Abstract
Immune escape mutations selected by human leukocyte antigen class I-restricted CD8+ cytotoxic T lymphocytes (CTLs) can result in biologically and clinically relevant costs to HIV-1 replicative fitness. This phenomenon may be exploited to design an HIV-1 vaccine capable of stimulating effective CTL responses against highly conserved, mutationally constrained viral regions, where immune escape could occur only at substantial functional costs. Such a vaccine might ‘channel’ HIV-1 evolution towards a less-fit state, thus lowering viral load set points, attenuating the infection course and potentially reducing the risk of transmission. A major barrier to this approach, however, is the accumulation of immune escape variants at the population level, possibly leading to the loss of immunogenic CTL epitopes and diminished vaccine-induced cellular immune responses as the epidemic progresses. Here, we review the evidence supporting CTL-driven replicative defects in HIV-1 and consider the implications of this work for CTL-based vaccines designed to attenuate the infection course.
Keywords: CD8+ T lymphocyte, CTL, HIV-1, HLA, human leukocyte antigen class I, immune escape, replication capacity, vaccine design, viral fitness
Combination antiretroviral therapy is currently our best hope to combat the HIV epidemic [1–3]. However, with over 33 million individuals infected and a global incidence of 2.5 million new cases per year [201], the need for an effective HIV vaccine remains critical. Evidence of reduced infection in the RV144 (Thai) HIV vaccine trial has renewed optimism in the field [4], but vaccine-elicited sterilizing immunity remains a distant goal due to key knowledge gaps in HIV immunobiology [5–7]. Vaccine-mediated stimulation of adaptive immune responses, including human leukocyte antigen (HLA) class I-restricted CD8+ cytotoxic T lymphocyte (CTL) responses, aimed at reducing viral load set point and rate of disease progression upon HIV infection, is perhaps a more realistic target [5,7]. Although the CTL-based vaccine concept suffered a setback with failure of the STEP trial in 2007 [8], recent data indicate that this vaccine was associated with reduced plasma viremia in participants expressing certain HLA alleles [9]. In addition, the STEP vaccine may have blocked infection by HIV variants most closely resembling the vaccine antigen [10], although rapid, vaccine-induced CTL escape [11] cannot be ruled out [10]. Taken together, results suggest that effective vaccine-induced CTL responses may be achievable. However, in order for the field to advance beyond the STEP and RV144 trials, further elucidation of their mechanism(s) of action will be essential.
The long-term benefit of ‘protective’ HLA class I alleles, such as B*27, B*57 and B*5801 [12–15], is likely mediated by their tendency to mediate early, robust CTL responses against highly conserved HIV epitopes, most notably in the structural p24Gag protein [16–18]. However, a major challenge to CTL-mediated control of HIV [13,16,19–23] is the selection of HLA-restricted escape mutations [24–33] that allow HIV to evade immune detection. Mutational escape occurs along generally reproducible pathways that are predictable based on host HLA allele expression [34–39]. Furthermore, in at least some cases, the advantages of CTL escape for the virus are offset by a measurable (and equally reproducible) cost to HIV protein function and/or viral replication capacity [40–45]. Thus, it has been hypothesized that immune-mediated HIV attenuation may contribute to lower plasma viremia and a more benign disease course in some individuals.
Cytotoxic T lymphocyte-based vaccine strategies targeting conserved regions of HIV are currently favored due to the presumed limited ability of the virus to escape (thereby enabling sustained immune recognition of infected cells) and/or the anticipated fitness costs associated with escape mutations that may occur [46–48]. However, this approach is limited by two key knowledge gaps. First, it is unclear whether regions of HIV outside of the p24Gag capsid protein encode appropriate conserved and immunogenic regions suitable for vaccine inclusion. Second, besides a few well-studied p24Gag epitopes restricted by classical protective HLA alleles, the extent to which CTL escape mutations confer biologically relevant fitness costs is unclear. Here, we summarize evidence supporting the ability of CTL responses to induce replicative defects in HIV-1 and consider the implications of this work for CTL-based vaccines designed to attenuate the disease course.
Impact of host HLA on HIV-1 fitness: a review of the evidence
Multiple lines of evidence indicate that immune-mediated attenuation can occur during natural HIV infection. Fitness costs of certain CTL escape mutations are observed indirectly through their reversion upon transmission to a host lacking the restricting HLA allele [33,49–51]. Similarly, reversion explains why certain immunogenic epitopes remain highly conserved at the population level [52]. In general, escape mutations selected by protective HLA alleles and/or within highly conserved CTL epitopes (e.g., the B*57-associated T242N mutation in p24Gag [33]) tend to revert, although there are reports of stably transmitted escape mutations restricted by protective alleles [53–57] as well as escape mutations selected by nonprotective alleles that readily revert [30].
In vitro studies assessing the replicative capacity of HIV reference strains engineered to express single (or combinations of) escape mutations have provided direct evidence of immune-associated attenuation [41–43,58–61]. Perhaps most striking, an HIVNL4–3 strain encoding the B*27-associated R264K mutation in the KK10-Gag epitope [62] (which typically occurs in chronic infection and is associated with disease progression when selected in B*27+ individuals [29]) exhibited a profound in vitro replication defect [42], indicating that a single CTL escape mutation can result in severe HIV attenuation. In contrast, the T242N mutation in the TW10-Gag epitope (which is often selected within weeks of infection in B*57/B*5801-expressing individuals [33]) conferred a modest in vitro fitness cost [41,43]. However, further reductions in replicative capacity were seen when T242N was combined with mutations in additional B*57-restricted p24Gag epitopes generally selected in vivo [59,60], demonstrating that immune-driven attenuation can be additive. In vitro analysis has also identified pairs of ‘negatively correlated’ mutations (e.g., T242N and M250I in Gag) [43], suggesting that certain combinations of CTL escape mutations may be particularly deleterious in vivo. To date, in vitro studies have focused primarily on escape mutations in p24Gag restricted by protective HLA alleles. However, recent reports of B*13- and B*35-restricted escape mutations in p1Gag [63], and Nef [64], respectively, provide evidence for HLA-associated attenuation in regions outside of the HIV capsid protein. Moreover, fitness defects resulting from escape mutations in A*7401- [65] and Cw*03- [66] restricted p24Gag epitopes have also been demonstrated, illustrating that alleles not typically associated with HIV control may contribute to viral attenuation.
Importantly, bioinformatic analyses [38,67–69], coupled with experimental validation have facilitated the identification of key secondary mutations that restore replicative defects associated with the primary escape mutation [17,41,42,58]. These ‘compensatory’ mutations can occur in close proximity to the primary escape site (e.g., S165N with A163G in B*5703-KF11 [17]; E260D with R264K in B*27-KK10 [58]; H219Q, I223V and M228I with T242N in B*57-TW10 [41,43], all in p24Gag) or a substantial distance away (e.g., S173A with R264K in B*27-KK10) [42]. Critically, compensatory mutations can serve to stabilize primary escape mutations in vivo upon transmission to individuals lacking the restricting HLA allele [56,57]. Indeed, there is concern that their presence may help to drive the accumulation of escape mutations in the population [17,55–57], with profound consequences for the epidemic.
