Abstract
The role of gelsolin, a calcium-dependent actin-severing protein, in mediating collagen phagocytosis, is not defined. We examined α2β1 integrin-mediated phagocytosis in fibroblasts from wild-type (WT) and gelsolin knockout (Gsn-) mice. After initial contact with collagen beads, collagen binding and internalization were 60% lower in Gsn- than WT cells. This deficiency was restored by transfection with gelsolin or with β1 integrin-activating antibodies. WT cells showed robust rac activation and increased [Ca2+]i during early contact with collagen beads, but Gsn- cells showed very limited responses. Transfected gelsolin in Gsn- cells restored rac activation after collagen binding. Transfection of Gsn- cells with active rac increased collagen binding to WT levels. Chelation of intracellular calcium inhibited collagen binding and rac activation, whereas calcium ionophore induced rac activation in WT and Gsn- cells. We conclude that the ability of gelsolin to remodel actin filaments is important for collagen-induced calcium entry; calcium in turn is required for rac activation, which subsequently enhances collagen binding to unoccupied α2β1 integrins.
INTRODUCTION
Phagocytosis is essential for the uptake and degradation of microorganisms and damaged cells but is also a critical process in remodeling of connective tissue matrices. In fibroblasts, the remodeling of the extracellular matrix by phagocytosis of collagen fibrils is dependent on the α2β1 integrin (Lee et al., 1996; Arora et al., 2000), a collagen receptor that mediates initial cellular recognition and binding to matrix collagens. Because initial particle attachment to surface receptors triggers actin-mediated binding and internalization in many cell types (Tjelle et al., 2000), it is likely that actin rearrangements in collagen phagocytic processes depend on actin-severing and -capping proteins of which gelsolin is a well-characterized example (Weeds and Maciver, 1993). Notably, the type of receptors involved in various phagocytic processes is a crucial determinant of phagocytic mechanisms because gelsolin plays an important role in FcγR-mediated phagocytosis but not in complement-receptor-mediated phagocytosis (Serrander et al., 2000). Currently, the role of gelsolin in the initial collagen binding steps of α2β1 integrin-mediated phagocytosis is not defined.
We have developed a model of collagen phagocytosis in which ventral loading of cells on collagen-coated beads facilitates studies of the initial binding and spreading steps of phagocytosis (Arora et al., 2003). In this context, the cell spreading processes associated with collagen phagocytosis are likely dependent on activation of rac and cdc42 (Price et al., 1998), small GTPases that regulate the extension of filopodia and lamellipodia and whose effector interactions are precisely localized at integrin binding sites (Del Pozo et al., 2002). Downstream of rac in the pathways leading to actin assembly and cell spreading, actin-binding proteins such as gelsolin regulate filament length by capping the fast-growing end and by severing filaments under the control of calcium ions and phosphoinositides (Janmey and Stossel, 1987; Hartwig et al., 1995). Indeed, previous studies have shown that there is a tight interactive relationship between rac and gelsolin in the control of actin assembly and spreading (Azuma et al., 1998). Early passage cells from gelsolin knockout animals exhibit reduced motility and compensatory overexpression of rac, characteristics that are normalized when gelsolin is reexpressed in these cells. Thus, gelsolin levels may regulate rac expression (Azuma et al., 1998). Furthermore, whereas recruitment of rac to the cell membrane is similar in both cell types, downstream events are impeded in the Gsn- cells (Azuma et al., 1998). However, it is not known whether gelsolin impacts on rac activation and whether gelsolin and rac activation regulate collagen phagocytosis.
We have assessed the influence of gelsolin on rac activation and how this process in turn regulates the function of collagen receptors in phagocytosis. We examined these pathways with fibroblasts from gelsolin-null and matched background, wild-type mice. Notably, because rac activation and gelsolin-induced severing of actin are determined in part by calcium ion flux (Janmey and Stossel, 1987; Stossel 1993; Lian et al., 2001), we considered that the altered organization of subcortical actin filaments in gelsolin null cells (Witke et al., 1995) would impact on calcium signaling induced by collagen binding to integrins. In this context, the binding step of collagen phagocytosis in fibroblasts is regulated by calcium entry through integrin-gated channels and release from intracellular stores (Arora et al., 2001). Furthermore, previous work has shown that calcium signaling is strongly influenced by the arrangement of cortical actin filaments (Patterson et al., 1999; Wang et al., 2001) and that the organization of the cortical actin cytoskeleton impacts on the functional characteristics of single ion channels in the plasma membrane (Janmey 1998; Shumilina et al., 2003). Accordingly, we compared collagen-induced calcium signaling in wild-type and gelsolin null cells. Our results show greatly attenuated integrin-induced calcium signals in gelsolin-null cells compared with wild-type cells. The absence of these signals in turn blocks rac activation, a critical regulator of the initial binding step of collagen phagocytosis, which in turn is required for collagen binding and intracellular degradation.
MATERIALS AND METHODS
Reagents
Latex (2- and 6-μm-diameter) beads were purchased from Polysciences (Warrington, PA). Antibodies to β-actin (clone AC-15), bovine type 1 collagen (clone COL-1), fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse (GAM) antibody and tetra-methyl rhodamine isothiocyanate-phalloidin were from Sigma-Aldrich (St. Louis, MO). Rat monoclonal antimouse integrin antibody to α2β1 (clone VLA-2) integrin was obtained from Chemicon International (Temecula, CA). FITC-goat anti-rat was purchased from Cedarlane Laboratories (Hornby, ON, Canada). Antibodies to Arp3 and c-Myc (clone 9E10) were from Santa Cruz Biotechnology (Santa Cruz, CA). The affinitypurified polyclonal antibody to recombinant gelsolin has been described previously (Azuma et al., 1998). Antibodies to cortactin (clone 4F11), rac (clone 23A8), and PAK-PBD protein glutathione S-transferase beads were obtained from Upstate Biotechnology (Lake Placid, NY). Phycoerythrin-conjugated antibody to the α2 integrin chain was obtained from BD PharMingen (San Diego, CA).
Cell Preparation
Fibroblasts were obtained from either wild-type (WT) or gelsolin knockout (Gsn-) day 12 mouse fetuses and cultured as described previously (Witke et al., 1995). The embryos from which the fibroblasts were derived were genotyped by polymerase chain reaction of tails snips to confirm depletion of gelsolin. Cells were grown in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum and 10% antibiotics. The fibroblastic identity of the cells was verified by staining for intracellular vimentin and collagen as well as absence of desmin. Cells were grown at 37°C and were used at passage 4.
Collagen Bead Binding
Collagen-coated latex beads (2 μm) were applied to microbiological (i.e., nontissue culture) dishes and dried down for attachment as described previously (Arora et al., 2003) followed by washing with phosphate-buffered saline. The number of beads plated per dish was adjusted to produce final bead:cell ratios specific for each experiment. Cells were counted electronically, and the cell concentration was adjusted before plating cells on dishes containing collagen-coated beads. The plates were maintained at room temperature for 10 min to allow the cells to settle and subsequently washed with fresh medium at 37°C. Detached cells were removed by repeated washes. Those cells that were attached, spread and rapidly internalized the collagen beads (Arora et al., 2000).
