Abstract
OBJECTIVES
Although topical agents for the treatment of tinea capitis decrease viable fungal elements and reduce shedding, their use as a prophylactic agent has not been investigated. This study evaluated the effectiveness of a prophylactic ketoconazole shampoo (Nizoral 2%) protocol to reduce the number of clinically evident tinea capitis infections in a high-risk African American, urban population.
METHODS
We conducted a retrospective analysis of a ketoconazole prophylaxis protocol that was implemented at an urban pediatric clinic for medically fragile children. Patients at high risk for tinea capitis received twice-weekly ketoconazole shampoo. The primary outcome of the study was a reduction in the number of documented tinea capitis infections between the 12-month preprotocol and 12-month postprotocol periods. A secondary outcome included the evaluation of predisposing risk factors for acquiring tinea infections.
RESULTS
Ninety-seven patients, with a mean age of 8.06 years, were included. Most patients (78%) were African American. There were a total of 13 tinea capitis infections during the 12-month preprotocol period. During the 12-month postprotocol period, 41 infections were documented: 37 (90.2%) in the prophylaxis group and 4 (9.8%) in the nonprophylaxis group. The average numbers of per-patient infections in the postprotocol period were 0.79 and 0.08 in the prophylaxis and nonprophylaxis groups, respectively. Initiation of prophylaxis did not reduce tinea capitis infections (p=NS). Previous history of infection and a high level of care were significant predictors of infections (p<0.05).
CONCLUSIONS
Improved hygiene, adherence to prescribed treatment regimens, and prevention of recurrent environmental exposure to surviving fomites should be stressed in high-risk patients and supersede the need for an antifungal (ketoconazole shampoo) prophylaxis protocol.
INDEX TERMS: antifungal, ketoconazole, pediatric, prophylaxis, tinea capitis, topical
INTRODUCTION
Tinea capitis is a common fungal infection of the scalp, hair follicles, and hair shafts.1 Although it can affect any age group, it is particularly common in school-aged children.1 In the United States, tinea capitis is the most common dermatophyte infection of childhood, with 90% of infections caused by the anthropophilic fungi Trichophyton tonsurans.2,3 Tinea capitis is commonly spread by asymptomatic carriers and currently infected patients, or by surviving fomites on inanimate objects, such as combs.1 Several investigations of school-aged children suggest infection rates ranging between 3% and 13%.2–5 African American children in urban areas are significantly more likely to harbor pathogenic fungal elements in the scalp than other races. It has been hypothesized that this pronounced ethnic difference may be due to discordance in socioeconomic status, specific hair practices, and a genetic predisposition.3–5 Although systemic therapy, such as oral griseofulvin, effectively penetrates the infected hair shaft, topical shampoos, including ketoconazole and selenium sulfide, are recommended as adjunctive therapeutic options.6–9
Treatment with griseofulvin results in complete elimination of Trichophyton species from the scalp in less than 25% of patients.10 Previous infection has shown to be the strongest predictor of future infection. Topical agents decrease viable fungal elements and reduce shedding, which decreases the likelihood of reinfection and risk of serving as a vector for further spread of infection.8,9,11,12 Use of topical agents as an adjunct therapy should allow students to return to school more readily.6 Further infection control measures include discouraging the use of shared personal hygiene items, including combs and brushes. Although topical antifungal agents are given to asymptomatic children in the household of the infected patient, the relative effectiveness of this strategy is unknown.6,7
In a Medically Fragile Children's clinic in urban Columbia, South Carolina, an increase in the number of tinea capitis infections prompted clinicians to institute a protocol for prophylactic ketoconazole shampoo for patients at high risk of infection. Previous education and the use of standard treatment protocols had not curbed the rise in the number of infections in our clinic. To our knowledge, the efficacy of topical ketoconazole as a prophylactic agent has not been previously reported. We evaluated the effectiveness of a prophylactic ketoconazole shampoo (Nizoral 2%) protocol to reduce the number of clinically evident tinea capitis infections in a high-risk African American, urban population.
MATERIALS AND METHODS
Institutional review board approval was provided by the University of South Carolina and Palmetto Health Richland prior to data collection. The study was conducted in a Medically Fragile Children's clinic in urban Columbia, South Carolina. This clinic services chronically ill pediatric patients, predominantly African American, with debilitating diseases that frequently require health care intervention. Patients deemed as high risk for tinea capitis infection or those chosen based on physician discretion were selected to receive prophylactic therapy with a twice-weekly 2% ketoconazole shampoo per protocol. High risk was defined as a documented history of tinea capitis infection. Patients and caregivers were educated on the appropriate use of the medication, and on proper hygiene and strategies to reduce transmission, including sterilization of inanimate objects when appropriate. Initial supply and refills were provided by the clinic's pharmacy and recorded on the medication administration record. Actual adherence to the ketoconazole protocol was not measured.
