of available binding sites, and diseases (e.g., hepatic and renal impairment), and endogenous compounds may alter the drug-protein binding interaction.50,51 Changes in the composition and amount of circulating plasma proteins, such as albumin and α1-acid glycoprotein, can alter the distribution of drugs (Table 2). Additionally, albumin concentration in the blood is directly proportional to gestational age, reflecting both placental transport and fetal synthesis. Affinity of albumin for acidic drugs increases from birth to early infancy. At birth, α1-acid glycoprotein concentration, by which basic drugs are bound, is half that of the adult concentration.52,53 A reduction in the quantity of total plasma proteins (including albumin) in the neonate and young infant increases the free fraction of drug, thereby increasing the availability of the active compound.9 Increases in the free fraction of a drug may also increase drug distribution in the tissues and can produce adverse effects. Besides plasma protein binding, drugs can be bound reversibly by erythrocytes. Drugs that bind to erythrocytes may exhibit concentration-dependent uptake from plasma. Finally, tissue binding also plays an important role in drug distribution, but its pharmacological effect is unknown. Tissue binding has no influence on terminal drug elimination clearance but may affect the half-life of drugs.53
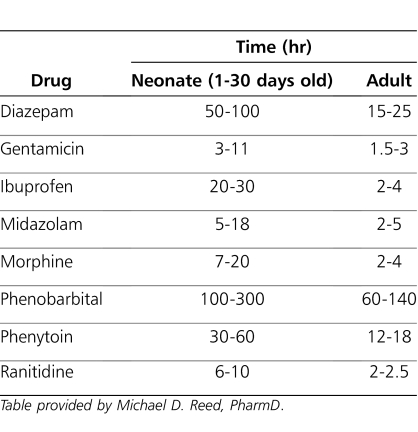
Table 4.
Comparison of Elimination Half-Life Times for Selected Drugs Administered to Neonates
Table 5.
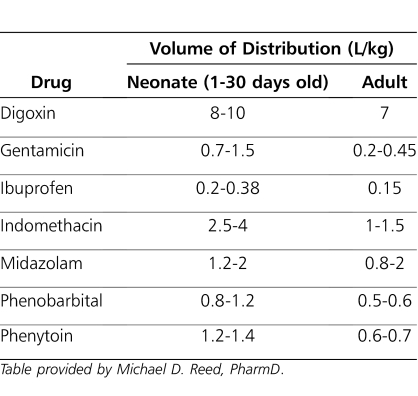
Comparative Distribution Volumes for Selected Drugs Commonly Administered to Neonates
There are a number of endogenous molecules (e.g., bilirubin and free fatty acids) that like drugs, may bind to plasma proteins and are capable of displacing drugs from these protein binding sites through competitive binding. Free fatty acids and unconjugated bilirubin are at higher concentrations in neonates. These substances can compete with medications that are protein bound for high-affinity binding sites on albumin. Displacement of the medication can cause an increase in free drug concentration, which may produce undesirable effects. Although the bilirubin binding affinity of albumin at birth is independent of gestational age, it is less in the newborn than in the adult. The binding affinity of albumin for bilirubin increases with age and reaches adult levels by approximately 5 months of age. A number of drugs (e.g., ampicillin, diazepam, phenytoin) are known to compete with and displace bilirubin from binding sites on the albumin protein, thus increasing a neonate's risk for developing kernicterus.54 Conversely, hyperbilirubinemia can reduce the protein binding of ampicillin, penicillin, and phenobarbital, increasing their potential for toxicity and necessitating dose modifications. Sulfonamides and medications that are highly bound to plasma protein (e.g., ceftriaxone) are contraindicated in neonates because they can displace bilirubin, which may cause kernicterus.55–59
There are also age-dependent changes in body composition (Table 3). As a consequence, physiologic space for drug distribution will display changes throughout life but are most prominent in early neonatal life. The ratio of total body water to body weight is greater in the newborn than in older children and adults (Table 3). Total body water decreases from 80% of body weight at birth to 60% after the first few months of life.4,9 It gradually decreases with age and reaches adult values by 12 years of age.
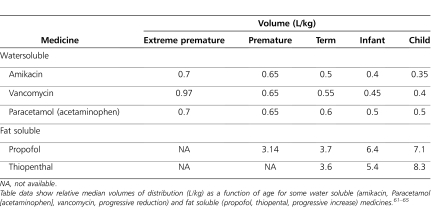
In the extreme preterm neonate, total body fat content can be as low as 1% of total body weight. In term neonates, 4-month-old infants, and adults, body fat is about 15%, 25%, and 20% of total body weight, respectively.58 The volumes of extracellular and intracellular water are also greater in neonates, infants, and children than in adults. Thus, hydrophilic drugs will have larger volumes of distribution in newborns and infants on a per kilogram of body weight basis than adults. Similarly, infants have a higher proportion of body fat than adults, which may cause them to have a larger volume of distribution for lipophilic drugs. However, the influence of age on the apparent volume of distribution is not as readily apparent for lipophilic drugs that are primarily distributed into tissue.4,9,60 Aminoglycoside antibiotics are highly water soluble; hence, the volume of distribution (L/kg) is larger in neonates than in children. Without the administration of larger doses per weight, peak serum concentrations may be subtherapeutic; hence, this is clinically important when the peak serum concentration is pharmacodynamically relevant (Table 6). Conversely, a highly lipophylic drug (e.g., propofol) will have a smaller volume of distribution in neonates, potentially resulting in higher drug concentrations in the affected compartment because of more limited distribution (Table 6).61–65 This may result in supratherapeutic serum concentrations and adverse effects.
Table 6.