In experiments to evaluate collagen bead internalization, FITC-collagen-coated beads were incubated with cells for timed incubation periods. Internalization was stopped by cooling on ice. Fluorescence from the extracellular beads was quenched by trypan blue, whereas internalized beads retained their bead-associated fluorescence.
Bead Incubation, Isolation, and Immunoblotting
Collagen-coated latex beads (6 μm) were attached to 100-mm nontissue culture plastic dishes. Cell suspensions were allowed to attach to beads for 20 min. Unattached, floating cells were aspirated and replaced with media warmed to 37°C to synchronize phagocytosis. Cells were collected at discrete time points thereafter. Cells and collagen-coated latex beads were collected with a cell scraper in homogenization buffer (1% Triton X-100, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.5 mM EGTA, 20 μg/ml aprotonin, 1 μg/ml Pefabloc, and 10 mM PIPES, pH 6.8). The samples were sonicated (2 s) and centrifuged for 3 min at 3000 × g to remove unbroken cells. The beads were sedimented and resuspended in medium. The supernatants were sonicated, centrifuged at 10,000 × g for 5 min and washed in cytoskeletal buffer. Aliquots (50 μl) were separated for bead counts to normalize immunoblots. Bead-associated proteins were recovered by suspension in Laemlli sample buffer. The samples were boiled and separated on SDS-PAGE gels. Immunoblotted samples were probed with appropriate antibodies and quantified by scanning densitometry. Data were normalized to bead counts, which were obtained by counting the total number of beads in each sample with a hemocytometer.
Actin Assembly
In permeabilized cells incubated with rhodamine actin monomer, we measured increases of rhodamine fluorescence due to incorporation into nascent actin filaments (Hartwig 1992; Hartwig et al., 1995; Azuma et al., 1998; Chan et al., 1998). Cells were permeabilized for 20 s by using 0.1 vol of OG buffer (PHEM buffer containing 2% octyl glucoside and 2 μM phalloidin). Permeabilization was stopped by diluting the detergent with buffer without detergent. Immediately thereafter, freshly sedimented rhodamine actin monomer (0.23 μM) in buffer containing 120 mM KCl, 2 mM MgCl2, 3 mM EGTA, 10 mM PIPES, and 0.1 mM ATP was added to the samples for 10 s followed by fixation with 3.7% formaldehyde. The samples were observed with a Nikon TE 300 microscope, and rhodamine fluorescence in single cells was quantified using the PCI Imaging program. For estimation of background correction, detergent treatments were omitted, fluorescence was quantified, and this background signal was subtracted from experimental samples.
Severing Assay
The ability of gelsolin to sever actin filaments was measured as described previously (Allen and Janmey, 1994). Briefly, rhodamine-phalloidin (1 μM; Molecular Probes, Eugene, OR) was added to actin filaments (0.4 μM), and the rate of fluorescence loss at 570 nm was measured fluorimetrically. Reduction of fluorescence is caused by the ability of gelsolin to sever actin and displace phalloidin after the addition of CaCl2 (1 mM). Affinity-purified rabbit muscle actin (1.0 mg/ml; Cytoskeleton, Denver, CO) was resuspended in polymerization buffer (50 mM KCl, 2 mM MgCl2, 0.5 mM ATP, 2 mM Tris, pH 8.0) and sedimented with an Airfuge (30,000 rpm for 20 min) to remove unpolymerized actin. The concentration of actin was determined by measuring the absorbance before and after ultracentrifugation using an absorption coefficient (E2901% = 6.3). Cell lysates from WT and Gsn- cells were prepared with detergent plus protease inhibitors in buffer containing 50 mM KCl, 2 mM MgCl2, 0.5 mM ATP, 2 mM Tris, pH 8.0, 1 mM EGTA, and 1% Triton X-100. The lysates were dialyzed with several changes of buffer containing 2 mM MgCl2, 50 mM KCl, 2 mM Tris-HCl, and 1 mM EGTA, 0.5 mM β-mercaptoethanol. The volume of the dialyzed cell lysate was adjusted to 400 μl in dialysis buffer. Labeled F-actin in polymerizing buffer (200 μl; 50 mM KCl, 2 mM MgCl2) was added to a final concentration of 400 nM. The severing assay was performed in the presence of calcium (2 mM CaCl2/1 mM EGTA).
Immunofluorescence, Confocal Microscopy, and Fluorescence Recovery after Photobleaching
Cells plated on beads were allowed to spread and bind to beads for 30 min. Cells were fixed with 3% formaldehyde in phosphate-buffered saline, permeabilized with 0.2% TritonX-100, and stained with polyclonal gelsolin antibody followed by FITC-tagged second antibody. The spatial distribution of gelsolin staining around beads was determined by confocal microscopy (Leica, Heidelberg, Germany; 40 × oil immersion lens). Transverse optical sections were obtained at 1-μm nominal thickness. In some experiments, cells transfected with mutant rac constructs containing a myc tag were allowed to spread and bind to collagen beads. Cells were fixed, permeabilized, and stained with mouse monoclonal myc antibody and FITC-conjugated goat anti-mouse antibody. For fluorescence recovery after photobleaching measurements, Gsn- and WT type cells were directly labeled with PE-α2-integrin antibody for 30 min, washed in HEPES-buffered DMEM supplemented with 10 mM glucose, 1 mM Mg2+, and 1 mM Ca2+. Preparations were kept at room temperature to minimize internalization of receptors. Fluorescently labeled receptors in small circular areas (4-μm2 bleach spots) were illuminated and photobleached (maximum intensity, 500 ms) with an argon laser at 488 nm. Fluorescence in the bleach spot was measured before bleaching and for 180 s after bleaching. The diffusion coefficient (D, × 10-10cm2/s) and the mobile fraction (%R) was calculated (Axelrod et al., 1976; Jacobson and Wojcieszyn 1984).
[Ca2+]i Measurements
Cells were loaded with fura 2/acetoxymethyl ester (3 μM) and plated on collagen-coated beads. [Ca2+]i in single, attached cells was estimated as described with a microscope-based, ratio fluorimeter (Arora et al., 1994). Experiments in which low (0.1 μM) calcium was used in the extracellular medium contained 1 mM MgCl2 to maintain integrin function.
Rac Activation
Equal numbers of Gsn- and WT cells were immunoblotted between passages 1-4. We found that rac levels were initially much higher in the Gsn- cells than the WT cells as described previously (Azuma et al., 1998), but by passage 4 they were equivalent. Accordingly, cells at passage 4 were serum starved and lysed; lysates were collected in lysis buffer with protease inhibitors (Upstate Biotechnology), clarified by low-speed centrifugation, and supernatants were incubated with a GST fusion-protein (Upstate Biotechnology) corresponding to the p21-binding domain (PBD, residues 67-150 of human PAK-1; expressed in Escherichia coli and bound to glutathione agarose). The samples were washed four times with wash buffer after an hour of incubation at 4°C. Pellets were boiled in 2 × Laemlli buffer. Samples were separated on 10% SDS-PAGE gels, transferred to nitrocellulose paper, and probed with rac antibody (clone 23A8; Upstate Biotechnology). Supernatants from samples were also run on SDS-PAGE gels and probed for β-actin, total rac, and gelsolin.