Immune-driven attenuation of HIV, and subsequent compensation, is also supported by studies examining the in vitro replication capacity of recombinant viruses encoding clinically derived plasma RNA Gag-protease sequences [70–72]. In a population-based analysis of subtype B sequences, host expression of protective HLA alleles B*13, B*57 and B*5801 was correlated with significant reductions in replication capacity in sequences derived from acute/early, but not chronic, infection [70]. Results from subtype C infection are consistent with this observation. In Bloemfontein, South Africa, higher in vitro replicative capacity of Gag-protease sequences from patients with advanced disease compared with those with preserved CD4 cell counts has been reported [73]. In Durban, South Africa, significantly lower Gag-protease replication capacities in acute/early-infected individuals with lower viral load set points [72] and sustained reductions in viral replication capacities in chronically infected individuals expressing protective HLA alleles, notably B*81, have been reported [71]. Although a comprehensive analysis of ex vivo HIV fitness will likely require whole viral isolates [74], replicative capacities of Gag-protease recombinant viruses nevertheless correlate well with those of clinical isolates [Wright J, Unpublished Data].
These HIV studies build from previous and complementary in vitro [61,75,76] and in vivo [77–81] evidence demonstrating early escape and immune-mediated viral attenuation in the SIV-macaque model. For example, rhesus macaques expressing the protective Mamu-A*01 allele induce a T47I escape mutation in the SIV Gag CM9 epitope that has been shown to decrease viral fitness [61,75], while secondary mutations located at I26V and/or I71V can restore viral fitness [76].
Taken together, these data suggest that early attenuation of HIV through the selection of fitness-reducing CTL escape mutations by protective HLA alleles may confer long-term clinical benefit, although these effects may be substantially diminished by the subsequent selection of compensatory mutations. In general, escape mutations occurring in highly conserved HIV regions tend to exhibit the most pronounced fitness defects, while escape in variable regions tend to be fitness-neutral [45]. Although in vitro studies have largely concentrated on p24Gag, recent evidence of immune-driven functional defects in p1Gag [63] and Nef [64], combined with numerous examples of clinically relevant functional costs associated with antiretroviral resistance mutations selected in Pol [82–87] and Env [88,89], suggest that further efforts to identify immune-driven replicative costs in natural HIV infection are warranted.
Biological & clinical benefits of immune-driven mutations in HIV
Transmission of attenuated HIV variants
Transmission of viral variants harboring B*57/B*5801-associated A146P and T242N escape mutations in Gag is associated with lower viremia and higher CD4 cell counts in HLA-mismatched recipients, indicating that infection with attenuated HIV may be advantageous to the new host [90]. A similar study of discordant couples in Zambia reported beneficial effects of transmitted Gag, but not Nef, escape mutations [91]. The fact that intrinsic properties of the transmitted/ founder viral sequence can modulate disease progression is consistent with a recent phylogenetic analysis reporting that HIV genotype largely determines the HIV viral load set point [92].
Spontaneous HIV control
Immune-mediated attenuation of an already ‘wimpy’ virus?
If acquisition of an attenuated HIV variant confers a survival advantage, possession of protective host genetic factors further enhances this benefit. Expression of HLA-B*57 has been associated with exceptional control of HIV viremia in vertically infected infants, even when escape mutations were transmitted from mother to child, suggesting that both selection and maintenance of fitness-attenuating mutations in p24Gag contribute to the beneficial effects of this HLA allele [93]. It is worth noting, however, that vertical transmission of the B*27 R264K mutation in the p24Gag KK10 epitope is associated with rapid disease progression in the infant [55], presumably as this mutation is almost always fully compensated by the presence of the upstream S173A mutation [42].
Similarly, HIV long-term nonprogression may be explained in part by sustained immune containment of an attenuated transmitted founder virus. Members of the Sydney Blood Bank Cohort, comprised of eight hemophiliacs who acquired an attenuated Nef/LTR-deleted strain of HIV through blood transfusion between 1981 and 1984, exhibited long-term HIV control for well over a decade or more [94]. Examination of viral isolates among those who eventually progressed to disease revealed in vivo evolution towards more fit variants [94–97], whereas favorable host genetic factors such as HLA-B*57 and CCR5Δ32 heterozygosity appeared to play an important role in individuals who displayed undetectable viral loads and limited CD4 cell decline into their third decade of infection, suggesting that host and viral factors acted in an additive fashion to maintain durable control [74].
Some cases of HIV ‘elite control’ (the ability to spontaneously suppress plasma HIV RNA to below limits of clinical detection [98]) may be explained by acquisition of a ‘wimpy’ virus followed by host-driven attenuation of this founder variant [74], possibly through nonconventional mutational pathways [99]. Consistent with this hypothesis, Gag-protease recombinant viruses constructed using plasma RNA sequences obtained from 18 HIV controllers at the earliest available postinfection time point already displayed reduced replication capacity compared with isolates from acutely infected persons who subsequently failed to control viremia [100]. In this study, transmitted drug resistance, HLA-associated polymorphisms and presumed de novo selection of CTL escape mutations likely contributed to effective HIV immune control. Larger case–control studies comparing Gag-protease [101] and reverse transcriptase (RT)-integrase [102] recombinant viruses revealed significantly lower replication capacities in elite controller-derived viruses compared with those from untreated chronic progressors, indicating that fitness defects are not limited to Gag. Even greater attenuation was observed in viruses derived from elite controllers who expressed protective HLA alleles [101,102]. Enhanced immune recognition of common CTL escape variants has been reported in HIV controllers [103], and detailed analyses of escape pathways in sequences from B*57 and B*5801-expressing elite controllers revealed a correlation between rare polymorphic variants within conserved immunodominant epitopes and reduced replication capacity [99]. Although the underlying mechanism(s) for elite control are complex and remain incompletely understood, these observations support effective immune-driven attenuation of an already weakened founder variant as a correlate of this phenotype. Immune containment of HIV may be possible, therefore, if vaccine responses can be harnessed to efficiently select unconventional escape mutations with greater fitness consequences.
Implications as the epidemic continues
The potential impact of immune-mediated HIV replicative fitness defects at the population level is another key issue. The ‘HLA footprinting’ hypothesis asserts that escape mutations, particularly those that incur no fitness cost, will accumulate over time in circulating HIV sequences [35,53,55,104,105], with potentially serious consequences. If immunogenic CTL epitopes were to diminish due to escape, our capacity to elicit natural and vaccine-induced cellular immune responses would similarly wane as the epidemic progressed. The more prevalent the HLA allele selecting the mutation (and the more genetically stable the viral escape variant), the faster this phenomenon may occur. Indeed, the relatively poor contribution of common HLA class I alleles to acute-phase CTL responses suggests that enrichment for some escape variants has already occurred in circulating HIV sequences [106]. More concerning, however, is the potential for population-level accumulation of escape mutations restricted by protective HLA alleles. The B*27-associated R264K mutation, which abrogates host response to the Gag-KK10 epitope, appears stable upon transmission in the presence of the compensatory mutation S173A, leading to speculation that B*27 may cease to provide significant benefit to future generations [55–57]. Similarly, the B*51-restricted I135T mutation, located at the C-terminus of the TI8 epitope in RT, is a fitness-neutral mutation [53,54,107] whose prevalence may be increasing in some populations, most notably Japan, where B*51 allele prevalence is high [53]. Although B*51 is considered to be protective in some populations [15,23], it is no longer associated with clinical benefit in Japan [108], presumably due to loss of TI8 as an immunogenic epitope in modern sequences [53,108]. A similar phenomenon may be occurring in South Africa, where the frequency of compensatory mutations that stabilize HLA B*57/5801-associated escape mutations may be increasing [72,109], possibly foreshadowing a diminishing effect of these alleles.