Volume of Distribution as a Function of Age for Some Water and Fat Soluble Medicines
Other factors that influence drug binding and distribution include variability in regional blood flow, organ perfusion, permeability of cell membranes, changes in acid-base balance, and cardiac output. The distribution of a drug throughout the body is mostly the phenotypic final result of passive diffusion, associated binding of the drug to tissue components, and the expression of transporters (e.g., P-gp) in the different tissues.66 The expression and localization of P-gp in specific tissues facilitate its ability to limit cellular uptake of xenobiotic substrates to these sites (e.g., the blood-brain barrier, hepatocytes, renal tubular cells, and enterocytes).66,67 Data for the ontogeny of the expression of P-gp in humans is limited. A study of the expression of P-gp in the central nervous system of tissue obtained post mortem from neonates born at 22 to 42 weeks of gestational age suggested a pattern of localization similar to that in adults.68 However, the level of expression of P-gp appeared to be lower than that in adults. The authors concluded that plasma membrane efflux pumps are expressed in a developmental, cell-specific fashion in the human central nervous system. The complementary pattern of plasma membrane efflux pumps at the blood-brain and blood-cerebral spinal fluid barriers may limit central nervous system uptake and retention of drugs and toxins in neonates.68 Additionally, the blood-brain barrier is immature in newborns and more permeable to drugs, and myelinization continues after birth, suggesting that the passive diffusion of drugs into the central nervous system is age dependent.53 This may lead to higher drug concentrations in the central nervous system of very young children, as reflected by the progressive increase in the brain phenobarbital-to-plasma phenobarbital ratio from 28 to 39 weeks of gestational age, demonstrating the increased transport of phenobarbital into the brain.69
Metabolism
The body usually converts drugs to metabolites that are more water soluble and subsequently are more easily excreted, mainly by the renal or hepatobiliary route. Alternatively, metabolism might convert compounds into either therapeutically active or toxic metabolites.70 Drug metabolism can occur in the gastrointestinal tract, kidneys, blood cells, and lungs, but the major site of drug metabolism is the liver.9,71
Developmental differences in the activity of intestinal drug-metabolizing enzymes that markedly alter the bioavailability of drugs are incompletely characterized.29 Duodenal biopsy specimens have shown that cytochrome P-450 (CYP) 3A4 is absent in the fetal duodenum, and CYP3A4 activity increases with age. In fact, there is almost a threefold increase in CYP3A4 activity between neonates and children at >12 years of age.72 Moreover, examination of duodenal and jejunal biopsy specimens from infants and children suggests that epoxide hydrolase and glutathione peroxidase activities demonstrate little age dependence, whereas the intestinal activity of CYP1A1 appears to increase with age.73 The activity of β-glucuronidase in the small intestine of infants has been reported to be much higher (as much as sevenfold) than in children.74 On the other hand, biopsy results of the distal duodenum suggest that glutathione S-transferase activity decreases from infancy through early adolescence, as reflected by reduced apparent oral clearance of busulfan, a substrate for this enzyme.75 These findings are clinically relevant because, depending on the routes of metabolism, the oral bioavailability of these enzymes' substrates may be different in neonates than in older children and adults. Using dextromethorphan as a probe to assess drug metabolism, Kearns and co-workers9 showed that intestinal drug metabolism progressively increases in the first year of life, with onset of CYP3A4 activity that is slower than that of CYP2D6.
Hepatic drug metabolism can be divided into phase I and phase II reactions. Phase I reactions (i.e., oxidation, reduction, and hydrolysis) are mediated mainly by CYP enzymes, while phase II reactions involve conjugation pathways (e.g., acetylation, glucuronidation, sulfation, and methylation).71,76,77 In general, the rate of drug elimination by biotransformation in neonates and infants is much slower than in adults. During the first week of life, there are rapid physiological postnatal changes in the liver blood flow, including increasing portal vein blood flow, and gradual closure of the ductus venosus shunt. In addition, the loss of the umbilical blood supply causes changes in hepatic oxygenation. These relevant changes may affect the capacity not only of hepatic drug metabolism but oral bioavailability in the neonate.78
Phase I reactions
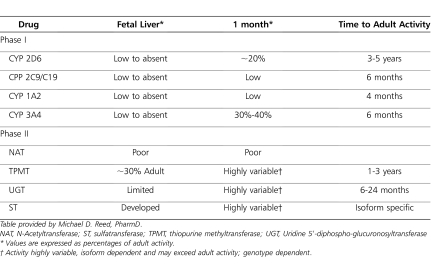
At birth, total hepatic cytochrome P450 concentration is approximately 30% that of adult values.77 All isoenzymes display specific age-dependent maturation; therefore, any generalization based on overall cytochrome activity is inappropriate and inaccurate (Table 7). CYP3A7 is the major isoform in the human embryonic, fetal, and newborn liver. In the period from late fetal to early neonatal life, there is a peak in CYP3A7 activity. Within 1–4 weeks of birth, there is a transition in isozymes in which CYP3A7 virtually disappears and CYP3A4 predominates.70–72 CYP3A4 expression and activity then reaches 30% to 50% of adult levels from 3 to 12 months of age.78 CYP2D6 represents only 2% of the total hepatic CYP content in adults.79 Studies of the catalytic activity of CYP2D6 in human fetal liver microsomes have shown negligible or minimal activity. CYP2D6 activity (dextromethorphan O-demethylation) has been noted in neonatal liver microsomes. In the first postnatal month, activity of CYP2D6 gradually increases.80 Tramadol disposition can also be used as a probe drug to simultaneously assess CYP2D6 and CYP3A4 ontogeny in the first months of life.81
Table 7.