Transfections
To obtain more direct proof for a functional relationship between gelsolin expression and collagen phagocytosis in fetal fibroblasts, Gsn- cells were transfected with a gelsolin expression vector. We expressed gelsolin in fetal Gsn- fibroblasts by subcloning gelsolin cDNA into the expression vector LK444 (Azuma et al., 1998). Gsn- cells were transfected using FuGENE6 transfection reagent (Roche Diagnostics, Indianapolis, IN). After titration experiments to determine the optimum concentration of vector, cells were transfected, incubated for 48 h, and subjected to bead binding assays. To estimate transfection efficiency and to serve as a transfection control, pEGFPluc was used (BD Biosciences Clontech, Palo Alto, CA), and the number of fluorescent cells was counted. Similarly, cells were transfected with constitutively active (Q61 c-myc) or dominant negative (N17 c-myc) forms of Rac (provided by A.K.).
Statistical analyses
For continuous variables, means and SEs of means were computed and differences between groups were evaluated by Student's unpaired t test or analysis of variance for multiple comparisons with statistical significance set at p < 0.05. Post hoc comparisons were performed with Tukey's test. For all experiments, at least three independent experiments were evaluated, each performed in triplicate.
RESULTS
Cell Characterization
The fibroblastic nature of cells cultured from WT and Gsn- embryos at passage 4 was established by immunostaining with antibodies to vimentin, collagen, and desmin. All cells resembled fibroblasts morphologically, stained positively for vimentin and collagen, but did not stain for desmin. Estimates of cell volume (determined by forward scatter by using flow cytometry) indicated that in suspension, the cells were very similar in size (500 ± 5 fluorescence units, Gsn- cells; 510 ± 9 fluorescence units, WT cells; p > 0.2). When cells were spread on tissue culture plates coated with collagen, there were larger projected surface areas in Gsn- cells (2873 ± 191 μm2) than in WT type cells (1799 ± 103 μm2;p < 0.01). Both cell types exhibited similar, overall morphologies, but there was much more abundant staining for actin filaments in the Gsn- cells, particularly in subcortical regions as described previously (Witke et al., 1995; our unpublished data). When normalized to total protein, total actin by immunoblotting was ∼1.5 times higher in Gsn- cells than in WT cells. We also measured cell surface and total α2β1 receptor immunostaining in Gsn- and WT type cells. Surface receptor staining for α2β1 in Gsn- and WT type cells was 3.9 ± 0.9 and 4.1 ± 0.75 fluorescence units, respectively (p > 0.2). Total α2β1 receptor staining was 14.8 ± 1.1 and 15.2 ± 1.8 fluorescence units in the Gsn- and WT-type cells, respectively (p > 0.2).
We established that collagen bead binding was specifically mediated by the α2β1 receptor in Gsn- and WT cells with the use of a blocking antibody, followed by incubation with fluorescent collagen-coated beads and counting of attached beads by fluorescence microscopy. At bead:cell ratios of 2:1, and when incubated in the presence of the α2β1 function-interfering antibody, there were similar percentages of cells with bound beads in the two different cell types (2.0 ± 0.9% in Gsn- and 2.5 ± 0.7% beads in WT; p > 0.2). In control experiments in which an irrelevant antibody was added, there were 12.0 ± 2.2 and 25.0 ± 3.9% cells with bound beads in the Gsn- and WT cells, respectively (p < 0.01). Collectively, these data indicate that Gsn- and WT cells use the α2β1 integrin for collagen bead binding, that there are no significant differences in collagen receptor abundance on the surface of Gsn- and WT cells but that after initial stimulation, WT cells are much more likely to bind collagen beads than Gsn- cells.
Involvement of Gelsolin in Collagen Binding and Degradation
We explored a possible role for gelsolin in the initial steps of collagen bead binding by estimating the abundance of gelsolin around collagen beads. After 10 min of incubation with beads, confocal microscopy showed pronounced localization of gelsolin around beads (Figure 1A). As expected, there was no immunostaining for gelsolin in Gsn- cells (our unpublished data). We compared collagen bead binding in Gsn- cells and WT cells at varying bead to cell ratios by flow cytometry and fluorescence microscopy. Because collagen-beads were the only matrix cells could bind on nontissue culture dishes, we counted the number of beads per cell and the number of Gsn- cells and WT cells remaining on the dishes after washing after a 30-min exposure to beads (Figure 1B). The initial contact of cells with beads induced much greater collagen bead binding in WT than Gsn- cells (60% fewer cells with bound collagen beads in Gsn- cells at the maximum bead:cell ratio of 16:1; p < 0.01). Evidently, contact-stimulated collagen binding was greatly enhanced in WT cells.
Figure 1.
Involvement of gelsolin in the initial binding step of collagen phagocytosis. (A) Fibroblasts from WT mice were plated on collagen-coated beads for 20 min, fixed, and immunostained with polyclonal antibody to gelsolin. Cells show enrichment of gelsolin around collagen beads. DIC, differential interference contrast microscopy of same cells immunostained for gelsolin. (B) Gsn- and WT cells were plated at indicated collagen bead:cell ratios, incubated for 30 min, and collagen bead binding was analyzed by flow cytometry. There was significantly lower percentage of cells binding beads in Gsn- cells compared with WT cells. Data are mean ± SEs of mean percentage of cells binding collagen beads for B and C (n = 4 independent samples/data point). (C) Gsn- cells transfected with cDNA gelsolin expression vector for 48 h show gelsolin expression at ∼50% of WT cells. When analyzed with collagen bead binding assay, there was 50% enhancement of collagen bead binding in transfected cells. (D and E) α2β1-mediated collagen bead binding and phagocytosis in Gsn- and WT cells. Cells were plated on collagen-coated beads at 8:1 (bead:cell ratios) at 37°C for the indicated times, put on ice, and collagen bead attachment (D) and phagocytosis (E) were determined. Internalized beads were discriminated by quenching extracellular bound beads with trypan blue. Fluorescent and quenched beads were counted in 60 cells/sample at each time point. The numbers of beads binding per cell were significantly lower in Gsn- cells (p < 0.05), which also showed delayed bead internalization.
We sought more direct proof for a functional relationship between gelsolin expression and collagen bead binding in fibroblasts by transfecting Gsn- cells with a gelsolin expression vector. Transfection efficiency was 55% as determined by cotransfection with a fluorescence expression vector (pEGFP). After 48 h of incubation, gelsolin expression was increased (∼50% of WT levels by immunoblotting; Figure 1C), indicating that in the successfully transfected cell population, collagen bead binding was restored to levels of WT. Consistent with this observation, in the whole population, collagen bead binding was increased by 50% in Gsn- cells transfected with the gelsolin expression vector compared with nontransfected cells (Figure 1C; bead:cell ratio = 16:1; p < 0.001).
In time-course experiments (bead:cell ratio = 8:1), the numbers of collagen beads bound per cell were significantly lower in Gsn- cells compared with WT at 60 and 120 min after bead loading (Figure 1D; p < 0.05) but the difference was reduced at 240 min, possibly because the cells were saturated with beads. Because collagen binding is followed by degradation of collagen in intracellular compartments (Arora et al., 2000), we examined collagen internalization in WT and Gsn- cells. Beads on the cell surface were distinguished from internalized beads by treating cells with trypan blue, a method that quenches fluorescence of extracellular beads and induces a color change to blue, whereas internalized beads retain fluorescence around the beads. At early times (10 and 20 min), there were very low numbers of internalized beads per cell (Figure 1E). At later times (60 min), the WT cells exhibited twofold more internalized beads per cell (p < 0.01).