A related and equally relevant question is the extent to which immune-driven defects in replicative fitness have influenced HIV pathogenesis over the epidemic’s course. HIV transmission is characterized by a severe genetic bottleneck [110–114] where a single transmitted founder virus typically establishes infection [113–116], although transmission of multiple viruses does occur less frequently [117–119]. Theoretically, this bottleneck can serve to reset viral fitness to a slightly lower level in each new host [120], allowing early CTL responses to reduce it further before compensatory mutations are selected anew [121]. In populations with relatively high immunogenetic diversity and where the majority of transmissions occur relatively early in the donor’s infection course (i.e., before compensatory mutations are selected), it is conceivable that host immune pressures could drive the gradual attenuation of virus over time [120,121]. Conversely, in populations with lower immunogenetic diversity, HIV virulence could increase over time as escape and compensatory mutations accumulate to fixation under strong directional selection [121].
Attempts have been made to study population-level changes in clinical disease markers (e.g., plasma viremia and CD4 counts) as indirect evidence for altered HIV pathogenesis over time; however, the lack of historic data, inherent limitations in conducting observational studies and changes in the technologies used to measure these parameters have made interpretation of these results challenging. Indeed, some reports suggest viral attenuation over time [120,122]; some contend that HIV may be becoming more pathogenic as the epidemic progresses [123,124]; while others report no evidence for major change [125–128]. Efforts to directly address temporal changes in replication capacity are fewer, and have yielded similarly conflicting results: one study comparing fitness in a small number of historic (1986–1989) and modern Belgian isolates supported viral attenuation [120]; while another, conducted in Amsterdam, observed the opposite effect [129]. To our knowledge, no studies have yet attempted to integrate HIV sequence, host HLA and viral fitness measurements to assess the impact of immune-driven defects on HIV pathogenesis over the epidemic’s course.
Implications for vaccine design
Our current understanding of immune-driven HIV replicative defects can be summarized as follows. After the appearance of antiviral cellular immune responses, HLA-restricted escape variants emerge under strong positive selection [130,131]. Certain CTL escape mutations occur at biologically relevant costs to viral replicative capacity [43,60,70,99,132], the effects of which may be cumulative [59,60]. These replicative defects may be fully or partially restored over time through the selection of compensatory mutations at secondary sites [17,42,62,70,133]. Moreover, in some [30,33], but certainly not all [33,55–57] cases, HLA-associated mutations selected in previous host(s) may revert to wild-type in the recipient if the latter does not express the relevant HLA allele; a process that is dependent on the fitness cost of the mutation and the presence of stabilizing compensatory mutations [17,56]. Acquisition of an attenuated founder virus can provide clinical benefit to the recipient [90,91], which may subsequently diminish upon reversion or compensation. If the recipient’s immune system is able to successfully control the incoming virus (or induce additional fitness-reducing escape mutations), these survival benefits may extend into the long term, as evidenced by the HIV controller phenotype [74,99,101].
Lessons learned from natural infection can be translated into priority vaccine research areas moving forward. In the absence of sterilizing immunity, the goal of a CTL-based vaccine will be to facilitate long-term immune containment of HIV viremia [7,134]. Analogous to the potent effects of combination antiretroviral therapy, a CTL vaccine should elicit simultaneous, early, robust and long-lasting responses against multiple epitopes in regions where immune escape is (ideally) impossible or (more realistically) tightly constrained. In this way, HIV can only achieve partial immune evasion at a substantial fitness cost. Furthermore, it is important to remember that in many cases, an individual’s CTL repertoire retains at least some ability to recognize commonly occurring escape variants through cross-reactivity [103] and/or de novo generation of variant-specific responses [135,136]. Observations that increased cross-reactive CTL correlate with control [103,137] and that ‘unconventional’ CTL escape mutations confer greater replicative defects [99] support the incorporation of common HLA-associated polymorphisms into vaccine design. Doing so may prime CTL responses against both the wild-type and the most commonly selected variant, thus driving evolution of the incoming virus down mutational pathways with greater functional consequences.
Currently, only a small number of CTL escape mutations are known to be associated with HIV attenuation, and these are biased in favor of protective HLA alleles and epitopes located in Gag. It is therefore critical to identify additional attenuation-inducing CTL escape mutations and to ensure that these epitopes are included in future HIV vaccine strategies. Of equal importance will be the definition of CTL epitopes that can escape rapidly with no cost to HIV fitness, and to ensure that these ‘decoy’ epitopes are removed from future vaccine antigens through deletion or mutation. A deeper knowledge of antigenic sequences that can elicit attenuation-inducing CTL responses by a broad array of host HLA alleles should be coupled with exciting new knowledge related to the types of vectors that best generate robust effector-memory T-cell phenotypes, including herpesvirus-based approaches [138,139]. While T cell-based vaccines are unlikely to prevent initial infection, this strategy might be able to provide lasting vaccine-mediated protection against HIV pathogenesis and progression to AIDS.
Future perspective
The observation that some CTL escape pathways may be mutually exclusive merits further attention. Mutational antagonism has been described in the context of drug resistance: for example, the K65R mutation in RT decreases susceptibility to nucleoside RT inhibitors (NRTIs) (except zidovudine), but there is an extremely high barrier to its selection in the presence of other thymidine analog mutations (e.g., T215F/Y) due to substantial functional costs to RT when these mutations occur together [140,141]. This observation has led to the strategic recommendation that certain NRTIs should be combined therapeutically to prolong antiviral control by raising the mutational barrier or driving HIV evolution down pathways that attenuate RT function [142]. Similar data are now emerging in the context of CTL escape. One recent study identified 18 antagonistic pairs of escape mutations in p24Gag that resulted in severe fitness defects, revealing strategic epitope combinations for vaccine consideration [143]. Interestingly, antagonistic mutations were generally restricted by B*57 and newly identified protective alleles B*14 and B*52 [144], suggesting that certain protective allele combinations may enhance immune control to a greater extent than the sum of their individual effects [143]. Similarly, escape pathways in two B*35-restricted Nef epitopes were found to occur together rarely in vivo and to significantly reduce Nef-mediated HLA class I downregulation activity when introduced simultaneously into a reference strain background [64]. Further work in this area could illuminate vaccine targets that exploit mutational barriers and antagonistic escape pathways for clinical benefit.
Improving our understanding of the properties of HIV founder strains is also essential. Although HIV transmission is at least to some extent influenced by frequency-dependent [145] and stochastic [146] processes, founder viruses may be enriched for properties that facilitate productive establishment of infection in the new host, which may not be the same features that confer maximum fitness in the donor virus pool [112,147]. For example, transmitted viral envelopes tend to exhibit CCR5-tropism [148], enhanced sensitivities to antibody-mediated neutralization [110], and more closely resemble ancestral rather than contemporaneous sequences in the donor [149,150]. Recent reconstructions of complete transmitted founder HIV-1 genomes are consistent with the transmission bottleneck [114,116,151], although it remains unclear whether transmitted viral sequences could share certain features related to sensitivity to cellular immune responses (e.g., particular epitope sequences). If such signatures of transmitted viruses can be elucidated, CTL vaccines might be designed that exploit these features.