Ontogenic Patterns of Pharmacologically Important Phase I/II Drug Metabolizing Enzymes
The maturation of CYP1A2 is delayed compared to other CYP isoforms. Use of imipramine demethylation as a marker reaction of CYP1A2 activity showed there was minimal production of desmethylimipramine by fetal liver. The rate of demethylation began to rise in neonatal tissue at approximately 8 to 28 days following birth and reached adult levels by 1 year of age.82,83 Similarly, the use of caffeine N-3-demethylation in vitro as a marker for CYP1A2 showed a rise in CYP1A2 activity during this period, reaching a plateau at approximately 120 days.84 Expression of CYP2C protein and its activity in fetal liver appears to be negligible. CYP2C mRNA and protein expression are significantly elevated as early as the first postnatal day. The protein expression increases during the first week of life, CYP2C activity (tolbutamide hydroxylation) surges after the first week and remains at about 40% of the adult level during the first year.77 The ontogeny of CYP2E1 follows a pattern similar to that of CYP2C and CYP2D6, with very low levels in the human fetus followed by a rapid postnatal development that may be controlled by the degree of methylation at the 5′ end of the CYP2E1 gene.77,85,86 Besides CYP isoenzyme-specific ontogeny, other phase I enzymes like esterases might also display ontogeny. We are unaware of any in vitro assessment of esterase ontogeny, but propacetamol (acetaminophen) might be used as an in vivo test probe to assess age-dependent esterase activity as it is a prodrug of paracetamol and is hydrolyzed by esterases after intravenous administration.87,88
Phase II reactions
Phase II reactions are synthetic reactions that include glucuronidation, sulfation, methylation, or acetylation.86,89 Less information is available for the impact of ontogeny on phase II enzymes than phase I enzymes, but it appears that the activities of phase II enzymes are also age-dependent (Table 7). Limited activity of uridine 5′-diphospho-glucuronosyltransferase (UGT) to a variety of compounds, including bilirubin, steroid hormones, and planar phenols, has been demonstrated in catalytic studies using human fetal and neonatal liver microsomes.4,78,86,90 Activity in fetal and 10-day-old neonate liver tissue is generally less than 10% to 30% of that found in adult hepatic tissue. Studies of human neonatal glucuronidation from post mortem liver samples demonstrated that hepatic glucuronidation is immature in the neonates.78,90 Immature bilirubin UGT manifests clinically in almost all neonates in some degree of unconjugated hyperbilirubinemia. The inability of neonates to conjugate chloramphenicol results in the “gray baby” syndrome, which leads to the death of hundreds of newborns. Neonatal glucuronidation of acetaminophen (a substrate for UGT1A6 and UGT1A9) and morphine (a UGT2B7 substrate) is decreased in newborns and young children compared with that in adolescents and adults. The activity reaches adult values between 2 and 6 months for morphine.9,91 Sulfation requires the transfer of a sulfate group from 3′-phosphoadenosine 5′-phosphosulfate to a substrate that is catalyzed by a family of sulfotransferase enzymes. Catalytic studies with human fetal liver cytosolic fractions have demonstrated that there is significant sulfotransferase activity toward numerous substances present from mid-gestation.78,86,89 Paracetamol is either sulfated or glucuronidated and thus provides us with a drug substrate to simultaneously assess ontogeny of sulfation and glucuronidation in neonates and young infants.92
Elimination
Renal excretion is a major route of elimination for many drugs. Drugs that are nonvolatile, water soluble, and have low molecular weight are generally eliminated by renal excretion. Renal clearance is defined as the volume of plasma that is cleared of drug per unit of time through the kidneys. It is the sum of three processes: glomerular filtration, tubular secretion, and active or passive tubular reabsorption.93 When predicting renal clearance of a drug, one must consider that glomerular filtration, secretion, and reabsorption processes are simultaneously taking place and are maturing in their specific patterns.53 Maturation of renal function begins during fetal organogenesis and is completed by early childhood. Nephrogenesis begins at 9 weeks of gestation and is completed by 34 weeks of gestation. During pregnancy however, fetal growth retardation, the administration of nephrotoxic drug or substance to the mother, or renal/urologic fetal malformations may negatively influence nephrogenesis, which can be potentially reflected in renal drug clearance. Additionally, the renal function in preterm infants is physiologically still reduced due to ongoing nephrogenesis.71,94–96
At birth, kidneys are anatomically and functionally immature, and as a result, the renal function in the newborn is limited. The glomerular filtration rate (GFR) is approximately 2 to 4 mL per minute per 1.73 m2 in term neonates, but it may be as low as 0.6 to 0.8 mL per minute per 1.73 m2 in preterm neonates. The GFR increases rapidly during the first 2 weeks of life because of a postnatal drop in renal vascular resistance and an increase in renal blood flow. Afterward, it rises steadily until adult values are reached at 8 to 12 months of age.95 Other factors that influence the GFR are vasoactive systems (e.g., renin-angiotensin system), plasma protein concentration, arteriolar resistance, and the increase in surface area of the glomerular membrane.95,97
Tubular secretion is an active transport process that is independent of plasma protein binding. However, it does depend on blood flow, the affinity of drug carrier proteins in the proximal tubule, the rate of transport across the tubular membrane, and the rate of delivery of the drug to the site of secretion.88 Tubular secretion is immature at birth and approaches adult values by 7–12 months of age. Maturation for premature infants may be different from that for term infants because of limited tubular function in preterm infants.49,97 In the neonate, tubular secretion can be important for the renal elimination of some drugs (e.g., penicillins, cephalosporins, and digoxin).
Tubular reabsorption is potentially a passive process.97 It depends on physiochemical characteristics (i.e., lipid versus water solublility and acidic versus basic drugs) and the pH of fluids in the proximal and distal tubules. Tubular reabsorption remains relatively immature at birth, especially in preterm infants. It has been demonstrated that the development and maturation of the glomerular permeability functions and renal tubular reabsorption are gradual and continuous processes from birth to adolescence with a peak maturation between 1 and 3 years.9,98
Pharmacodynamıcs
PD refers to the relationship between drug concentration at the effect site (receptor) and pharmacological response and thereby involves receptor binding, postreceptor effects, and chemical interactions. PD, along with pharmacokinetics, helps to explain the relationship between a drug dose and a response (the drug effects).99,100 In general, the pharmacological response depends on the drug binding to its target (e.g., a specific receptor). A drug receptor is a biological component that specifically interacts with a drug molecule initiating a pharmacological response. The concentration of the drug at the receptor site influences the drug's therapeutic or toxic responses, and thereby, pharmacokinetics play a key role in the definition of proper treatment in the newborn (e.g., midazolam interaction with the GABA receptor, and morphine interaction with the mu-opioid receptor). However, it is very clear that the simple knowledge of the pharmacokinetic profile of a given drug alone cannot be used to define optimal dosing regimens. Pharmacokinetic knowledge about a drug(s) combined with a thorough understanding of its pharmacodynamic characteristics is critical to developing an optimal approach to dosing any drug.10,100,101
In contrast to the information available for developmental aspects of pharmacokinetics, the effect of human ontogeny on the PD of drugs in neonates has not been well established. Theoretically, age-dependent variations in receptor number and receptor affinity for drugs could influence the response of drugs, but little information exists about the effect of human ontogeny on interactions between drugs and receptors and the consequence of these interactions. Therefore, any assessment of PD in children must take into consideration the efficacy or safety of a given drug with respect to age-dependent differences. In order to highlight both the feasibility and the difficulties of performing pharmacodynamic studies in neonates, the following examples (e.g., dopamine as inotropic, ibuprofen to induce closure of patent ductus arteriosus, aminoglycosides/vancomycin to treat infections, opioids or benzodiazepines for sedation/analgesia) illustrate the complexity of the drug/effect relationship in early life.