Actin and Actin Binding Proteins in Collagen Bead Binding
The initial binding step of collagen phagocytosis involves protrusion and extension of the leading edge of the cell during spreading on collagen-coated beads (Arora et al., 2003). To determine whether collagen bead binding required intact actin filaments, we treated cells with cytochalasin D (1 μM) for 30 min before incubation with collagen beads. Both Gsn- and WT cells showed a >10-fold reduction in the percentage of cells binding to collagen beads (Figure 2A). We next examined actin assembly in response to initial contact and spreading over collagen beads after 2, 10, and 20 min. Cells were subsequently permeabilized, incubated with rhodamine actin, and fluorescence was quantified by image analysis around attached beads in Gsn- and WT cells (Figure 2B). There was rapid actin incorporation of actin monomer into filaments at 2 and 10 min in WT cells, whereas actin assembly was greatly reduced in Gsn- cells. Control experiments with WT cells showed that Alexaλ488 phalloidin colocalized with newly incorporated rhodamine actin monomers (Figure 2B, inset) and that preincubation of cells with cytochalasin D (1 μM) blocked incorporation of actin monomers into nascent filaments (our unpublished data). The rate of actin assembly is also partly dependent on the availability of filament barbed ends. Because barbed ends can be generated by the severing activity of actin binding proteins like gelsolin, we compared severing activity in WT and Gsn- cells. As expected, basal severing activity was more rapid in the WT cells (WT, 12.6 fluorescence units/s, R2 = 0.77; Gsn-, 7.4 fluorescence units/s, R2 = 0.83. Severing activity after bead binding was for WT, 15.2 fluorescence units/s, R2 = 0.76 and for Gsn-, 8.0 fluorescence units/s, R2 = 0.79).
Figure 2.
Involvement of actin filaments and actin binding proteins in collagen bead binding. (A) Incubation of cells with cytochalasin D reduces the percentage of cells binding collagen beads in both WT and Gsn- cells by >10-fold. Data are mean ± SEs of mean percentage of cells binding collagen beads (n = 4 independent samples/data point). (B) De novo actin assembly around beads. Cells were allowed to bind to collagen beads for 2, 10, and 20 min and subsequently treated with 0.2% OG-PHEM buffer for 2 s, incubated with rhodamine-actin monomers, fixed, and stained with FITC-phalloidin. There was reduced and much slower incorporation of actin monomers into actin filaments in Gsn- cells. Inset, WT cells incubated with collagen beads for 2 min show staining of actin filaments with FITC-phalloidin (left), rhodamine actin monomer incorporation into nascent filaments around beads (middle), and phase contrast showing collagen beads. (C) Accumulation of actin and actin binding proteins during early events of bead binding. Collagen-bead associated proteins were isolated at indicated time points and immunoblotted. From immunoblots normalized for bead counts and protein abundance (by Bio-Rad assay), there was marked reduction in amounts of actin, cortactin, and Arp3 associated with collagen beads in Gsn- cells. Data are mean ± SEs of blot density adjusted for number of beads in sample (n = 3/sample). Immunoblotting of whole cell lysates from unstimulated Gsn- and WT cells is shown for corresponding proteins at right side of panel.
The extension of cell membrane that is involved in collagen bead binding likely requires actin binding proteins such as Arp2/3 (for its actin nucleating activity) and cortactin (for stabilization of the actin network around the bound collagen). Because the above data showed substantial differences in collagen bead-binding between Gsn- and WT cells (Figure 1), we examined the relative abundance of Arp 3 and cortactin that accumulated at the collagen bead-binding site by immunoblotting for bead-associated proteins (Arora et al., 2000). From immunoblots normalized for equal number of bead counts, there were increasing amounts of actin, cortactin, and Arp3 associated with beads at 0, 2, and 10 min in WT cells, which decreased toward the initial level by 20 min (Figure 2C). In Gsn- samples, there was little change in the amounts of actin, cortactin, and Arp3 around the beads. As these differences in Arp 3 and cortactin accumulation around beads could be attributable to variations in the total protein content, whole cell lysates from unstimulated cells were immunoblotted, but we found no difference in the abundance of cortactin and Arp3 in Gsn- and WT cells (Figure 2C, insets). These data show that binding of collagen beads to cells via collagen receptors (α2β1 integrin) promotes efficient actin assembly in WT but not in Gsn- cells. Because these differences could be due to differences in secretion of intracellular α2β1 integrins to the plasma membrane in cells binding to beads, we first determined total β1-integrin content by Western blotting the total cell lysates (Figure 2C, inset) and then determined the relative abundance of β1-integrin on the beads at different time points. There was no difference of either total β1-integrin or bead-associated β1-integrin in the Gsn- and WT cells (Figure 2C). For all the experiments in which bead extracts were used, data were normalized by bead counts for each data point.
Impact of Gelsolin on Integrin Function
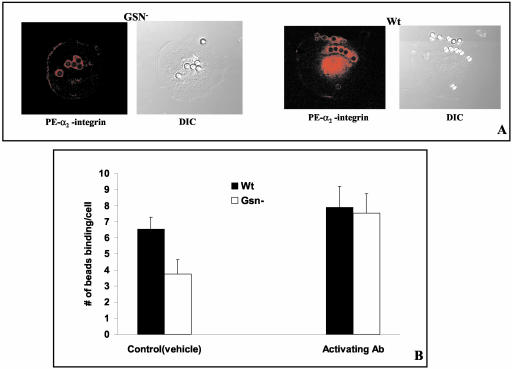
Collagen binding to cells promotes aggregation and cross-linking of α2β1. We compared aggregation of the α2 integrin in WT and Gsn- cells in response to collagen bead binding by immunofluorescence staining of vital cells and optical sectioning by confocal microscopy. There was no difference in the fluorescence of α2-integrin (labeled with PE-conjugated antibody) around beads attached to the surface of Gsn- and WT cells (Figure 3A), suggesting that once collagen beads initially bound, integrin clustering and avidity were not affected by gelsolin. Collagen receptor mobility and diffusion coefficient were studied by fluorescence recovery after photobleaching and were very similar in the two cell types (percentage of mobile fraction, 55% in WT and 56% in Gsn-; diffusion coefficients, 2.25 ± 0.85 × 10-10cm2/s in WT and 2.44 ± 0.98 × 10-10cm2/s in Gsn-). These data suggest that Gsn- cells have equivalent collagen receptor avidity and mobility in the membrane as WT cells.
Figure 3.
Influence of gelsolin on integrin function. (A) Recruitment of α2-integrin (labeled with PE-conjugated antibody) around bound collagen beads was similar in Gsn- and WT cells, suggesting that integrin avidity was not affected by Gelsolin. (B) For assessment of antibody-induced integrin activation, Gsn- and WT cells were incubated with beads at 8:1 (bead:cell ratio) in the presence of β1-integrin activating antibody (9EG7) and analyzed by fluorescence microscopy. There was 50% enhancement in collagen bead binding in Gsn- cells but no change in WT cells (p < 0.01). Data are mean ± SEs of mean number of beads bound per cell.
