Abstract
Immunosuppressive regimens, which include antithymocyte globulin (ATG), are widely used for the treatment of severe aplastic anemia (SAA). However, bradycardia has been reported only as a rare side effect of ATG therapy in the manufacturer's product information and, in rare cases, in the adult literature. We present an adolescent with SAA and preexisting bradycardia who underwent immunosuppression therapy with ATG, methylprednisolone, and tacrolimus and developed profound sinus bradycardia with successive doses of ATG.
INDEX TERMS: antithymocyte globulin, aplastic anemia, Atgam, bradycardia
INTRODUCTION
Treatment with antithymocyte globulin (ATG), corticosteroids, and cyclosporine or tacrolimus has become the standard of care for severe aplastic anemia (SAA) in the absence of an HLA-matched sibling donor. ATG is a monomeric immunoglobulin G (IgG) obtained from the serum of horses or rabbits immunized with human thymus lymphocytes. It is a lymphocyte-selective immunosuppressant, as demonstrated by its ability to reduce the number of circulating lymphocytes.1 The most commonly reported side effects of treatment with ATG are fever, chills, leukopenia, thrombocytopenia, and dermatologic manifestations (rashes, urticaria, pruritis, wheal, and flare).1 Adverse cardiovascular reactions that have been reported in association with ATG include myocarditis, “cardiac irregularity,” chest pain, hypertension, hypotension, tachycardia, and bradycardia.1 Based on data published by the manufacturer, most of these adverse cardiovascular effects have been reported in fewer than 5% of patients receiving ATG. One recent case report described a delayed onset cardiopulmonary reaction, including bradycardia, in a 60-year-old male after ATG infusion.2 A separate case series described significant sinus bradycardia that occurred in a 63-year-old female on day 3 of ATG treatment.3 Despite these data, bradycardia during treatment with ATG is not reported frequently in peer-reviewed literature and is not well recognized by clinicians. We present the case of an adolescent who had mild sinus bradycardia at the time he was diagnosed with SAA, which was dramatically exacerbated during ATG treatment. The patient's bradycardia was also noted to improve concurrently with improvement in the underlying disease process, suggesting the possibility of a common immune-mediated mechanism for both SAA and sinus bradycardia in this patient.
CASE REPORT
A 14-year-old African American male was found to be pancytopenic on routine laboratory evaluation, with a white blood cell count of 1.8 × 109 cells/L, a hemoglobin concentration of 6.4 g/dL, and a platelet count of 17 000 × 109 cells/L. Results of chemistry studies, including liver functions, were normal. Bone marrow biopsy and aspirate tests were performed and results confirmed a diagnosis of SAA.
At the time the patient was diagnosed with SAA, he exhibited asymptomatic sinus bradycardia with a ventricular rate of 46 to 50 beats per minute (bpm) by electrocardiogram. According to his pediatrician's records, the patient had documented heart rates of 76 to 80 bpm over a 5-year period prior to this presentation. An echocardiogram demonstrated normal anatomy with a fractional shortening of 50%. He was noted to have a mildly depressed free T4 level of 0.68 ng/dL (normal range, 0.8 to 2.0 ng/dL), despite a normal level of thyroid-stimulating hormone. After the patient received thyroid hormone supplementation, the free T4 test was repeated and the result showed an increased level to 0.82 ng/dL with a concurrent decrease in thyroid-stimulating hormone, suggesting patient compliance with treatment. Holter monitoring at that time demonstrated an average heart rate of 63 bpm with a range of 40 to 140 bpm. Overall, the patient spent more than 60% of the monitoring period with a heart rate of less than 60 bpm, and his average and minimum heart rates were both at or below the age-related low normal range.4,5 The patient was trim but not particularly athletic, so that exercise could not sufficiently explain his bradycardia.