Dopamine is a catecholamine with wide ranging effects peripherally as well as in the central nervous system. It is commonly used to support blood pressure and perfusion in the very ill neonate by interaction with dopamine receptors. Seri et al102 studied the effects of dopamine on renal, mesenteric, and cerebral blood flow in 23 nonhypotensive preterm neonates (birth weight, 981 ± 314 grams; postnatal age, <2 days) with color Doppler ultrasonography. Dopamine was given at a dose of 6.1 ± 3 mcg/kg/min to manage oliguria or impaired peripheral perfusion or both. Dopamine significantly increased blood pressure and urine output (2.98 ± 1.18 versus 1.68 ± 0.45; p < 0.05). Dopamine decreased the pulsatility index in the renal artery without affecting the pulsatility index in the superior mesenteric and medial cerebral arteries. Thus, dopamine increased the renal blood while mesenteric and cerebral blood remained unchanged. That study indicates a functionally mature renal but not mesenteric and vasodilatory dopaminergic response in the preterm neonate. There is also a lack of effect of small (2 mcg/kg/min) to medium (8 mcg/kg/min) large dose dopamine on cerebral hemodynamics in the nonhypotensive preterm neonate. Those authors recommended that small doses of dopamine should not be used to augment mesenteric blood flow in nonhypotensive preterm neonates with necrotizing enterocolitis. The differences in both the pharmacokinetics (i.e., concentration/time) and PD (i.e., concentration/effect) of dopamine are apparant because dopamine receptors display age-dependent distribution, and receptor expression displays regional differences. Moreover, it still can be debated to what extent blood pressure and/or urine output are indeed robust and relevant PD outcome variables in this population.
For antibiotics, the pharmacodynamic targets in adults and neonates are likely similar because the concentration/effect relates to the pathogens involved. When beta-lactams are used, the free-drug concentration should be maintained above the minimum inhibitory concentration (MIC) level for the entire dosing interval. If possible, the maximum peak serum concentration (Cmax) of aminoglycosides-to-MIC ratio should be ≥8. The pharmacokinetic/PD target area under the serum concentration curve (AUC) for vancomycin-to-MIC ratio of more than 400 is advocated for clinical effectiveness in adults. Unfortunately, current vancomycin dosing in neonates will be achieved only if MIC is <2 mg/L. The difficulties relate mainly to the feasibility of the adaptations suggested.103 Larger aminoglycoside doses with longer intervals between consecutive administrations combined with still limited predictability in renal clearance may result in toxicity due to either unanticipated impaired clearance (e.g., unknown renal dysfunction, perinatal asphyxia) or specific patients with higher sensitivity who develop toxicity (e.g., ototoxicity due to C1494T mitochondrial DNA mutation).104
Ibuprofen, a nonsteroidal anti-inflammatory agent that induces closure of the patent ductus arteriosus (PDA) in neonates, is another example of a medication that can cause a clincally significant alteration in the pharmacokinetic and PD of other medications. Hirt et al105 studied ibuprofen pharmacokinetics in preterm neonates with PDA and aimed to establish relationships between doses, plasma concentrations, and ibuprofen efficacy and safety. Sixty-six very-low-birth-weight infants with gestational ages from 25 to 34 weeks and postnatal ages ranging from 1 to 11 days with respiratory distress syndrome and hemodynamically significant PDA were treated with ibuprofen-lysine. Median intravenous daily doses of the drug were 10, 5, and 5 mg/kg on 3 consecutive days. A total of 129 serum samples were evaluated. A relationship was shown between plasma ibuprofen concentration and PDA closure rate. Based on the observations collected, a dosing scheme was proposed to achieve effective plasma concentrations, which were irrespective of gestational age. Three intravenous doses (10, 5, 5 mg/kg) were given at 24-hour intervals for neonates at <70 hours of life; 14, 7, 7 mg/kg for neonates between 70 and 108 hours of age; and 18, 9, 9 mg/kg for neonates between 108 and 180 hours of life. Clearly this dose scheme needs prospective validation. The practice of extrapolating a given ibuprofen serum concentration to success of PDA closure or appearance of side effects does not take potential age-related differences into account. Functional PDA closure due to smooth muscle constriction and permanent anatomic closure due to vascular remodeling are the results of a complex interaction of different mechanisms. Prostaglandins, oxygen, nitric oxide, and various other factors play a key role in ductal closure. An understanding of the role of these factors, involved both in the maintenance of vascular tone of the ductus in fetal life as well as stimulation of ductal closure in postnatal life, and the cardiovascular and respiratory consequences of a patent ductus arteriosus, is important for the clinician involved in management of premature neonates.106 Prostanoid receptors display ontogeny,107 while others have suggested using urinary PGE2 as a pharmacodynamic biomarker to quantify the reduction in PGE2 synthesis following ibuprofen administration in preterm neonates.108
Finally, the PD of potent analgesics and sedatives (e.g., morphine, midazolam) illustrate the complex interaction between pharmacokinetics and PD and outcomes within the neonatal population. There are robust outcome data for the need to administer appropriate doses of opioids or analgesics during and after surgery. The administration of opioids after surgery results in reduced mortality and morbidity. In contrast, there is insufficient evidence to recommend routine use of opioids in mechanically ventilated newborns. Opioids should be used selectively, when indicated by clinical judgment and evaluation of pain indicators. If sedation is required, morphine is safer than midazolam because the administration of midazolam for this indication is associated with poorer (intraventricular hemorrhage) outcome.109,110
Both compounds also nicely illustrate the complex interaction between maturational pharmacokinetics and PD. Morphine is an effective analgesic, but its glucuronide metabolites (morphine-3 and morphine-6 glucuronide) are also of PD relevance. Consequently, the phenotypic effects of such compounds in neonates will be the result of both pharmacokinetics (primary renal elimination, metabolic elimination, renal elimination of the metabolites) and PD (receptor expression, post receptor signaling), necessitating an integrated approach and modelling.111,112
CONCLUSIONS
Providing safe and effective drug therapy in the newborn requires an understanding and consideration of the developmental aspects in both the pharmacokinetics and the PD. Newborns are neither small adults nor little children, and several maturation processes overlap with disease characteristics. These developmental aspects make it difficult and even unsafe to dose medications in neonates by simply scaling adult doses on the basis of the weight and/or body surface area. Neonatal drug therapy should be based on a critical interpretation of the available data, an understanding of fetal development and maturational processes, and an understanding of how diseases may affect drug biodisposition in this specific population. Although there is a significant volume of literature that describes the ontogeny and maturational aspects of pharmacokinetics in the fetus and neonate, there continues to be a need for greater clarity of the ontogeny of isozymes and the influence of pharmacogenomics in the neonate. There is also a need to explore potential maturational aspects of PD based on age-adapted dosing regimen.