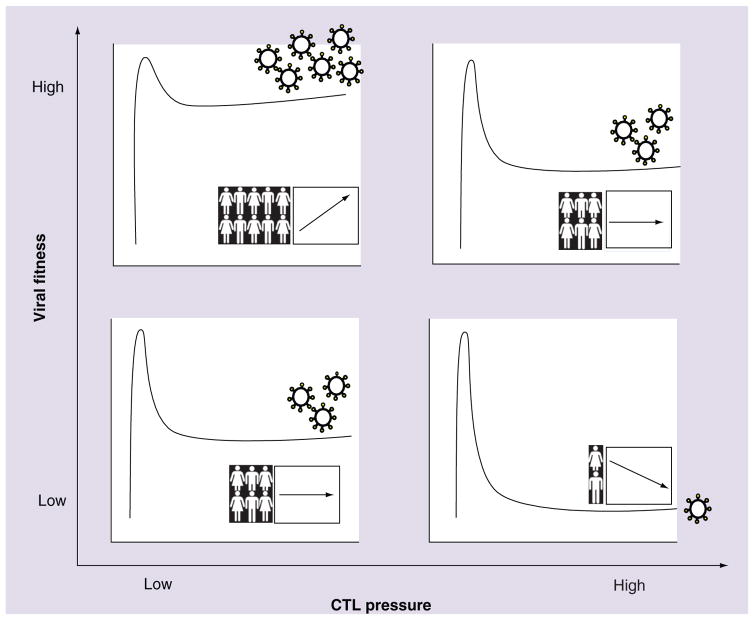
Finally, it is important to model the potential consequences of a fitness-reducing vaccine strategy for both the patient and the population. On the individual level, vaccine-induced CTL responses mediating prolonged viral control may minimize HIV-induced immune destruction and reduce the incidence of in vivo selection of compensatory mutations, although these outcomes will be largely dependent on the level of viral load suppression obtained. At the population level, vaccine-induced HIV control could reduce the risk of transmission and lead to the dissemination of attenuated viral variants in the population. Of course, any vaccine strategy designed to reduce viral fitness must acknowledge the inherent drawbacks associated with transmission of escape variants and their potential accumulation at the population level [53]. Furthermore, recent evidence that the risk of superinfection may be enhanced in individuals infected with attenuated HIV isolates raises an additional caveat [152], although this has not been consistently observed [153]. A graphic summarizing the potential individual and population-level implications of immune-mediated attenuation of HIV-1 replicative fitness is presented in Figure 1.
Figure 1. Individual and population-level implications of immune-mediated attenuation of HIV-1.
The extent to which immune-mediated fitness defects influence the clinical course of HIV infection, and the potential implications of immune-mediated HIV attenuation to the future of the epidemic, depend on various factors including the relative fitness of the variant acquired at transmission (y-axis) as well as the effectiveness of the host CTL response (x-axis). We present four simplified, hypothetical scenarios illustrating these combined effects on outcome for the individual (illustrated here as the kinetic curves of acute-phase HIV viremia and subsequent set point stabilization) and for the population (graph insets illustrating hypothetical trends in HIV incidence [human figures] and pathogenesis [arrows] over time). Upper-left quadrant: high transmitted viral fitness combined with poor host CTL responses yields high viral load set points in infected individuals. Upper-left quadrant inset: continued transmission of highly fit variants in the absence of immune selection pressure could result in increasing HIV pathogenesis (and potentially incidence) over the epidemic’s course. Lower-right quadrant: low transmitted viral fitness combined with highly effective host CTL responses yields successful immune control of HIV-1. Lower-right quadrant inset: continued passage of attenuated variants through hosts capable of mounting highly effective natural (or vaccine-induced) immune responses could result in decreasing HIV pathogenesis (and potentially decreased transmission risk) over time. Upper-right quadrant (and lower-left quadrant), plus insets: intermediate average plasma viral loads and stable population-level outcomes are envisioned in cases of high transmitted viral fitness and effective CTL responses (and vice versa).
CTL: Cytotoxic T lymphocyte.
Our ultimate goal of a global HIV-1 vaccine remains a major challenge. Evidence from natural infection supports biologically and clinically relevant attenuation of HIV-1 replicative fitness through immune escape at certain key epitopes. Vaccine-induced stimulation of effective immune responses against highly conserved, mutationally constrained epitopes, where escape could only occur at substantial replicative costs, represents a worthwhile strategy. Such a vaccine could ‘channel’ HIV-1 evolution down fitness-reducing pathways, thus lowering the viral load set point, attenuating the infection course and potentially reducing the risk of transmission. Continued improvement in our understanding of the individual and population-level consequences of CTL escape and compensation on HIV replicative fitness will be essential to achieve this goal.
Executive summary.
Impact of host HLA on HIV-1 fitness: a review of the evidence
HLA-restricted cytotoxic T lymphocyte (CTL) responses drive the selection of immune escape mutations that can reduce HIV-1 fitness.
In general, fitness-reducing escape mutations occur in conserved viral regions (e.g., p24Gag) and are selected by ‘protective’ HLA class I alleles (e.g., B*27 and B*57), although exceptions apply.
Immune-driven fitness defects may be at least partially restored by the selection of secondary (‘compensatory’) mutations over the disease course.
Biological & clinical benefits of immune-driven mutations
Transmission of immune-attenuated HIV-1 may provide clinical benefit to recipients, however this benefit may diminish over time due to reversion of attenuating mutations and/or evolution to fitter forms.
Long-term HIV control is in some cases mediated by prolonged immune containment or further host-driven attenuation of an already weakened transmitted viral variant, suggesting that host and viral factors are additively responsible for durable control.
Implications as the epidemic continues
Escape mutations selected by common HLA alleles (or those stabilized by compensatory mutations) may accumulate at the population level, possibly resulting in diminishing natural and vaccine-induced CTL responses to key immunogenic epitopes as the epidemic progresses.
The consequences of immune-driven attenuation on HIV-1 pathogenesis over the epidemic’s course remains a critical question.
Implications for vaccine design
Targeting immune responses to conserved regions raises the mutational barrier to escape, yielding sustained immune recognition or escape at high fitness costs.
Such a ‘fitness-reducing’ vaccine strategy could theoretically reduce the viral set point, slow disease progression and reduce HIV-1 transmission risk.
Priming the CTL response to both wild-type and common escape variants may facilitate evolution of nonconventional escape mutations with greater fitness costs.
Future perspective
Elucidation of antagonistic combinatorial mutational pathways resulting in severe fitness defects could be exploited for vaccine design.
Identification of signature features of transmitted viruses could reveal novel vaccine targets.
Population-level accumulation of immune escape variants represents a major potential consequence that would need to be considered.