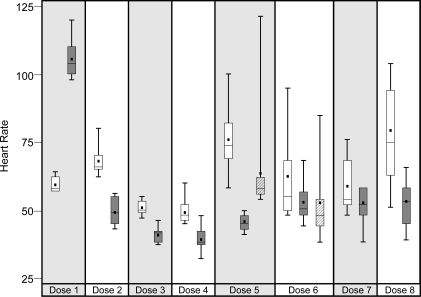
No HLA-matched-related donor was found, and therefore the decision was made to begin immunosuppressive therapy. The patient was admitted 1 month after diagnosis to begin treatment according to a modification of a previously published report,6 with equine-derived ATG (20 mg/kg, intravenously [IV] once daily, infused over 8 hours [Pharmacia & Upjohn Co.]), methylprednisolone (0.5 mg/kg, IV every 6 hours throughout ATG therapy), tacrolimus (0.03 mg/kg, by mouth, twice daily and adjusted to maintain a trough level of 6 to 8 ng/ml), and granulocyte colony-stimulating factor (5 mcg/kg, subcutaneously, once daily after completion of ATG therapy). The patient also received premedication with acetaminophen and diphenhydramine prior to each ATG infusion. During the initial infusion of ATG, the patient developed fever, rigors, tachycardia, and hypertension that were believed to represent an acute reaction to ATG. The infusion was slowed, and the patient was treated with meperidine, acetaminophen, and diphenhydramine, with subsequent improvement of his symptoms. With the remaining doses of ATG, the patient was noted to have an exacerbation of his bradycardia, with a heart rate as low as 32 bpm that lasted between 6 and 14 hours (Figure). Despite maintaining a normal blood pressure and adequate perfusion, the patient's degree of bradycardia prompted the use of isoproterenol during doses 5 and 6 of ATG and the use of glycopyrrolate during doses 6 and 7. The use of 2 different medications was based on provider preference at the time. Isoproterenol was infused at a rate of 0.01 mcg/kg/min and was started just after ATG completion on both occasions. It was continued for 14 and 8 hours during doses 5 and 6, respectively, until the patient's heart rate was consistently above 50 bpm. Glycopyrrolate, 200 mcg, was given once prior to dose 6 in an attempt to prevent bradycardia. During dose 7, 200 mcg of glycopyrrolate was given every 8 hours during ATG infusion.
In the weeks following immunosuppressive therapy, the patient's heart rate ranged from 60 to 109 bpm. This was a statistically significant change (p<0.01) compared to the patient's heart rate based on data collected from clinic visits, electrocardiograms, Holter monitoring, and inpatient admissions before and immediately after treatment with ATG. During the same time period, statistically significant increases in the patient's white blood cell count, absolute neutrophil count, hemoglobin, and platelet counts were also observed (all were p≤0.01), suggesting a concurrent improvement in the underlying disease process. One year after completion of ATG, but still receiving tacrolimus, the patient's hemogram normalized except for a platelet count still at 70 × 109 cells/L. His heart rate has been 60 to 65 bpm at rest.
DISCUSSION
Our patient developed significantly progressive sinus bradycardia compared with baseline heart rates during 5 of 8 ATG infusions. The timing, duration, and severity of the events were variable. However, the events appeared to become less severe with each subsequent dose of ATG.
Given that multiple medications were administered in conjunction with ATG, it is difficult to completely attribute the worsening bradycardia to the ATG infusions. The temporal relationship between ATG administration and the episodes of profound bradycardia provides the most compelling evidence of a causal relationship. Methylprednisolone has been reported to cause sinus bradycardia.7 However, our patient received IV methylprednisolone every 6 hours, and the bradycardia appeared to occur only in conjunction with ATG administration. Additionally, based on a previous case series, bradycardia secondary to steroid therapy is reported to last at least 72 hours.7 That our patient's heart rate returned to baseline values between ATG infusions argues against methylprednisolone as a cause of bradycardia. Meperidine was also felt to potentially contribute to the bradycardia. For this reason, it was discontinued early in the immunosuppressive course, and the last dose was administered just prior to the third ATG infusion. The persistence of bradycardia after discontinuation of meperidine makes it unlikely to be causal. It was believed that other medications were less likely to be contributory, and no temporal relationship could be demonstrated between any other medication and the bradycardic episodes.
There are some confounding factors that must be considered when comparing heart rates before and during ATG administration. One consideration is that administration of ATG requires the patient to be in bed. Prior to ATG, this patient was able to ambulate, and therefore, the differences in heart rates may have been secondary to changes between ambulatory and sedentary states. Another factor to take into account is the timing of the ATG infusions, which resulted in the completion of each infusion while the patient was sleeping. Although one might postulate that the sleep state may have contributed to the worsening of bradycardia, more significant bradycardia was noted during ATG infusions than during Holter monitoring during sleep and while awake prior to immunotherapy. This suggests that there was a true exacerbation of the patient's bradycardia during ATG administration. Finally, the use of isoproterenol and glycopyrrolate makes interpretation of the data more difficult. As expected, the duration of bradycardia was shortened when these agents were used, and their use may have prevented bradycardia during ATG doses 6 and 7.