ACKNOWLEDGEMENTS
The clinical research of K. Allegaert was supported by the Fund for Scientific Research, Flanders (Belgium) (FWO Vlaanderen) and a Fundamental Clinical Investigatorship (1800209N). The clinical research of C. Tayman was supported by the Scientific and Technological Research Council of Turkey (TUBITAK).
ABBREVIATIONS
- AUC
area under the serum concentration curve
- Cmax
maximum peak serum concentration
- CYP
cytochrome P-450
- GFR
glomerular filtration rate
- MIC
minimum inhibitory concentration
- NICU
neonatal intensive care unit
- PD
pharmacodynamic
- PDA
patent ductus arteriosus
- P-gp
glycoprotein
- UGT
uridine 5′-diphospho-glucuronosyltransferase
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Avanel S, Bomkratz A, Dassieu G, et al. The incidence of prescription without marketing product license in a neonatal intensive care unit. Arch Pediatr. 2000;7(2):143–147. doi: 10.1016/s0929-693x(00)88083-5. [DOI] [PubMed] [Google Scholar]
- 2.Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for drug Investigation in Children. BMJ. 2000;320(7227):79–82. doi: 10.1136/bmj.320.7227.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Anker J. Managing drug safely. Semin Fetal Neonatal Med. 2005;10(1):73–81. doi: 10.1016/j.siny.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Rakhmanina NY, van den Anker JN. Pharmacological research in pediatrics: from neonates to adolescents. Adv Drug Deliv Rev. 2006;58(1):4–14. doi: 10.1016/j.addr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Blumer JL, Reed MD. Principle of neonatal pharmacology. In: Yaffe SJ, Aranda JV, editors. Neonatal and Pediatric Pharmacology. 3rd ed. Philadelphia: WB Saunders & Co; 2005. pp. 146–158. [Google Scholar]
- 6.Wadsworth SJ, Suh B. In vitro displacement of bilirubin by antibiotics and 2-hydroxybenzoylglycine in newborns. Antimicrob Agents Chemother. 1988;32(10):1571–1575. doi: 10.1128/aac.32.10.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollard AJ, Rylance G. Inappropriate prescribing of promethazine in infants. Arch Dis Child. 1994;70(4):357. doi: 10.1136/adc.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glover ML, Reed MD. Propylene glycol: the safe diluent that continues to cause harm. Pharmacotherapy. 1996;16(4):690–693. [PubMed] [Google Scholar]
- 9.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 10.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55(5):667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson AJ. Introduction to Clinical Pharmacology. In: Atkinsin AJ, Daniels CE, Dedrick RL, Grudzinskas CV, Markey SP, editors. Principles of Clinical Pharmacology. 2nd ed. New York: Academic Press; 2001. pp. 1–6. [Google Scholar]
- 12.Menard D. Functional development of the human gastrointestinal tract: hormone- and growth factor-mediated regulatory mechanisms. Can J Gastroenterol. 2004;18(1):39–44. doi: 10.1155/2004/640897. [DOI] [PubMed] [Google Scholar]
- 13.Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. J Parenteral Enteral Nutr. 1999;23(5):3–6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- 14.Kelly EJ, Newell SJ, Brownlee KG, et al. Gastric acid secretion in preterm infants. Early Hum Dev. 1993;35(3):215–220. doi: 10.1016/0378-3782(93)90108-7. [DOI] [PubMed] [Google Scholar]
- 15.Huang NN, High RH. Comparison of serum levels following the administration of oral and parenteral preparation of penicillin to infants and children of various age groups. J Pediatr. 1953;42(6):657–658. doi: 10.1016/s0022-3476(53)80422-1. [DOI] [PubMed] [Google Scholar]
- 16.Morselli PL. Antiepileptic drugs. In: Morselli PL, editor. Drug Disposition During Development. New York: Spectrum;; 1977. pp. 311–360. [Google Scholar]
- 17.Dumon RC, Rudolph CD. Development of gastrointestinal motility in the infant and children. Pediatr Clin North Am. 1994;23(4):655–671. [PubMed] [Google Scholar]
- 18.Carlos MA, Babyn PS, Macron MA, et al. changes in gastric emptying in early postnatal life. J Pediatr. 1997;130(6):931–937. doi: 10.1016/s0022-3476(97)70279-8. [DOI] [PubMed] [Google Scholar]
- 19.Staelens S, van den Driessche M, Barclay D, et al. Gastric emptying in healthy neborns fed an intact protein formula, a partially and extensively hydrolyzed formula. Clin Nutr. 2008;27(2):264–268. doi: 10.1016/j.clnu.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Berseth CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. 1989;115(4):646–651. doi: 10.1016/s0022-3476(89)80302-6. [DOI] [PubMed] [Google Scholar]
- 21.Tawil YA, Berseth CL. Geatational age and postnatal maturation of duodenal motor responses to intragastric feeding. J Pediatr. 1996;129(3):374–381. doi: 10.1016/s0022-3476(96)70069-0. [DOI] [PubMed] [Google Scholar]
- 22.Heimann G. Enteral absorption and bioavailability in children in relation to age. Eur J Clin Pharmacol. 1980;18(1):43–50. doi: 10.1007/BF00561477. [DOI] [PubMed] [Google Scholar]
- 23.Grand RJ, Watkins JB, Torti FM. Development of the human gastrointestinal tract: a review. Gastroenterology. 1976;70(5):790–810. [PubMed] [Google Scholar]
- 24.Yanowitz TD, Yao AC, Pettigrew KD, et al. Postnatal hemodynamic changes in very-low-birthweight infants. J Appl Physiol. 1999;87(1):370–380. doi: 10.1152/jappl.1999.87.1.370. [DOI] [PubMed] [Google Scholar]