To design a fitness-reducing HIV vaccine, greater understanding of immune-driven fitness costs and compensation at both the individual and population levels is required.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
We gratefully acknowledge research support from the Canadian Institutes for Health Research (CIHR), Simon Fraser University and Microsoft Research. DRC is a recipient of the Canada-HOPE fellowship from CIHR and Sanofi-Aventis. JK Wright is funded by the National Research Foundation of South Africa. MA Brockman is a Canada Research Chair, Tier 2. ZL Brumme is supported by a CIHR New Investigator Award. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mayer KH, Venkatesh KK. Antiretroviral therapy as HIV prevention: status and prospects. Am J Public Health. 2010;100(10):1867–1876. doi: 10.2105/AJPH.2009.184796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320(5877):760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 6.Berkley S, Bertram K, Delfraissy JF, et al. The 2010 scientific strategic plan of the Global HIV Vaccine Enterprise. Nat Med. 2010;16(9):981–989. doi: 10.1038/nm0910-981. [DOI] [PubMed] [Google Scholar]
- 7.Johnston MI, Fauci AS. An HIV vaccine – challenges and prospects. N Engl J Med. 2008;359(9):888–890. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- 8.HIV vaccine failure prompts Merck to halt trial. Nature. 2007;449(7161):390. doi: 10.1038/449390c. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald DW, Janes H, Robertson M, et al. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the STEP study) J Infect Dis. 2011;203(6):765–772. doi: 10.1093/infdis/jiq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪.Rolland M, Tovanabutra S, Decamp AC, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17(3):366–371. doi: 10.1038/nm.2316. Breakthrough HIV-1 sequences in recipients of a cytotoxic T lymphocyte (CTL)-based vaccine diverged from the vaccine epitope sequences to a greater extent compared with those of placebo recipients, suggesting that the vaccine blocked infection by variants most similar to the vaccine sequence. This represented the first evidence of selective pressure from vaccine-induced CTL responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts MR, Exley B, Price DA, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci USA. 2005;102(12):4512–4517. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 14.Costello C, Tang J, Rivers C, et al. HLA-B*5703 independently associated with slower HIV-1 disease progression in Rwandan women. AIDS. 1999;13(14):1990–1991. doi: 10.1097/00002030-199910010-00031. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien SJ, Gao X, Carrington M. HLA and AIDS: a cautionary tale. Trends Mol Med. 2001;7(9):379–381. doi: 10.1016/s1471-4914(01)02131-1. [DOI] [PubMed] [Google Scholar]
- 16.Altfeld M, Kalife ET, Qi Y, et al. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med. 2006;3(10):E403. doi: 10.1371/journal.pmed.0030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford H, Prado JG, Leslie A, et al. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J Virol. 2007;81(15):8346–8351. doi: 10.1128/JVI.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulder PJ, Bunce M, Krausa P, et al. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12(18):1691–1698. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- 19.Walker BD, Chakrabarti S, Moss B, et al. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 20.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 23.Kaslow RA, Carrington M, Apple R, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2(4):405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 24.Phillips RE, Rowland-Jones S, Nixon DF, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 25.Rowland-Jones SL, Phillips RE, Nixon DF, et al. Human immunodeficiency virus variants that escape cytotoxic T-cell recognition. AIDS Res Hum Retroviruses. 1992;8(8):1353–1354. doi: 10.1089/aid.1992.8.1353. [DOI] [PubMed] [Google Scholar]
- 26.Klenerman P, Rowland-Jones S, McAdam S, et al. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 1994;369(6479):403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 27.Price DA, Goulder PJ, Klenerman P, et al. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94(5):1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulder PJ, Walker BD. The great escape – AIDS viruses and immune control. Nat Med. 1999;5(11):1233–1235. doi: 10.1038/15184. [DOI] [PubMed] [Google Scholar]
- 29.Goulder PJ, Phillips RE, Colbert RA, et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3(2):212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 30.Allen TM, Altfeld M, Yu XG, et al. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J Virol. 2004;78(13):7069–7078. doi: 10.1128/JVI.78.13.7069-7078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draenert R, Le Gall S, Pfafferott KJ, et al. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J Exp Med. 2004;199(7):905–915. doi: 10.1084/jem.20031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borrow P, Lewicki H, Wei X, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3(2):205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 33.Leslie AJ, Pfafferott KJ, Chetty P, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10(3):282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 34.Allen TM, Altfeld M, Geer SC, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol. 2005;79(21):13239–13249. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296(5572):1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau CM, Daniels MG, Carlson JM, et al. HLA Class-I driven evolution of human immunodeficiency virus type 1 subtype C proteome: immune escape and viral load. J Virol. 2008;82(13):6434–6446. doi: 10.1128/JVI.02455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brumme ZL, Brumme CJ, Heckerman D, et al. Evidence of differential HLA class I-mediated viral evolution in functional and accessory/regulatory genes of HIV-1. PLoS Pathog. 2007;3(7):e94. doi: 10.1371/journal.ppat.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brumme ZL, John M, Carlson JM, et al. HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef Proteins. PLoS One. 2009;4(8):E6687. doi: 10.1371/journal.pone.0006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John M, Heckerman D, James I, et al. Adaptive interactions between HLA and HIV-1: highly divergent selection imposed by HLA class I molecules with common supertype motifs. J Immunol. 2010;184(8):4368–4377. doi: 10.4049/jimmunol.0903745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, McNevin J, Zhao H, et al. Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: fitness-balanced escape. J Virol. 2007;81(22):12179–12188. doi: 10.1128/JVI.01277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brockman MA, Schneidewind A, Lahaie M, et al. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J Virol. 2007;81(22):12608–12618. doi: 10.1128/JVI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneidewind A, Brockman MA, Yang R, et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007;81(22):12382–12393. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Picado J, Prado JG, Fry EE, et al. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol. 2006;80(7):3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey JR, Zhang H, Wegweiser BW, et al. Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J Infect Dis. 2007;196(1):50–55. doi: 10.1086/518515. [DOI] [PubMed] [Google Scholar]
- 45▪.Troyer RM, McNevin J, Liu Y, et al. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog. 2009;5(4):E1000365. doi: 10.1371/journal.ppat.1000365. CTL escape mutations in conserved Gag epitopes significantly reduced HIV-1 fitness, whereas mutations in the variable Env epitopes tended to be fitness-neutral. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altfeld M, Allen TM. Hitting HIV where it hurts: an alternative approach to HIV vaccine design. Trends Immunol. 2006;27(11):504–510. doi: 10.1016/j.it.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Allen TM, Altfeld M. Crippling HIV one mutation at a time. J Exp Med. 2008;205(5):1003–1007. doi: 10.1084/jem.20080569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3(11):E157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedrich TC, Dodds EJ, Yant LJ, et al. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat Med. 2004;10(3):275–281. doi: 10.1038/nm998. [DOI] [PubMed] [Google Scholar]