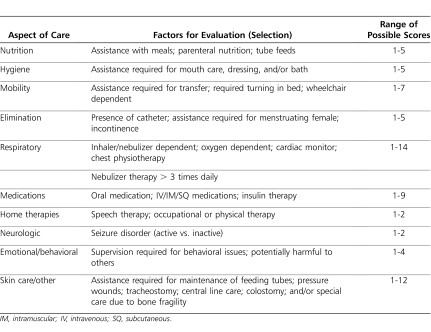
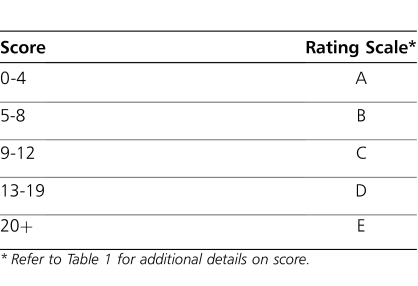
This retrospective study analyzed the difference in number of documented tinea capitis infections, including both reinfection and new infections, between the 12-month preprotocol and 12-month postprotocol periods. Data collected included patient demographics, prophylactic ketoconazole treatment data, and number of tinea capitis infections. Secondary outcomes included the evaluation of predisposing risk factors for acquiring tinea infections (e.g., previous history of infection, complexity of care, wheelchair requirements). Complexity of care (COC) was defined using a clinic-specific scoring system, rating patients as more complex based on their ambulation status, number of required clinic visits, feeding status, motor status, and respiratory status (Tables 1 and 2). A power calculation was not performed because all patients enrolled in the clinic during the study period were eligible for study inclusion. Descriptive statistics were applied to the data. A one-sided t test was used on continuous data with a predetermined alpha of ≤0.05.
Table 1.
Complexity of Care Scoring System
Table 2.
Complexity of Care Rating Scale
RESULTS
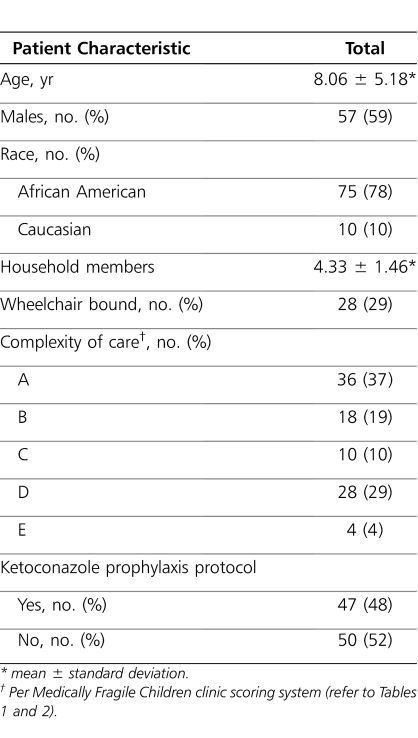
A total of 97 patients with a mean age of 8.06 years (range, 1-21 years) were included in the retrospective analysis (Table 3). Most patients (78%) were African American. A total of 47 patients (48%) received the prophylactic ketoconazole regimen, and 50 (52%) continued with standard of care hygiene practices without prophylaxis. Attendance at scheduled clinic visits was nearly 100% in both groups. A total of 13 tinea capitis infections were documented during the 12-month preprotocol period. All of these infections occurred in patients who were subsequently selected for the ketoconazole prophylaxis protocol. During the 12-month postprotocol initiation period, a total of 41 infections were documented; 37 (90.2%) of these occurred in the ketoconazole prophylaxis group, compared with 4 (9.8%) in the nonprophylaxis group. The 37 infections in the group that received ketoconazole occurred in 16 unique patients. The average number of tinea capitis infections in the 12-month postprotocol period was 0.79 in the ketoconazole prophylaxis group, compared with 0.08 in the nonprophylaxis group. Initiation of the prophylaxis protocol was not associated with a reduction in tinea capitis infections (p=NS). Of the 42 patients with a COC score of C, D, or E (i.e., higher complexity of care), 12 (29%) acquired a tinea capitis infection during the 12-month postprotocol period, compared with 8 of 54 (15%) of those patients with a COC score of A or B. Previous history of tinea infection and patients with a higher COC score were significant predictors of subsequent infections (p<0.05). Ambulatory status determined as wheelchair requirement, age, and sex was not associated with further infections.
Table 3.
Patient Demographics (n=97)
DISCUSSION
Despite implementation of a prophylactic ketoconazole protocol in our urban pediatric clinic, a reduction in subsequent tinea infections among clinic patients was not observed. Topical agents, including ketoconazole, have been shown to reduce fungal elements and shedding of spores when given as an adjuvant to systemic therapy.8,9,11,12 Protracted courses of topical therapy may reduce spreading and decrease the likelihood of patients becoming asymptomatic carriers following clinical resolution.6,8,9 However, topical agents alone would not be expected to eliminate all fungal elements because these agents do not effectively penetrate the hair shaft.1,8,9 A recent epidemiologic investigation of culture-confirmed Trichophyton species on the scalp suggests that both endemic and epidemic spread is expected to occur throughout a population.3 No culture data or genetic analyses are available to identify the causal pathogens in our study. Asymptomatic carriers were not identified or screened in our population, because only clinically evident disease was documented. No further conclusions can be made on the prospective source of spread in our study patients.