- 25.Martinussen M, Brubakk AM, Vik T, et al. Mesenteric blood flow velocity and its relation to transitional circulatory adaptation in appropriate for gestational age preterm infants. Pediatr Res. 1996;39(2):275–280. doi: 10.1203/00006450-199602000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Martinussen M, Brubakk AM, Linker DT, et al. Mesenteric blood flow velocity and its relation to circulatory adaptation during the first week of life in healthy term infants. Pediatr Res. 1994;36(3):334–339. doi: 10.1203/00006450-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Mcclean P, Weaver LT. Ontogeny of human pancreatic exocrine function. Arch Dis Child. 1993;68(1):62–65. doi: 10.1136/adc.68.1_spec_no.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suchy FJ, Bucuvalas JC, Novak DA. Determinants of bile formation during development: ontogeny of hepatic bile acid metabolism and transport. Semin Liver Dis. 1987;7(2):77–84. doi: 10.1055/s-2008-1040567. [DOI] [PubMed] [Google Scholar]
- 29.Hall SD, Thummel KE, Watkins PB, et al. Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos. 1999;27(2):161–166. [PubMed] [Google Scholar]
- 30.Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34((1-2)):47–54. doi: 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- 31.Johnson WW. P-glycoprotein mediated efflux as a major factor in the varians of absorption and distrubition of drugs: modulation of chemotheraphy resistans. Methods Find Exp Clin Pharmacol. 2002;24(8):501–514. doi: 10.1358/mf.2002.24.8.705071. [DOI] [PubMed] [Google Scholar]
- 32.Anthony V, Skach WR. Moleculer mechanism of P-glycoprotein assembly into cellular membranes. Curr Prot Peptide Sci. 2002;3(5):485–501. doi: 10.2174/1389203023380503. [DOI] [PubMed] [Google Scholar]
- 33.Edward CA, Parret AM. Probiotics, prebiotics and symbiotics: approches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(5):1052–1057. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 34.Rowland IR. Factors effecting metabolitic activity of the intestinal microflora. Drug Metab Rev. 1988;19((3-4)):243–261. doi: 10.3109/03602538808994135. [DOI] [PubMed] [Google Scholar]
- 35.Illet KF, Tee LB, Reeves PT, et al. Metabolism of drug and other xenobiotics in the gut lumen and wall. Pharmacol Ther. 1990;46(1):67–93. doi: 10.1016/0163-7258(90)90036-2. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47(1):3–10. doi: 10.1097/MPG.0b013e31816a8cca. [DOI] [PubMed] [Google Scholar]
- 37.Seth V, Beotra A, Bagga A, et al. Drug therapy in malnutrition. İndian Pediatr. 1992;29(11):1341–1346. [PubMed] [Google Scholar]
- 38.Choonara I. Percutaneous drug absorbtion and administration. Ach Dis Child. 1994;71(2):73–74. doi: 10.1136/fn.71.2.f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntyre J, Choonara I. Drug toxicity in the neonate. Biol Neonate. 2004;86(4):218–221. doi: 10.1159/000079656. [DOI] [PubMed] [Google Scholar]
- 40.Greenbalt DJ, Koch-Weaser J. Intramuscular injections of drugs. N Engl J Med. 1976;295(10):542–546. doi: 10.1056/NEJM197609022951006. [DOI] [PubMed] [Google Scholar]
- 41.Carry MR, Ringel SP, Starcevich JM. Distribution of capillaries in normal and diseased human skeletal muscle. Muscle Nerve. 1986;9(5):445–454. doi: 10.1002/mus.880090510. [DOI] [PubMed] [Google Scholar]
- 42.Kafetzis DA, Sinaniotis CA, Papadatos CJ, et al. Pharmacokinetics of amikacin in infants and pre-school children. Acta Paediatr Scand. 1979;68(3):419–422. doi: 10.1111/j.1651-2227.1979.tb05030.x. [DOI] [PubMed] [Google Scholar]
- 43.Sheng KT, Huang NN, Promadhattavedi V. Serum concentrations of cephalothin in infants and children and placental transmission of the antibiotic. Antimicrob Agents Chemother. 1964;10:200–216. doi: 10.1128/AAC.10.2.200. [DOI] [PubMed] [Google Scholar]
- 44.Thorsson L, Edsbacker S, Kallen A, et al. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbuhaler. Br J Clin Pharmacol. 2001;52(5):529–538. doi: 10.1046/j.0306-5251.2001.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiller-Scotland CF, Hlawa R, Gebhart J. Experimental data for total deposition in the respiratory tract of children. Toxicol Lett. 1994;72((1-3)):137–144. doi: 10.1016/0378-4274(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 46.van Lingen RA, Dienum HT, Quack CME, et al. Multiple dose pharmacokinetics of rectally administered acetaminophen interm infants. Clin Pharmacol Ther. 1999;66(5):509–515. doi: 10.1016/S0009-9236(99)70014-7. [DOI] [PubMed] [Google Scholar]
- 47.Williams CN. Role of rectal formulations: suppositoires. Scand J Gastroenterol. 1990;25(172):60–62. doi: 10.3109/00365529009091913. [DOI] [PubMed] [Google Scholar]
- 48.Fisher RS, Ho J. Potential new methods for antiepileptic drug delivery. CNS Drugs. 2003;16(9):579–593. doi: 10.2165/00023210-200216090-00001. [DOI] [PubMed] [Google Scholar]
- 49.Bartelink IH, Rademaker CM, Schobben AF, et al. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45(11):1077–1097. doi: 10.2165/00003088-200645110-00003. [DOI] [PubMed] [Google Scholar]
- 50.Strolin Benedetti M, Baltes EL. Drug metabolism and disposition in children. Fundam Clin Pharmacol. 2003;17(3):281–299. doi: 10.1046/j.1472-8206.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 51.Kearns GL. Impact of developmental pharmacology on pediatric study desing; overcoming the challenges. J Allergy Clin Immunol. 2000;106(3):128–139. doi: 10.1067/mai.2000.109419. [DOI] [PubMed] [Google Scholar]
- 52.Ginsberg G, Hattis D, Miller R, et al. Pediatric Pharmacokinetic data: Implications for enviromental risk assessment for children. Pediatrics. 2004;113(4):973–983. [PubMed] [Google Scholar]
- 53.Mahmood I. Pediatric Pharmacology and Pharmacokinetics. Rockville, MD: Pine House Publishers;; 2008. Developmental pharmacology: impact on pharmacokinetics and PD of drugs; pp. 68–107. [Google Scholar]