- 50.Treurnicht FK, Seoighe C, Martin DP, et al. Adaptive changes in HIV-1 subtype C proteins during early infection are driven by changes in HLA-associated immune pressure. Virology. 2010;396(2):213–225. doi: 10.1016/j.virol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews PC, Prendergast A, Leslie A, et al. Central role of reverting mutations in HLA associations with human immunodeficiency virus set point. J Virol. 2008;82(17):8548–8559. doi: 10.1128/JVI.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frater AJ, Brown H, Oxenius A, et al. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J Virol. 2007;81(12):6742–6751. doi: 10.1128/JVI.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Kawashima Y, Pfafferott K, Frater J, et al. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 2009;458(7238):641–645. doi: 10.1038/nature07746. CTL-driven escape mutations restricted by prevalent HLA alleles may be accumulating over time in the population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brumme ZL, Brumme CJ, Carlson J, et al. Marked epitope and allele-specific differences in rates of mutation in HIV-1 Gag, Pol and Nef CTL epitopes in acute/early HIV-1 infection. J Virol. 2008;82(18):9216–9227. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goulder PJ, Brander C, Tang Y, et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001;412(6844):334–338. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- 56.Schneidewind A, Brumme ZL, Brumme CJ, et al. Transmission and long-term stability of compensated CD8 escape mutations. J Virol. 2009;83(8):3993–3997. doi: 10.1128/JVI.01108-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornelissen M, Hoogland FM, Back NK, et al. Multiple transmissions of a stable human leukocyte antigen-B27 cytotoxic T-cell-escape strain of HIV-1 in the Netherlands. AIDS. 2009;23(12):1495–1500. doi: 10.1097/QAD.0b013e32832d9267. [DOI] [PubMed] [Google Scholar]
- 58.Schneidewind A, Brockman MA, Sidney J, et al. Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J Virol. 2008;82(11):5594–5605. doi: 10.1128/JVI.02356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boutwell CL, Rowley CF, Essex M. Reduced viral replication capacity of human immunodeficiency virus type 1 subtype C caused by cytotoxic-T-lymphocyte escape mutations in HLA-B57 epitopes of capsid protein. J Virol. 2009;83(6):2460–2468. doi: 10.1128/JVI.01970-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crawford H, Lumm W, Leslie A, et al. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J Exp Med. 2009;206(4):909–921. doi: 10.1084/jem.20081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peyerl FW, Bazick HS, Newberg MH, Barouch DH, Sodroski J, Letvin NL. Fitness costs limit viral escape from cytotoxic T lymphocytes at a structurally constrained epitope. J Virol. 2004;78(24):13901–13910. doi: 10.1128/JVI.78.24.13901-13910.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelleher AD, Long C, Holmes EC, et al. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J Exp Med. 2001;193(3):375–386. doi: 10.1084/jem.193.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prado JG, Honeyborne I, Brierley I, Puertas MC, Martinez-Picado J, Goulder PJ. Functional consequences of human immunodeficiency virus escape from an HLA-B*13-restricted CD8+ T-cell epitope in p1 Gag protein. J Virol. 2009;83(2):1018–1025. doi: 10.1128/JVI.01882-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueno T, Motozono C, Dohki S, et al. CTL-mediated selective pressure influences dynamic evolution and pathogenic functions of HIV-1 Nef. J Immunol. 2008;180(2):1107–1116. doi: 10.4049/jimmunol.180.2.1107. [DOI] [PubMed] [Google Scholar]
- 65.Matthews PC, Adland E, Listgarten J, et al. HLA-A*7401-mediated control of HIV viremia is independent of its linkage disequilibrium with HLA-B*5703. J Immunol. 2011;186(10):5675–5686. doi: 10.4049/jimmunol.1003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honeyborne I, Codoner FM, Leslie A, et al. HLA-Cw*03-restricted CD8+ T-cell responses targeting the HIV-1 gag major homology region drive virus immune escape and fitness constraints compensated for by intracodon variation. J Virol. 2010;84(21):11279–11288. doi: 10.1128/JVI.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolland M, Carlson JM, Manocheewa S, et al. Amino-acid co-variation in HIV-1 Gag subtype C: HLA-mediated selection pressure and compensatory dynamics. PLoS One. 2010;5(9):E12463. doi: 10.1371/journal.pone.0012463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poon AF, Swenson LC, Dong WW, et al. Phylogenetic analysis of population-based and deep sequencing data to identify coevolving sites in the NEF gene of HIV-1. Mol Biol Evol. 2009;27(4):819–832. doi: 10.1093/molbev/msp289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlson JM, Brumme ZL, Rousseau CM, et al. Phylogenetic dependency networks: inferring patterns of CTL escape and codon covariation in HIV-1 Gag. PLoS Comput Biol. 2008;4(11):E1000225. doi: 10.1371/journal.pcbi.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70▪.Brockman MA, Brumme ZL, Brumme CJ, et al. Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated for in chronic infection. J Virol. 2010;84(22):11937–11949. doi: 10.1128/JVI.01086-10. Immune escape mutations in subtype B Gag-protease sequences selected by protective HLA class I alleles were associated with fitness defects in acute/ early, but not chronic infection, suggesting that replicative defects are largely compensated over the disease course. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright JK, Brumme ZL, Carlson JM, et al. Gag-protease-mediated replication capacity in HIV-1 subtype C chronic infection: associations with HLA type and clinical parameters. J Virol. 2010;84(20):10820–10831. doi: 10.1128/JVI.01084-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright JK, Novitsky V, Brockman MA, et al. Influence of Gag-protease-mediated replication capacity on disease progression in individuals recently infected with HIV-1 subtype C. J Virol. 2011;85(8):3996–4006. doi: 10.1128/JVI.02520-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang KH, Goedhals D, Carlson JM, et al. Progression to AIDS in South Africa is associated with both reverting and compensatory viral mutations. PLoS One. 2011;6(4):E19018. doi: 10.1371/journal.pone.0019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Lobritz MA, Lassen KG, Arts EJ. HIV-1 replicative fitness in elite controllers. Curr Opin HIV AIDS. 2011;6(3):214–220. doi: 10.1097/COH.0b013e3283454cf5. Elite control may be explained by the acquisition of an attenuated virus in combination with further host-driven attenuation and favorable host genetic factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peyerl FW, Barouch DH, Yeh WW, et al. Simian-human immunodeficiency virus escape from cytotoxic T-lymphocyte recognition at a structurally constrained epitope. J Virol. 2003;77(23):12572–12578. doi: 10.1128/JVI.77.23.12572-12578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeh WW, Cale EM, Jaru-Ampornpan P, Lord CI, Peyerl FW, Letvin NL. Compensatory substitutions restore normal core assembly in simian immunodeficiency virus isolates with Gag epitope cytotoxic T-lymphocyte escape mutations. J Virol. 2006;80(16):8168–8177. doi: 10.1128/JVI.00068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Connor DH, Allen TM, Vogel TU, et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat Med. 2002;8(5):493–499. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 78.Matano T, Kobayashi M, Igarashi H, et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J Exp Med. 2004;199(12):1709–1718. doi: 10.1084/jem.20040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez CS, Stratov I, De Rose R, et al. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J Virol. 2005;79(9):5721–5731. doi: 10.1128/JVI.79.9.5721-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loh L, Petravic J, Batten CJ, Davenport MP, Kent SJ. Vaccination and timing influence SIV immune escape viral dynamics in vivo. PLoS Pathog. 2008;4(1):E12. doi: 10.1371/journal.ppat.0040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawada M, Tsukamoto T, Yamamoto H, et al. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol. 2008;82(20):10199–10206. doi: 10.1128/JVI.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turner D, Brenner BG, Routy JP, Petrella M, Wainberg MA. Rationale for maintenance of the M184v resistance mutation in human immunodeficiency virus type 1 reverse transcriptase in treatment experienced patients. New Microbiol. 2004;27(2 Suppl 1):31–39. [PubMed] [Google Scholar]