Because integrin function can also be modulated by alterations of affinity, we used an activating antibody (9EG7; Lenter et al., 1993) to β1-integrin to assess whether we could “rescue” the gelsolin-dependent reduction of collagen binding (bead:cell = 8:1). Whereas treatment with activating antibody caused only a minimal increase in WT cells (Figure 3B), it strongly enhanced collagen bead binding in Gsn- cells (to levels of WT levels), indicating that many of the β1-integrins in Gsn- cells are not constitutively activated and that the signals regulating β1-integrin affinity are reliant in part on gelsolin.
Rac Activation in Collagen Bead Binding
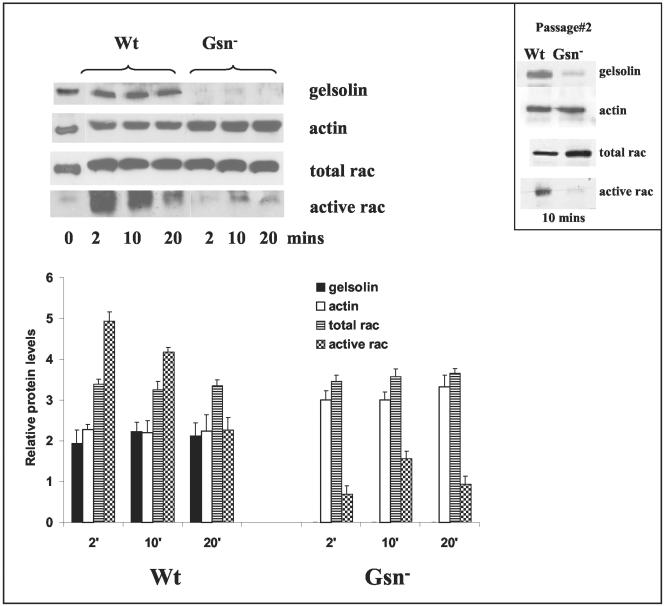
Rac is activated in response to fibroblast spreading (Price et al., 1998), regulates lamellipodia formation and membrane ruffling in fibroblasts (Etienne-Manneville and Hall, 2002), and mediates particle internalization in macrophages (Caron and Hall, 1998). Previous studies have also shown that gelsolin is an effector of rac-mediated actin dynamics, that gelsolin acts downstream of rac, and that gelsolin deficiency causes increased rac expression in early passage cells (Azuma et al., 1998). Accordingly, we passaged Gsn- and WT cells so that total expression of rac was equivalent in the two cell types. Because the ventrally loaded collagen phagocytosis model requires extension of lamellipodia for bead internalization (Arora et al., 2003), we anticipated increased rac activity in response to spreading on collagen beads. Although total rac levels were equivalent, there was greatly reduced rac activity in Gsn- cells compared with WT cells after spreading on collagen beads (Figure 4). For all experiments Gsn- and WT cells used were at the same passage 4. We also performed experiments to detect rac activation at earlier passages and found greatly reduced rac activity in Gsn- cells (Figure 4, inset). We determined whether this deficiency was indeed attributable to gelsolin. Gsn- and WT cells were transfected with cDNA constructs containing constitutively active rac (rac Q61) or dominant negative rac (rac N17), both of which express a c-myc tag. After 48 h, transfected cells were plated on collagen-coated beads (2 μm), washed, and fixed after 1 h of spreading, and distinguished by immunostaining for c-myc. Expression of active Rac enhanced more than twofold collagen bead binding in Gsn- cells, raising it to levels similar to that of untransfected WT cells. The dominant negative rac construct reduced collagen bead binding to very low levels in both Gsn- and WT cells (Figure 5, A and B).
Figure 4.
Gelsolin impacts on rac activation. Gsn- and WT cell cultures at passage 4 showed equal expression levels of rac. For rac activation assay, cells were serum starved overnight and were stimulated with collagen-coated beads for the times indicated. Cell lysates were clarified by centrifugation and incubated with PAK-PBD-agarose beads for 1 h at 4°C. Beads were washed four times and analyzed on SDS-PAGE gels and probed with rac antibody. In spite of equal amounts of rac in the WT and Gsn- cells, rac activation was very low in Gsn- cells compared with WT. Histograms show mean ± SEs of blot density for indicated proteins after adjustment for total protein content (by Bio-Rad). Inset, results of similar experiment on cells from passage 2.
Figure 5.
Rac activation overcomes the antagonizing effect of Gsn- on collagen bead binding. (A and B) Gsn- and WT cells were transfected with cDNA constructs for constitutively active rac (rac Q61) or for a dominant negative rac (rac N17) both of which express a c-myc tag. Cells were plated on collagen-coated beads for 1 h, washed, and fixed. Transfected cells were distinguished by immunostaining for c-myc. Rac activation enhances more than twofold collagen bead binding in Gsn- cells and increases collagen bead binding to levels similar to that of untransfected WT cells. The dominant negative rac construct reduces collagen bead binding to very low levels in both Gsn- and WT cells. (C) Immunoblots for indicated proteins using PAK binding assay described in Figure 4. Gsn- cells were transfected with gelsolin expression vector as indicated and incubated with collagen beads (or not). Note that only in cells transfected with gelsolin and incubated with collagen beads is there detectable activation of rac.
We next examined the role of gelsolin in rac activation during collagen bead binding. Gsn- cells were transfected with a cDNA expression construct for gelsolin, and rac activation was assayed. The binding of collagen beads induced activation of rac in the presence of gelsolin but not when gelsolin was absent (Figure 5C). To determine the level of the signaling sequence at which rac is required for collagen bead binding, we transfected WT cells with dominant negative rac (racN17-myc) and performed bead binding assays in the presence of either β1-integrin activating antibody (9EG7) or irrelevant antibody (nebulin; NB2). The number of bound beads was counted in myc-stained cells. There was no difference in the numbers of beads binding to cells treated with activating antibody or with the irrelevant antibody (8.0 ± 1.0; 8.5 ± 1.5; p > 0.2). These data suggest that collagen-bead binding through integrins is downstream of rac.