- 54.de Zwart LL, Haenen HE, Versantvoort CH, et al. Role of biokinetics in risk assessment of drugs and chemicals in children. Regul Toxicol Pharmacol. 2004;39(3):282–309. doi: 10.1016/j.yrtph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Amin SB, Harte T, Scholer L, et al. Intravenous lipid and bilirubin-albumin binding variables in premature infants. Pediatrics. 2009;124(1):211–217. doi: 10.1542/peds.2008-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pullen J, Stolk LM, Degraeuwe PL, et al. Protein binding of flucloxacillin in neonates. Ther Drug Monit. 2007;29(3):279–283. doi: 10.1097/FTD.0b013e318063e30f. [DOI] [PubMed] [Google Scholar]
- 57.Ambat MT, Ostrea EM, Jr, Aranda JV. Effect of ibuprofen L-lysinate on bilirubin binding to albumin as measured by saturation index and horseradish peroxidase assays. J Perinatol. 2008;28(4):287–290. doi: 10.1038/sj.jp.7211925. [DOI] [PubMed] [Google Scholar]
- 58.Tetelbaum M, Finkelstein Y, Nava-Ocampa AA, et al. Back to basics: understanding drugs in children: pharmacokinetic maturation. Pediatr Rev. 2005;26(9):321–328. doi: 10.1542/pir.26-9-321. [DOI] [PubMed] [Google Scholar]
- 59.Monte SV, Prescott WA, Johnson KK, et al. Safety of ceftriaxone sodium at extremes of age. Expert Opin Drug Saf. 2008;7(5):515–523. doi: 10.1517/14740338.7.5.515. [DOI] [PubMed] [Google Scholar]
- 60.Modi N. Clinical implications of postnatal alterations in body water distribution. Semin Neonatol. 2003;8(4):301–316. doi: 10.1016/S1084-2756(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 61.Touw DJ, Westerman EM, Sprij AJ. Therapeutic drug monitoring of aminoglycosides in neonates. Clin Pharmacokinet. 2009;48(2):71–88. doi: 10.2165/00003088-200948020-00001. [DOI] [PubMed] [Google Scholar]
- 62.Anderson BJ, Allegaert K, Van den Anker JN, et al. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol. 2007;63(1):75–84. doi: 10.1111/j.1365-2125.2006.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson BJ, Pons G, Autret-Leca E, et al. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth. 2005;15(4):282–292. doi: 10.1111/j.1460-9592.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- 64.Allegaert K, de Hoon J, Verbesselt R, et al. Maturational pharmacokinetics of single intravenous bolus of propofol. Paediatr Anaesth. 2007;17(11):1028–1034. doi: 10.1111/j.1460-9592.2007.02285.x. [DOI] [PubMed] [Google Scholar]
- 65.Garg DC, Goldberg RN, Woo-Ming RB, et al. Pharmacokinetics of thiopental in the asphyxiated neonate. Dev Pharmacol Ther. 1988;11(4):213–218. doi: 10.1159/000457691. [DOI] [PubMed] [Google Scholar]
- 66.Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42(1):59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 67.Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci. 2006;8(3):311–321. doi: 10.31887/DCNS.2006.8.3/fgirardin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daood M, Tsai C, Ahdab-Barmada M, et al. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics. 2008;39(4):211–218. doi: 10.1055/s-0028-1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Painter MJ, Pippenger C, Wasterlain C, et al. Phenobarbital and phenytoin in neonatal seizures: metabolism and tissue distribution. Neurology. 1981;31(9):1107–1112. doi: 10.1212/wnl.31.9.1107. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson GR. Pharmacokinetics: the dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill;; 2001. pp. 3–29. [Google Scholar]
- 71.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: Part 1. Clin Pharmacokinet. 2002;41(12):959–998. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]
- 72.Johnson TN, Tanner MS, Christopher J, et al. Enterocytic CYP3A4 in a pediatric population: developmental changes and the effect of coeliac disease and cystic fibrosis. Br J Clin Pharmacol. 2001;51(5):451–460. doi: 10.1046/j.1365-2125.2001.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stahlberg MR, Hiatene E, Maki M. Mucosal biotransformation rates in the small intestine of children. Gut. 1988;29(8):1058–1063. doi: 10.1136/gut.29.8.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antonowicz I, Shwachman H, Sotoo I. Beta-galactosidase and beta-glucuronidase activities in intestinal mucosa of infants and children. Pediatrics. 1971;47(4):737–744. [PubMed] [Google Scholar]
- 75.Gibbs JB, Liacouras CA, Baldassano RN, et al. Up regulation of glutathione S-transferase activity in enterocytes of young children. Drug Metab Dispos. 1999;27(12):1466–1469. [PubMed] [Google Scholar]
- 76.McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300(2):361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- 77.Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300(2):355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 78.Gow PJ, Ghabrial H, Smallwood RA, et al. Neonatal hepatic drug elimination. Pharmacol Toxicol. 2001;88(1):3–15. doi: 10.1034/j.1600-0773.2001.088001003.x. [DOI] [PubMed] [Google Scholar]
- 79.Allegaert K, van den Anker J, Naulers G, et al. Determinants of drug metabolism in early neonatal life. Curr Clin Pharmacol. 2007;2(1):23–29. doi: 10.2174/157488407779422294. [DOI] [PubMed] [Google Scholar]
- 80.Treluyer JM, Jacqz-Aigrain E, Alvarez F, et al. Expression of CYP2D6 in developing human liver. Eur J Biochem. 1991;202(2):583–588. doi: 10.1111/j.1432-1033.1991.tb16411.x. [DOI] [PubMed] [Google Scholar]
- 81.Allegaert K, Anderson B, Verbesselt R, et al. Tramadol disposition in the very young: an attempt to assess in vivo cytochrome P450 2D6 activity. Br J Anaesth. 2005;95(2):231–229. doi: 10.1093/bja/aei170. [DOI] [PubMed] [Google Scholar]
- 82.Sonnier M, Cresteil T. Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem. 1998;251(3):893–898. doi: 10.1046/j.1432-1327.1998.2510893.x. [DOI] [PubMed] [Google Scholar]
- 83.Cresteil T. Onset of xenobiotic metabolism in children: toxicological implications. Food Addit Contam. 1998;15:45–51. doi: 10.1080/02652039809374614. [DOI] [PubMed] [Google Scholar]