Overall, our population was at high risk for tinea infections, with several strong predictors and known risk factors present, including an African American majority and a previously documented tinea infection. Only high-risk patients in our clinic received prophylactic ketoconazole per protocol, possibly creating a subpopulation of patients at risk for harboring and potentially spreading disease. The clinic is often at capacity, and several patients may interact in the playroom and holding areas simultaneously. Fomites have been shown to contaminate inanimate objects, thus resulting in additional spread.1 Standard cleaning procedures were continued during the protocol period; however, specific interventions in the playroom and holding areas were not noted. The rate of infection was very low in those not receiving the prophylactic ketoconazole, with only 8% (4 of 50) of patients having infections during the postimplementation period. Although this may be misrepresented through a selection bias in those receiving prophylactic ketoconazole per protocol, the authors do not feel that implementation of a universal prophylaxis campaign for all patients would result in improved outcomes. The high rate of reinfection in the study protocol arm does not support this rationale.
Compliance is essential for successful eradication during treatment of active infections as well as prevention of spread.6,7 Medication adherence, both for the prophylactic regimens and during the treatment courses of active infections, may be questioned in our population. As a state-funded program, the patients received medications at each visit free of charge. Although compliance with scheduled clinic visits was nearly 100% in the study period, adherence to the protocol at home was not measured. The socioeconomic status of these patients' families and caregivers may also play a role because crowded households, including foster homes, with varying standards for hygiene are common.1,11,12 It was recommended that all household members, including pets, receive a course of treatment and household objects (e.g., bedding, furniture) be thoroughly disinfected; however, this was not mandated as part of the protocol.
CONCLUSION
Based on the results of this study, routine ketoconazole prophylaxis was discontinued at our clinic. The authors conclude that improved hygiene, adherence to prescribed treatment regimens, and prevention of recurrent environmental exposure play a significant role in infection control and supersede the need for a blanket antifungal prophylaxis protocol. Topical agents, including ketoconazole, continue to have a pivotal role as an adjunct to systemic therapy for the treatment and suppression of tinea capitis.
ACKNOWLEDGEMENTS
At the time this manuscript was prepared and submitted, Dr Winters was a resident at Palmetto Health Richland, Columbia South Carolina, and Dr Carlson was a student pharmacist at the University of South Carolina College of Pharmacy, Columbia, South Carolina. Data were presented in part at the 2008 American College of Clinical Pharmacy Spring Research Forum and Meeting on April 8, 2008, in Phoenix, Arizona. We would like to thank members of the Palmetto Health Medically Fragile Children's program for their contributions to this study and health and maintenance of our patients.
ABBREVIATIONS
- COC
Complexity of care
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Mohrenschlager M, Seidl HP, Ring J, Abeck D. Pediatric tinea capitis: recognition and management. Am J Clin Dermatol. 2005;6(4):203–213. doi: 10.2165/00128071-200506040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sharma V, Hall JC, Knapp JF, et al. Scalp colonization by Trichophyton tonsurans in an urban pediatric clinic. Arch Dermatol. 1988;124(10):1511–1513. [PubMed] [Google Scholar]
- 3.Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966–973. doi: 10.1542/peds.2009-2522. [DOI] [PubMed] [Google Scholar]
- 4.Ghannoum M, Isham N, Hajjeh R, et al. Tinea capitis in Cleveland: survey of elementary school students. J Am Acad Dermatol. 2003;48(2):189–193. doi: 10.1067/mjd.2003.109. [DOI] [PubMed] [Google Scholar]
- 5.Williams JV, Honig PJ, McGinley KJ, Leyden JJ. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96(2):265–267. [PubMed] [Google Scholar]
- 6.Chan YC, Friedlander SF. New treatments for tinea capitis. Curr Opin Infect Dis. 2004;17:97–103. doi: 10.1097/00001432-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kakourou T, Uksal U. European Society for Pediatric Dermatology. Guidelines for the management of tinea capitis in children. Pediatr Dermatol. 2010;27(3):226–228. doi: 10.1111/j.1525-1470.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 8.Allen HB, Honig PJ, Leyden JJ, McGinley KJ. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1992;69:81–83. [PubMed] [Google Scholar]
- 9.Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol. 2000;39:302–304. doi: 10.1046/j.1365-4362.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman SM, Wright KJ, Navarre H. Griseofulvin has only a modest impact on eradicating carriage of Trichophyton tonsurans. J Pediatr Pharmacol Ther. 2009;14(2):94–99. doi: 10.5863/1551-6776-14.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greer DL. Treatment of symptom-free carriers in the management of tinea capitis. Lancet. 1996;348:350–351. doi: 10.1016/s0140-6736(05)64987-2. [DOI] [PubMed] [Google Scholar]
- 12.Neil G, Hanslo D. Control of the carrier state of scalp dermatophytes. Pediatr Infect Dis J. 1990;9:57–58. doi: 10.1097/00006454-199001000-00013. [DOI] [PubMed] [Google Scholar]