Role of Calcium
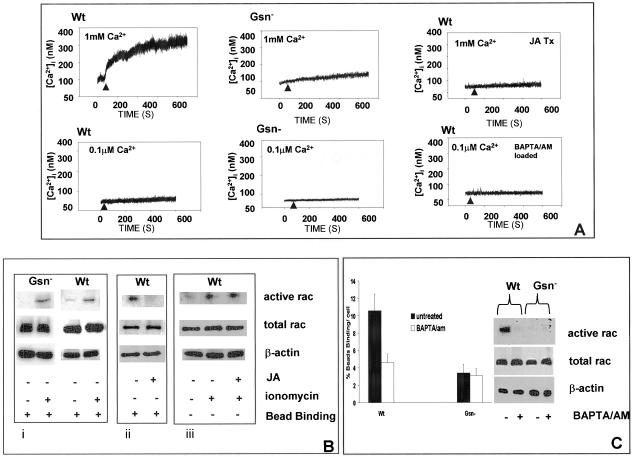
Gelsolin is a calcium-activated actin-binding protein that requires elevated Ca2+ for remodeling of actin filaments in the processes that accompany cell motility and phagocytosis (Stossel 1993). We first examined the mechanism by which calcium regulates gelsolin and effects rac activation in collagen bead binding assays. Free intracellular calcium ion concentration was measured in single, Gsn-, and WT cells by using fura 2 and ratio fluorimetry. Only cells that were spreading and attaching to collagen beads were measured. Collagen bead binding induced 300 ± 65 nM increases in [Ca2+]i above basal levels in WT cells (n = 5 cells; ∼300% increase above baseline; p < 0.001) compared with 50 ± 25 nM in Gsn- cells (Figure 6A; n = 5 cells; ∼50% increase above baseline). Because Gsn- cells exhibited much more prominent subcortical actin filaments than WT cells, we treated WT cells with jasplakinolide (1 μM; 15 min) to promote subcortical actin filament assembly as described previously (Wang et al., 2001) in an effort to replicate the prominent subcortical actin filaments of Gsn- cells. In these cells, collagen bead-induced increases of [Ca2+]i were sharply reduced (<20 nM [Ca2+]i). We also evaluated whether preincubation of cells with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid/acetoxymethyl ester (BAPTA/AM) (3 μM; 25 min in 0.1 μM CaCl2 and 1 mM MgCl2) could block increases of [Ca2+]i stimulated by cell attachment to collagen beads. As expected, this treatment completely inhibited collagen-induced increases of [Ca2+]i. To verify the source of the [Ca2+]i increase, we reduced extracellular Ca2+ (0.1 μM CaCl2 including 1 mM MgCl2) in the buffer. There were minimal increases of intracellular calcium after collagen bead binding, suggesting that the principal source of the calcium for initiating signaling is extracellular.
Figure 6.
Role of calcium in rac activation. (A) Intracellular calcium transients were measured in single, fura 2/AM (3 μM)-loaded cells, detected by ratio fluorimetry at 346 nm/380 nm (excitation) and 510 nm (emission) in the presence (1 mM) or absence (0.1 μM) of CaCl2. Loaded cells were plated on collagen-coated beads and measurements were done after initial cell-bead binding and only in cells with attached beads. WT cells show robust calcium transients compared with Gsn- cells. To determine the importance of subcortical actin filaments on collagen-induced calcium increases, cells were incubated with JA (1 μM for 15 min) and during attachment to collagen beads, [Ca2+]i was measured by ratio fluorimetry. WT cells were also loaded with BAPTA/AM (3 μM in buffer containing 0.1 μM CaCl2) and measured by ratio fluorimetry. (B) Role of calcium in regulation of rac. i) Gsn- and WT cells were treated with ionomycin (2 μM; indicated by +) for 20 min, and rac activation was measured with PAK binding assay in the presence of collagen beads as described in Figure 4. ii) Effect of actin polymerization on activation of rac measured after bead binding in the JA- and vehicle-treated WT cells. iii) JA-treated WT cells were further treated with or without ionomycin showing effect on rac activation. (C) Collagen bead binding was measured in WT and Gsn- cells in low calcium buffer (0.1 μM CaCl2) with and without preincubation with BAPTA/AM as described above. Rac activation assays were performed as in B with or without BAPTA/AM preincubation.
We determined whether [Ca2+]i plays a role in activation of rac. We used ionomycin (1 μM; a calcium ionophore) to increase [Ca2+]i and then measured rac activation after ionomycin treatment. Both WT cells and Gsn- cells showed significant rac activation after bead binding (Figure 6B, i). To determine whether actin polymerization induced by jasplakinolide (JA) affects rac activation, we treated WT type cells with JA or vehicle (15min) in bead binding assay and found that subcortical actin polymerization prevented rac activation (Figure 6B, ii). Furthermore, treatment with ionomycin of JA-treated cells overcomes the effect of JA treatment on rac activation (Figure 6B, iii), suggesting that calcium influx through calcium channels plays an important role in rac activation.
We next assessed whether reduced intracellular calcium levels would affect the binding of cells to collagen beads in the two cell types. There was a more than twofold reduction of collagen bead binding in WT cells after BAPTA/AM treatment compared with controls, whereas collagen bead binding in Gsn- cells was not affected by BAPTA/AM and was comparable with WT cells with BAPTA/AM treatment (Figure 6C; 4:1 bead:cell ratio in low calcium buffer). We next assayed for rac activation in WT and Gsn- cells when preincubated with BAPTA/AM and then spread on collagen beads Preincubation with BAPTA/AM blocked rac activation in WT cells, consistent with the reduced collagen bead binding. There was no detectable rac activation in Gsn- cells with or without BAPTA/AM treatment (Figure 6C).
DISCUSSION
We have examined here the role of gelsolin in the binding and internalization events of collagen phagocytosis, a critical process for physiological remodeling of connective tissues. Previous data have shown that gelsolin is important for osteoclast-induced bone resorption (Chellaiah et al., 2000). Our findings indicate that gelsolin plays an important regulatory role in the α2β1-mediated initial steps of collagen fibril attachment and engulfment; ultimately, deficiency of gelsolin leads to inhibition of collagen degradation by the intracellular pathway. These findings are analogous to inhibition of phagocytosis by the Fc receptor in gelsolin-deficient macrophages (Serrander et al., 2000). In macrophages, engulfment of IgG-opsonized particles by Fc receptors requires extension of pseudopods, the coordinated assembly of actin and the recruitment of actin-binding proteins to the particle binding site (Allen and Aderem, 1996). In contrast, complement-opsonized particles seem to sink into the cell membrane (Kaplan, 1977) and notably, actin assembly and gelsolin are not essential for complement-mediated phagocytosis (Serrander et al., 2000). In fibroblasts engaged in matrix protein degradation by phagocytosis through the α2β1 integrin, gelsolin-mediated actin filament assembly is a critical step. This process requires a “spreading type” of phagocytosis in which the cells extend cell processes to attach and engulf the collagen beads. Whereas the larger surface area of gelsolin null cells suggests that spreading over collagen may be enhanced in the absence of gelsolin, it seems that α2β1 integrin-mediated binding to collagen beads is decreased. Therefore, gelsolin is an important determinant of internalization in the collagen phagocytic pathway.
We found a requirement of the α2β1 integrin for collagen binding in both WT and gelsolin-null cells. Collagen receptor abundance and recruitment of α2 integrin (labeled with PE-conjugated antibody) around beads were similar in gelsolin null and WT cells. Photobleaching experiments also showed that collagen receptor mobility and diffusion coefficients were nearly identical in these two cell types. Therefore, the cytoskeletal elements that regulate the mobility of collagen receptors in the plane of the plasma membrane are functional in the absence of gelsolin. However, integrin affinity modulation is also a critical regulatory system that determines the binding of cells to extracellular matrix proteins (Mould, 1996). Affinity can be modulated experimentally by antibodies to activation epitopes in the integrin molecules. Notably, the stimulatory anti-β1 monoclonal antibody 9EG7 (Lenter et al., 1993) can increase ligand binding by twofold in other systems (Mould, 1996). We found that 9EG7 enhanced collagen bead binding (by 50%) in gelsolin null cells to the level of collagen binding in WT cells. This finding suggests that in gelsolin null cells, a much smaller percentage of the α2β1 integrins is activated compared with WT cells. Conceivably, either the actin filament modifying activity of gelsolin or its recruitment of signaling proteins to the plasma membrane (possibly through phospholipid-protein interactions; Chellaiah et al., 2001) are involved in this regulation.