- 83.Wainberg MA. The impact of the M184V substitution on drug resistance and viral fitness. Expert Rev Anti Infect Ther. 2004;2(1):147–151. doi: 10.1586/14787210.2.1.147. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Dykes C, Domaoal RA, Koval CE, Bambara RA, Demeter LM. The HIV-1 reverse transcriptase mutants G190S and G190A, which confer resistance to non-nucleoside reverse transcriptase inhibitors, demonstrate reductions in RNase H activity and DNA synthesis from tRNA(Lys, 3) that correlate with reductions in replication efficiency. Virology. 2006;348(2):462–474. doi: 10.1016/j.virol.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quinones-Mateu ME, Tadele M, Parera M, et al. Insertions in the reverse transcriptase increase both drug resistance and viral fitness in a human immunodeficiency virus type 1 isolate harboring the multi-nucleoside reverse transcriptase inhibitor resistance 69 insertion complex mutation. J Virol. 2002;76(20):10546–10552. doi: 10.1128/JVI.76.20.10546-10552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Croteau G, Doyon L, Thibeault D, McKercher G, Pilote L, Lamarre D. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J Virol. 1997;71(2):1089–1096. doi: 10.1128/jvi.71.2.1089-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paredes R, Sagar M, Marconi VC, et al. In vivo fitness cost of the M184V mutation in multidrug-resistant human immunodeficiency virus type 1 in the absence of lamivudine. J Virol. 2009;83(4):2038–2043. doi: 10.1128/JVI.02154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armand-Ugon M, Quinones-Mateu ME, Gutierez A, et al. Reduced fitness of HIV-1 resistant to CXCR4 antagonists. Antivir Ther. 2003;8(1):1–8. [PubMed] [Google Scholar]
- 89.Lu J, Sista P, Giguel F, Greenberg M, Kuritzkes DR. Relative replicative fitness of human immunodeficiency virus type 1 mutants resistant to enfuvirtide (T-20) J Virol. 2004;78(9):4628–4637. doi: 10.1128/JVI.78.9.4628-4637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chopera DR, Woodman Z, Mlisana K, et al. Transmission of HIV-1 CTL escape variants provides HLA-mismatched recipients with a survival advantage. PLoS Pathog. 2008;4(3):E1000033. doi: 10.1371/journal.ppat.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91▪.Goepfert PA, Lumm W, Farmer P, et al. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med. 2008;205(5):1009–1017. doi: 10.1084/jem.20072457. Increasing numbers of HLA-B-associated escape mutations in or adjacent to Gag epitopes of transmitted viruses conferred a benefit to recipients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92▪.Alizon S, von Wyl V, Stadler T, et al. Phylogenetic approach reveals that virus genotype largely determines HIV set-point viral load. PLoS Pathog. 2010;6(9):E1001123. doi: 10.1371/journal.ppat.1001123. Viral genotype is a major contributor to viral load set point. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneidewind A, Tang Y, Brockman MA, et al. Maternal transmission of human immunodeficiency virus escape mutations subverts HLA-B57 immunodominance but facilitates viral control in the haploidentical infant. J Virol. 2009;83(17):8616–8627. doi: 10.1128/JVI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Learmont JC, Geczy AF, Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340(22):1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 95.Birch MR, Learmont JC, Dyer WB, et al. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC) J Clin Virol. 2001;22(3):263–270. doi: 10.1016/s1386-6532(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 96.Rhodes D, Solomon A, Bolton W, et al. Identification of a new recipient in the Sydney Blood Bank Cohort: a long-term HIV type 1-infected seroindeterminate individual. AIDS Res Hum Retroviruses. 1999;15(16):1433–1439. doi: 10.1089/088922299309946. [DOI] [PubMed] [Google Scholar]
- 97.Zaunders J, Dyer WB, Churchill M. The Sydney Blood Bank Cohort: implications for viral fitness as a cause of elite control. Curr Opin HIV AIDS. 2011;6(3):151–156. doi: 10.1097/COH.0b013e3283454d5b. [DOI] [PubMed] [Google Scholar]
- 98.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 99▪.Miura T, Brockman MA, Schneidewind A, et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J Virol. 2009;83(6):2743–2755. doi: 10.1128/JVI.02265-08. HLA-B*57/5801-positive elite controllers select nonconventional escape mutations in Gag with significant fitness costs and possess the ability to mount strong immune responses to these mutated epitopes, indicating that both these mechanisms are important for durable viral control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100▪.Miura T, Brumme ZL, Brockman MA, et al. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J Virol. 2010;84(15):7581–7591. doi: 10.1128/JVI.00286-10. Recombinant viruses encoding acute/early Gag-protease sequences from individuals who subsequently became elite controllers were attenuated due to transmitted drug resistance mutations and/or transmitted (or potentially newly selected) HLA-driven mutations, indicating that early virus attenuation may contribute to viral control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miura T, Brockman MA, Brumme ZL, et al. HLA-associated alterations in replication capacity of chimeric NL4–3 viruses carrying gag-protease from elite controllers of human immunodeficiency virus type 1. J Virol. 2009;83(1):140–149. doi: 10.1128/JVI.01471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brumme ZL, Li C, Miura T, et al. Reduced replication capacity of NL4–3 recombinant viruses encoding reverse transcriptase-integrase sequences from HIV-1 elite controllers. J Acquir Immune Defic Syndr. 2011;56(2):100–108. doi: 10.1097/QAI.0b013e3181fe9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turnbull EL, Lopes AR, Jones NA, et al. HIV-1 epitope-specific CD8+ T cell responses strongly associated with delayed disease progression cross-recognize epitope variants efficiently. J Immunol. 2006;176(10):6130–6146. doi: 10.4049/jimmunol.176.10.6130. [DOI] [PubMed] [Google Scholar]
- 104.Brander C, Walker BD. Gradual adaptation of HIV to human host populations: good or bad news? Nat Med. 2003;9(11):1359–1362. doi: 10.1038/nm941. [DOI] [PubMed] [Google Scholar]
- 105.Leslie A, Kavanagh D, Honeyborne I, et al. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J Exp Med. 2005;201(6):891–902. doi: 10.1084/jem.20041455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Streeck H, Jolin JS, Qi Y, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009;83(15):7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawashima Y, Kuse N, Gatanaga H, et al. Long-term control of HIV-1 in hemophiliacs carrying slow-progressing allele HLA-B*5101. J Virol. 2010;84(14):7151–7160. doi: 10.1128/JVI.00171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koga M, Kawana-Tachikawa A, Heckerman D, et al. Changes in impact of HLA class I allele expression on HIV-1 plasma virus loads at a population level over time. Microbiol Immunol. 2010;54(4):196–205. doi: 10.1111/j.1348-0421.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- 109.Chopera DR, Mlotshwa M, Woodman Z, et al. Virological and immunological factors associated with HIV-1 differential disease progression in HLA-B*58:01 positive individuals. J Virol. 2011;85(14):7070–7080. doi: 10.1128/JVI.02543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Derdeyn CA, Decker JM, Bibollet-Ruche F, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303(5666):2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 111.Derdeyn CA, Hunter E. Viral characteristics of transmitted HIV. Curr Opin HIV AIDS. 2008;3(1):16–21. doi: 10.1097/COH.0b013e3282f2982c. [DOI] [PubMed] [Google Scholar]