To the best of our knowledge, bradycardia has been reported only as a rare side effect of ATG in the manufacturer's published product information and in 2 cases from the adult literature. It is possible that this patient's underlying bradycardia may have made him more susceptible to bradycardia from ATG. However, we suspect that bradycardia may be a more significant side effect than previously reported.
Also, it is of interest that our patient did not appear to have had bradycardia prior to his diagnosis of SAA, and there was a significant improvement both in bradycardia and in the underlying disease process after immunosuppression. We hypothesize that the immunity-mediated process leading to SAA may also have had a previously undescribed effect on the cardiac conduction system leading to sinus bradycardia. While autoimmune effects on the cardiac conduction system have been previously reported,8 the isolated effect on the sinoatrial node and the apparent reversible process observed in this patient are unique. Although his presumably autoimmune hypothyroidism likely contributed to his bradycardia, the patient's heart rate was well below the baseline established by his pediatrician, even after thyroid levels were normalized. Evaluation of large cohorts of patients with SAA will be needed to address the possibility that bradycardia may be part of the autoimmune process and to characterize its impact on ATG toxicity.
SUMMARY
Although the condition is rare, clinicians should be aware that ATG may cause bradycardia. This relationship may be especially true for patients with preexisting arrhythmias. Immunity-mediated processes such as those leading to SAA may also have a previously undescribed effect on the cardiac conduction system leading to sinus bradycardia. Evaluation of large cohorts of patients with SAA and other patients treated with ATG will be needed to address the issues of cardiac toxicity and the relationship between preexisting bradycardia and autoimmune disease.
ABBREVIATIONS
- ATG
antithymocyte globulin
- IgG
immunoglobulin G
- SAA
severe aplastic anemia
Footnotes
DISCLOSURE The authors have no conflicts of interest or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
Figure 1.
Box plot depicting the heart rate changes during each dose of ATG. Wilcoxon rank sum test results for differences in distribution are statistically significant (p≤0.01) for Dose 1, Dose 2, Dose 3, Dose 4, Dose 5, and Dose 8.
□ Prior to ATG; During and after ATG; □/// With Isoproterenol
REFERENCES
- 1.Pfizer Inc. Atgam (lymphocyte immune globulin, antithymocyte globulin [equine] sterile solution) 2011. http://labeling.pfizer.com/ShowLabeling.aspx?id=525. June 20.
- 2.Loushin MK, Hasinoff IK, Belani KG. A delayed cardiopulmonary reaction to an intravenous immunosuppressant thymoglobulin after pancreas transplant. Anesth Analg. 2001;93:1260–1261. doi: 10.1097/00000539-200111000-00044. [DOI] [PubMed] [Google Scholar]
- 3.Kao SY, Xu W, Brandwein JM, et al. Outcomes of older patients (≥60 years) with acquired aplastic anaemia treated with immunosuppressive therapy. Br J Haematol. 2008;143(5):738–743. doi: 10.1111/j.1365-2141.2008.07389.x. [DOI] [PubMed] [Google Scholar]
- 4.Heragu NP, Scott WA. Heart rate variability in healthy children and in those with congenital heart disease both before and after operation. Am J Cardiol. 1999;83:1654–1657. doi: 10.1016/s0002-9149(99)00173-3. [DOI] [PubMed] [Google Scholar]
- 5.Nagashima M, Matsushima M, Ogawa A, et al. Cardiac arrhythmias in healthy children revealed by 24-hour ambulatory ECG monitoring. Pediatr Cardiol. 1987;8(2):103–108. doi: 10.1007/BF02079464. [DOI] [PubMed] [Google Scholar]
- 6.Frickhofen N, Kaltwasser JP, Schrezemeier H, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297–1304. doi: 10.1056/NEJM199105093241901. [DOI] [PubMed] [Google Scholar]
- 7.Akikusa JD, Feldman BM, Gross GJ, et al. Sinus bradycardia after intravenous pulse methylprednisolone. Pediatrics. 2007;119:e778–e782. doi: 10.1542/peds.2006-0029. [DOI] [PubMed] [Google Scholar]
- 8.Ristic AD, Maisch B. Cardiac rhythm and conduction disturbances: what is the role of autoimmune mechanisms? Herz. 2000;25(3):181–188. doi: 10.1007/s000590050005. [DOI] [PubMed] [Google Scholar]