Small GTPases of the Rho family have been widely implicated in coordinating actin assembly in response to extracellular signals, including phagocytosis through Fc receptors in macrophages (Caron and Hall 1998; Patel et al., 2002). In fibroblasts spreading on fibronectin (Price et al., 1998) and in platelets spreading on collagen (Suzuki-Inoue et al., 2001), rac is activated. Our data showed strong activation of rac in gelsolin WT cells that were binding and internalizing collagen beads; experiments with dominant negative rac constructs confirmed that similar to the Fc receptor, binding of collagen through α2β1 integrins requires activation of rac. Whereas previous data have shown that gelsolin levels regulate rac expression (Azuma et al., 1998), our experiments demonstrate that even when Rac levels between WT and gelsolin null cells were equilibrated by passaging, collagen receptor ligation was insufficient to activate rac in the absence of gelsolin. Conversely, we were able to overcome the inhibitory effect of gelsolin deficiency on collagen binding by transfecting gelsolin null cells with a constitutively active rac. Thus our data point to gelsolin, or a gelsolin substrate, as a central regulatory molecule in the activation of rac and the α2β1 integrin. In this context previous studies have shown that the small GTP-binding protein rac promotes dissociation of gelsolin from actin filaments in neutrophils (Arcaro, 1998) and in collagen phagocytosis this might be attributable to effects of gelsolin on the turnover of phosphoinositides.
Gelsolin is actively involved in mediating actin assembly and disassembly, processes that are required for the initial binding and extension of lamellae for collagen internalization (Arora et al., 2003). Actin assembly requires capture of actin subunits to form nuclei that can elongate at the barbed ends of filaments. Several actin nucleation pathways have been identified, including stimulation of the Arp2/3 complex to nucleate actin assembly (Machesky and Insall, 1999). Another physiologically relevant pathway involves gelsolin-mediated severing of actin filaments followed by uncapping of barbed ends to create nucleation sites (Witke et al., 1995; Azuma et al., 1998). The importance of gelsolin in actin filament assembly has been demonstrated in recent work indicating that gelsolin-induced severing and capping of actin filaments is upstream of Arp2/3-induced nucleation of filaments (Falet et al., 2002). In support of this view, we found that gelsolin is required for the efficient recruitment of Arp3 and cortactin, two proteins of the actin nucleating machinery, to collagen-coated beads during phagocytosis.
Modulation of actin dynamics by gelsolin is presumably dependent on activation of gelsolin by calcium (Janmey 1998). Accordingly, we measured collagen-induced calcium influx and discovered that gelsolin deficiency largely inhibits the increase of intracellular free calcium ion concentration that is required for efficient collagen binding (Arora et al., 2001). These findings indicate that gelsolin may indirectly regulate collagen-induced calcium influx through cation-permeable channels as has been demonstrated recently for sodium channel activity by capped actin filaments (Shumilina et al., 2003).
We asked whether the increased [Ca2+]i stimulated by collagen binding in WT cells (Arora et al., 2001) may be important for rac activation in fibroblasts as has been suggested for rac activation in neutrophils (Lian et al., 2001), and whether calcium is important for collagen binding. Ionophore-stimulated entry of calcium by ionomycin did indeed induce rac activation in gelsolin null cells to levels that were similar to WT cells. Furthermore, blockade of increases of [Ca2+]i with BAPTA/AM in both Gsn- and WT cells strongly inhibited collagen bead binding, establishing a central role for increased calcium to enhance collagen binding. Notably, because the gelsolin null cells exhibited abundant subcortical actin filaments, we considered that actin filaments may uncouple activation of calcium channels in response to collagen stimulation as has been shown earlier for other ligands (Patterson et al., 1999; Wang et al., 2001). Consistent with this notion, when we used jasplakinolide to induce subcortical actin filament assembly in WT cells (Wang et al., 2001), we were able to attenuate markedly collagen-induced increases of [Ca2+]i in WT cells.
Previously, we have shown that collagen phagocytosis is an actin and Ca2+-dependent process (Arora et al., 2001). Here, we show that gelsolin, which constitutes a significant fraction of the total cellular proteins, is an important candidate for mediating Ca2+-dependent actin remodeling in collagen phagocytosis. Although it is clear that rac can act upstream of gelsolin (Azuma et al., 1998), in collagen phagocytosis gelsolin may also exert important modulatory effects on rac activity. Specifically, the ability of gelsolin to remodel actin filaments seems important for calcium signaling, which, in response to collagen bead stimulation, is required for rac activation. Indeed, we were able to bypass this deficiency in gelsolin null cells either by treatment with integrin-activating antibodies or by transfection with constitutively active rac. Gelsolin evidently plays a crucial role in regulating the binding step of collagen phagocytosis.
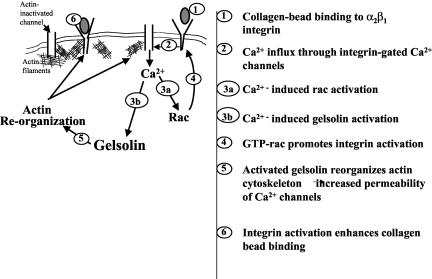
The model that we propose for gelsolin regulation of the collagen binding step of phagocytosis invokes actin filaments as pivotal substrates for gelsolin that also regulate collagen-induced calcium increases and rac activation (Figure 7). For initially bound collagen to activate increased binding by more unoccupied α2β1 integrins, there must be generation of a calcium signal. This signal is regulated by subcortical actin filaments and, in the absence of gelsolin, the organization of these filaments precludes efficient generation of a calcium signal. In WT cells, the collagen-induced calcium signal serves to activate rac which in turn leads to integrin activation and enhanced collagen binding at unoccupied receptors. Consequently, collagen binding is enhanced in WT cells because more α2β1 integrins are activated. A notable feature of this model is that although gelsolin can act downstream of rac (Azuma et al., 1998), its substrate (i.e., actin) can have profound effects on other signaling pathways that operate functionally upstream of rac, thereby constituting a positive feedback loop.
Figure 7.
Role of gelsolin in collagen phagocytosis. Gelsolin is required for remodeling of subcortical actin filaments that can regulate collagen-induced calcium entry. Calcium in turn regulates rac activation, which is required for enhanced binding of collagen by collagen receptors.
Acknowledgments
We thank Wilson Lee for help with flow cytometry, Laura Silver for assistance in the preparation of the manuscript, and Cheung Lo for cell culture. This work was supported by Canadian Institutes of Health Research operating, group and major equipment grants (to A.K., M.G., and C.A.M.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-07-0468. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-07-0468.