- 112.Keele BF, Derdeyn CA. Genetic and antigenic features of the transmitted virus. Curr Opin HIV AIDS. 2009;4(5):352–357. doi: 10.1097/COH.0b013e32832d9fef. [DOI] [PubMed] [Google Scholar]
- 113.Salazar-Gonzalez JF, Bailes E, Pham KT, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol. 2008;82(8):3952–3970. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/ founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206(6):1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herbeck JT, Rolland M, Liu Y, et al. Demographic processes affect HIV-1 evolution in primary infection before the onset of selective processes. J Virol. 2011 doi: 10.1128/JVI.02697-10. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bar KJ, Li H, Chamberland A, et al. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J Virol. 2010;84(12):6241–6247. doi: 10.1128/JVI.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83(8):3556–3567. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Bar KJ, Wang S, et al. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 2010;6(5):E1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arien KK, Troyer RM, Gali Y, Colebunders RL, Arts EJ, Vanham G. Replicative fitness of historical and recent HIV-1 isolates suggests HIV-1 attenuation over time. AIDS. 2005;19(15):1555–1564. doi: 10.1097/01.aids.0000185989.16477.91. [DOI] [PubMed] [Google Scholar]
- 121.Arien KK, Vanham G, Arts EJ. Is HIV-1 evolving to a less virulent form in humans? Nat Rev Microbiol. 2007;5(2):141–151. doi: 10.1038/nrmicro1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keet IP, Veugelers PJ, Koot M, et al. Temporal trends of the natural history of HIV-1 infection following seroconversion between 1984 and 1993. AIDS. 1996;10(13):1601–1602. doi: 10.1097/00002030-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 123.Gras L, Jurriaans S, Bakker M, et al. Viral load levels measured at set-point have risen over the last decade of the HIV epidemic in The Netherlands. PLoS One. 2009;4(10):E7365. doi: 10.1371/journal.pone.0007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dorrucci M, Rezza G, Porter K, Phillips A. Temporal trends in postseroconversion CD4 cell count and HIV load: the concerted action on seroconversion to AIDS and death in Europe collaboration, 1985–2002. J Infect Dis. 2007;195(4):525–534. doi: 10.1086/510911. [DOI] [PubMed] [Google Scholar]
- 125.Muller V, Ledergerber B, Perrin L, et al. Stable virulence levels in the HIV epidemic of Switzerland over two decades. AIDS. 2006;20(6):889–894. doi: 10.1097/01.aids.0000218553.51908.6b. [DOI] [PubMed] [Google Scholar]
- 126.Time from HIV-1 seroconversion to AIDS death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355(9210):1131–1137. [PubMed] [Google Scholar]
- 127.Troude P, Chaix ML, Tran L, et al. No evidence of a change in HIV-1 virulence since 1996 in France. AIDS. 2009;23(10):1261–1267. doi: 10.1097/QAD.0b013e32832b51ef. [DOI] [PubMed] [Google Scholar]
- 128.Herbeck JT, Gottlieb GS, Li X, et al. Lack of Evidence for Changing Virulence of HIV-1 in North America. PLoS One. 2008;3(2):E1525. doi: 10.1371/journal.pone.0001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gali Y, Berkhout B, Vanham G, Bakker M, Back NK, Arien KK. Survey of the temporal changes in HIV-1 replicative fitness in the Amsterdam Cohort. Virology. 2007;364(1):140–146. doi: 10.1016/j.virol.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 130.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206(6):1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Y, McNevin J, Cao J, et al. Selection on the human immunodeficiency virus type 1 proteome following primary infection. J Virol. 2006;80(19):9519–9529. doi: 10.1128/JVI.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Navis M, Schellens I, van Baarle D, et al. Viral replication capacity as a correlate of HLA B57/B5801-associated nonprogressive HIV-1 infection. J Immunol. 2007;179(5):3133–3143. doi: 10.4049/jimmunol.179.5.3133. [DOI] [PubMed] [Google Scholar]
- 133.Davis BH, Poon AF, Whitlock MC. Compensatory mutations are repeatable and clustered within proteins. Proc Biol Sci. 2009;276(1663):1823–1827. doi: 10.1098/rspb.2008.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barouch DH, Korber B. HIV-1 vaccine development after STEP. Annu Rev Med. 2010;61:153–167. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Allen TM, Yu XG, Kalife ET, et al. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J Virol. 2005;79(20):12952–12960. doi: 10.1128/JVI.79.20.12952-12960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feeney ME, Tang Y, Pfafferott K, et al. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J Immunol. 2005;174(12):7524–7530. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 137.Gillespie GM, Kaul R, Dong T, et al. Cross-reactive cytotoxic T lymphocytes against a HIV-1 p24 epitope in slow progressors with B*57. AIDS. 2002;16(7):961–972. doi: 10.1097/00002030-200205030-00002. [DOI] [PubMed] [Google Scholar]
- 138.Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parikh UM, Bacheler L, Koontz D, Mellors JW. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J Virol. 2006;80(10):4971–4977. doi: 10.1128/JVI.80.10.4971-4977.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frankel FA, Invernizzi CF, Oliveira M, Wainberg MA. Diminished efficiency of HIV-1 reverse transcriptase containing the K65R and M184V drug resistance mutations. AIDS. 2007;21(6):665–675. doi: 10.1097/QAD.0b013e3280187505. [DOI] [PubMed] [Google Scholar]
- 142.Brenner BG, Coutsinos D. The K65R mutation in HIV-1 reverse transcriptase: genetic barriers, resistance profile and clinical implications. HIV Ther. 2009;3(6):583–594. doi: 10.2217/hiv.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143▪.Wang Y, Oniangue-Ndza C, Schneidewind A, et al. Control of HIV viral replication mediated by the synergistic effects of unique combinations of HLA class I alleles. Presented at: Keystone Symposium on Protection from HIV: Targeted Intervention Strategies.; Whistler, BC, Canada. 20–25 March 2011; Identification of pairs of non-covarying CTL escape mutations that may be particularly deleterious in combination and could therefore be simultaneously targeted by vaccines. [Google Scholar]
- 144.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frater AJ, Edwards CT, McCarthy N, et al. Passive sexual transmission of human immunodeficiency virus type 1 variants and adaptation in new hosts. J Virol. 2006;80(14):7226–7234. doi: 10.1128/JVI.02014-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marks AJ, Pillay D, McLean AR. The effect of intrinsic stochasticity on transmitted HIV drug resistance patterns. J Theor Biol. 2010;262(1):1–13. doi: 10.1016/j.jtbi.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 147.Sagar M. HIV-1 transmission biology: selection and characteristics of infecting viruses. J Infect Dis. 2010;202(Suppl 2):S289–S296. doi: 10.1086/655656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 149.Sagar M, Laeyendecker O, Lee S, et al. Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis. 2009;199(4):580–589. doi: 10.1086/596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herbeck JT, Nickle DC, Learn GH, et al. Human immunodeficiency virus type 1 ENV evolves toward ancestral states upon transmission to a new host. J Virol. 2006;80(4):1637–1644. doi: 10.1128/JVI.80.4.1637-1644.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fischer W, Ganusov VV, Giorgi EE, et al. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One. 2010;5(8):E12303. doi: 10.1371/journal.pone.0012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Kuyl AC, Kozaczynska K, Arien KK, et al. Analysis of infectious virus clones from two HIV-1 superinfection cases suggests that the primary strains have lower fitness. Retrovirology. 2010;7:60. doi: 10.1186/1742-4690-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang OO, Daar ES, Jamieson BD, et al. Human immunodeficiency virus type 1 clade B superinfection: evidence for differential immune containment of distinct clade B strains. J Virol. 2005;79(2):860–868. doi: 10.1128/JVI.79.2.860-868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.UNAIDS/WHO . AIDS Epidemic Update. 2010 www.unaids.org/globalreport.