- 84.Cazeneuve C, Pons G, Rey E, et al. Biotransformation of caffeine in human liver microsomes from foetuses, neonates, infants and adults. Br J Clin Pharmacol. 1994;37(5):405–412. doi: 10.1111/j.1365-2125.1994.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver. Hypermethylation control of gene expression during the neonatal period. Eur J Biochem. 1996;238(2):476–483. doi: 10.1111/j.1432-1033.1996.0476z.x. [DOI] [PubMed] [Google Scholar]
- 86.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 87.Leeder JS. Translating pharmacogenetics and pharmacogenomics into drug development for clinical pediatrics and beyond. Drug Discov Today. 2004;9(13):567–573. doi: 10.1016/S1359-6446(04)03129-0. [DOI] [PubMed] [Google Scholar]
- 88.Kapur G, Mattoo T, Aranda JV. Pharmacogenomics and renal drug disposition in the newborn. Semin Perinatol. 2004;28(2):32–40. doi: 10.1053/j.semperi.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 89.McCarver DG, Hiner RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300(2):3621–3666. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- 90.de Wildt SN, Kearns GL, Leeder JS, et al. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet. 1999;36(6):439–452. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 91.Bouwmeester NJ, Anderson BJ, Tibboel D, et al. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92(2):208–217. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 92.Allegaert K, van den Anker J, Tayman C, de Hoon J. Determinants of variability in clearance of exogenous compounds in neonates. Verh K Acad Geneeskd Belg. 2009;71(3):42–64. [PubMed] [Google Scholar]
- 93.Chen N, Aleksa K, Woodland C, et al. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21(2):160–168. doi: 10.1007/s00467-005-2105-4. [DOI] [PubMed] [Google Scholar]
- 94.Van den Anker JN. Pharmacokinetics and renal function in the preterm infant. Acta Paediatr. 1996;85(12):1393–1399. doi: 10.1111/j.1651-2227.1996.tb13942.x. [DOI] [PubMed] [Google Scholar]
- 95.Yared A, Ichikawa I. Glomerular Circulation and Function in Pediatric Nephrology. 3rd ed. Baltimore: Lippincott Williams & Wilkins;; 1994. pp. 39–55. [Google Scholar]
- 96.Bjorkman S. Prediction of cytochrome P450-mediated hepatic drug clearance in neonates, ınfants and children: how accurate are available scaling methods? Clin Pharmacokinet. 2006;45(1):1–11. doi: 10.2165/00003088-200645010-00001. [DOI] [PubMed] [Google Scholar]
- 97.Hayton WL. Maturation and growth of renal fuction: dosing renally cleared drugs in children. AAPS PharmSci. 2002;2(1):E3. doi: 10.1208/ps020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hua MJ, Kun HY, Jie CS, et al. Urinary microalbumine and retinol-binding protein assay for verifying children' nefron development and maturation. Clin Chim Acta. 1997;264(1):127–132. doi: 10.1016/s0009-8981(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 99.Danhof M, de Jongh J, De Lange EC, et al. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Ann Rev Pharmacol Toxicol. 2007;47:357–400. doi: 10.1146/annurev.pharmtox.47.120505.105154. [DOI] [PubMed] [Google Scholar]
- 100.Derendorf H, Lesko LJ, Chaikin P, et al. Pharmacokinetic/pharmacodynamic modeling in drug research and development. J Clin Pharmacol. 2000;40((12 Pt 2)):1399–1418. [PubMed] [Google Scholar]
- 101.Bellissant E, Courcier-Duplantier S, Blin O, et al. Role of pharmacokinetic-pharmacodynamic relationships in drug development. Therapie. 2002;57(4):347–357. [PubMed] [Google Scholar]
- 102.Seri I, Abbasi S, Wood DC, Gerdes JS. Regional hemodynamic effects of dopamine in the sick preterm neonates. J Pediatr. 1998;133(6):728–734. doi: 10.1016/s0022-3476(98)70141-6. [DOI] [PubMed] [Google Scholar]
- 103.Lutsar I, Metsvaht T. Understanding pharmacokinetics/pharmacodynamics in managing neonatal sepsis. Curr Opin Infect Dis. 2010;23(3):201–217. doi: 10.1097/QCO.0b013e328337bb42. [DOI] [PubMed] [Google Scholar]
- 104.Qian Y, Guan MX. Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrob Agents Chemother. 2009;53(11):4612–4618. doi: 10.1128/AAC.00965-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirt D, Overmeire IBV, Treluyer JM, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65(5):629–636. doi: 10.1111/j.1365-2125.2008.03118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiruvolu A, Jaleel MA. Pathophysiology of patent ductus arteriosus in premature neonates. Early Hum Dev. 2009;85(3):143–146. doi: 10.1016/j.earlhumdev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Wright DH, Abran D, Bhattacharya M, et al. Prostanoid receptors: ontogeny and implications in vascular physiology. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):1343–1360. doi: 10.1152/ajpregu.2001.281.5.R1343. [DOI] [PubMed] [Google Scholar]
- 108.Antonucci R, Cuzzolin L, Arceri A, et al. Changes in urinary PGE2 after ibuprofen treatment in preterm infants with patent ductus arteriosus. J Clin Pharmacol. 2009;65(3):223–230. doi: 10.1007/s00228-008-0586-3. [DOI] [PubMed] [Google Scholar]
- 109.Bellù R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Sys Rev. 2008. Jan 23;, CD004212. [DOI] [PMC free article] [PubMed]
- 110.Allegaert K, Veyckemans F, Tibboel D. Clinical practice: analgesia in neonates. Eur J Pediatr. 2009;168(7):765–770. doi: 10.1007/s00431-009-0932-1. [DOI] [PubMed] [Google Scholar]
- 111.Knibbe CA, Krekels EH, van den Anker JN, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet. 2009;48(6):371–385. doi: 10.2165/00003088-200948060-00003. [DOI] [PubMed] [Google Scholar]
- 112.Anderson BJ, Larsson P. A maturation model for midazolam clearance. Paediatr Anaesth. 2011;21(3):302–308. doi: 10.1111/j.1460-9592.2010.03364.x. [DOI] [PubMed] [Google Scholar]