References
- Allen, L.A., and Aderem, A. (1996). Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J. Exp. Med. 184, 627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, P.G., and Janmey, P.A. (1994). Gelsolin displaces phalloidin from actin filaments. A new fluorescence method shows that both Ca2+ and Mg2+ affect the rate at which gelsolin severs F-actin. J. Biol. Chem. 269, 32916-32923. [PubMed] [Google Scholar]
- Axelrod, D., Koppel, D.E., Schlessinger, J., Elson, E., and Webb, W.W. (1976). Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro, A. (1998). The small GTP-binding protein rac promotes the dissociation of gelsolin from actin filaments in the neutrophils. J. Biol. Chem. 273, 805-813. [DOI] [PubMed] [Google Scholar]
- Arora, P.D., Bibby, K.J., and McCulloch, C.A. (1994). Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J. Cell. Physiol. 161, 187-200. [DOI] [PubMed] [Google Scholar]
- Arora, P.D., Manolson, M.F., Downey, G.P., Sodek, J., and McCulloch, C.A. (2000). A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J. Biol. Chem. 275, 35432-43541. [DOI] [PubMed] [Google Scholar]
- Arora, P.D., Silvestri, L., Ganss, B. Sodek, J., and McCulloch, C.A. (2001). Mechanism of cyclosporin-induced inhibition of intracellular collagen degradation. J. Biol. Chem. 276, 14100-14109. [DOI] [PubMed] [Google Scholar]
- Arora, P.D., Fan, L. Sodek, J. Kapus, A., and McCulloch, C.A. (2003). Differential binding to dorsal and ventral cell surfaces of fibroblasts: effect on collagen phagocytosis. Exp. Cell Res. 286, 366-380. [DOI] [PubMed] [Google Scholar]
- Azuma, T., W. Witke, W. Stossel, T.P. Hartwig, J.H., and Kwiatkowski, D.J. (1998). Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J. 17, 1362-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron, E., and Hall, A. (1998). Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717-1721. [DOI] [PubMed] [Google Scholar]
- Chan, A.Y., Raft, S., Bailly, M., Wyckoff, J.B., Segall, J.E., and Condeelis, J.S. (1998). Epidermal growth factor stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J. Cell Sci. 111, 199-211. [DOI] [PubMed] [Google Scholar]
- Chellaiah, M.A., Biswas, R.S., Yuen, D., Alvarez, U.M., and Hruska, K.A. (2001). Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J. Biol. Chem. 276, 47434-47444. [DOI] [PubMed] [Google Scholar]
- Chellaiah, M.A., Soga, N. Swanson, S., McAllister, S., Alvarez, U., Wang, D., Dowdy, S.F., and Hruska, K.A. (2000). Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275, 11993-2002. [DOI] [PubMed] [Google Scholar]
- Del Pozo, M.A., Kiosses, W.B., Alderson, N.B., Meller, N., Hahn, K.M., and Schwartz, M.A. (2002). Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4, 232-239. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629-635. [DOI] [PubMed] [Google Scholar]
- Falet, H., Hoffmeister, K.M., Neujahr, R., Italiano, J.E., Jr., Stossel, T.P., South-wick, F.S., and Hartwig, J.H. (2002). Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc. Natl. Acad. Sci. USA 99, 16782-16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, J.H. (1992). Mechanisms of actin rearrangements mediating platelet activation. J. Cell Biol. 118, 1421-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, J.H., Bokoch, G.M., Carpenter, C.L., Janmey, P.A., Taylor, L.A., Toker, A., and Stossel, T.P. (1995). Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell 82, 643-653. [DOI] [PubMed] [Google Scholar]
- Jacobson, K., and Wojcieszyn, J. (1984). The translational mobility of substances within the cytoplasmic matrix. Proc. Natl. Acad. Sci. USA 81, 6747-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey, P.A., and Stossel, T.P. (1987). Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325, 362-364. [DOI] [PubMed] [Google Scholar]
- Janmey, P.A. (1998). The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev. 78, 763-781. [DOI] [PubMed] [Google Scholar]
- Kaplan, G. (1977). Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand. J. Immunol. 6, 797-807. [DOI] [PubMed] [Google Scholar]
- Lee, W., Sodek, J., and McCulloch, C.A. (1996). Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J. Cell Physiol. 168, 95-104. [DOI] [PubMed] [Google Scholar]
- Lenter, M., Uhlig, H., Hamann, A., Jeno, P., Imhof, B., and Vestweber, D. (1993). A mAb against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc. Natl. Acad. Sci. USA 90, 9051-9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian, J.P., Crossley, L., Zhan, Q., Huang, R., Coffer, P., Toker, A., Robinson, D., and Badwey, J.A. (2001). Antagonists of calcium fluxes and calmodulin block activation of the p21-activated protein kinases in neutrophils. J. Immunol. 166, 2643-2650. [DOI] [PubMed] [Google Scholar]
- Machesky, L.M., and Insall, R.H. (1999). Signaling to actin dynamics. J. Cell Biol. 146, 267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould, A.P. (1996). Getting integrins into shape: recent insights into how integrin activity is regulated by conformational changes. J. Cell Sci. 109, 2613-2618. [DOI] [PubMed] [Google Scholar]
- Patel, J.C., Hall, A., and Caron, E. (2002). Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol. Biol. Cell 13, 1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, R.L., van Rossum, D.B., and Gill, D.L. (1999). Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell 98, 487-499. [DOI] [PubMed] [Google Scholar]
- Price, L.S., Leng, J., Schwartz, M.A., and Bokoch, G.M. (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9, 1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrander, L., Skarman, P., Rasmussen, B., Witke, W., Lew, D.P., Krause, K.H., Stendahl, O., and Nusse, O. (2000). Selective inhibition of IgG-mediated phagocytosis in gelsolin-deficient murine neutrophils. J. Immunol. 165, 2451-2457. [DOI] [PubMed] [Google Scholar]
- Shumilina, E.V., Negulyaev, Y.A., Morachevskaya, E.A., Hinssen, H., and Khaitlina, S.Y. (2003). Regulation of sodium channel activity by capping of actin filaments. Mol. Biol. Cell 14, 1709-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel, T.P. (1993). On the crawling of animal cells. Science 260, 1086-1094. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue, K., Yatomi, Y., Asazuma, N., Kainoh, M., Tanaka, T., Satoh, K., and Y. Ozaki, Y. (2001). Rac, a small guanosine triphosphate-binding protein, and p21-activated kinase are activated during platelet spreading on collagen-coated surfaces: roles of integrin alpha(2)beta(1). Blood 98, 3708-3716. [DOI] [PubMed] [Google Scholar]
- Tjelle, T.E., Lovdal, T., and Berg, T. (2000). Phagosome dynamics and function. Bioessays 22, 255-263. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Ko, K.S., Kapus, A., McCulloch, C.A., and Ellen. R.P. (2001). A spirochete surface protein uncouples store-operated calcium channels in fi-broblasts: a novel cytotoxic mechanism. J. Biol. Chem. 276, 23056-23064. [DOI] [PubMed] [Google Scholar]
- Weeds, A., and Maciver, S. (1993). F-actin capping proteins. Curr. Opin. Cell Biol. 5, 63-69. [DOI] [PubMed] [Google Scholar]
- Witke, W., Sharpe, A.H., Hartwig, J.H., Azuma, T., Stossel, T.P., and D.J. Kwiatkowski, D.J. (1995). Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell 81, 41-51. [DOI] [PubMed] [Google Scholar]