Abstract
Background
Capecitabine is effective and indicated for the salvage treatment of metastatic breast cancer. Therefore, it is essential to evaluate the efficacy of capecitabine in the adjuvant setting. There have been two large randomized studies to determine whether patients with high-risk early breast cancer benefit from the addition of capecitabine to standard chemotherapy, but they have yielded inconsistent results. We first undertook a meta-analysis to evaluate the efficacy of the addition of capecitabine over standard treatment.
Methods
PubMed, EBSCO, Web of Science, conference proceedings and key trials were searched from 1998 to 2011. The hazard ratio (HR) was used to evaluate the efficacy of a taxane-anthracycline regimen and a taxane-anthracycline-capecitabine regimen in early breast cancer. All of the data from each study use either fixed-effects or random-effects by Stata.
Findings
We found significant improvement in the additional capecitabine arm versus control in disease-free survival (DFS) (HR = 0.83, 95% CI: 0.71–0.98, P = 0.027), overall survival (OS) (HR = 0.71, 95% CI: 0.57–0.88, P = 0.002), distant recurrence (HR = 0.79, 95% CI: 0.66–0.94, P = 0.008) and the death from breast cancer only (HR = 0.65, 95% CI: 0.51–0.83, P = 0.001). Meanwhile, the subgroup analysis revealed that capecitabine improved the DFS in triple negative (HR = 0.71, 95% CI: 0.53–0.96, P = 0.028), hormone receptor negative (HR = 0.73, CI: 0.56–0.94, P = 0.017) and HER2 negative (HR = 0.81, CI: 0.67–0.98, P = 0.034) patients.
Conclusion
Due to the synergistic effect of taxane and capecitabine, taxane-anthracycline-capecitabine regimen may effectively improve the efficacy in the adjuvant setting and may be a novel generation of adjuvant chemotherapy regimen. The results of the current meta-analysis support this hypothesis and indicate that taxane-based regimen with capecitabine may be an effective, convenient, and well tolerated regimen in patients with early breast cancer.
Introduction
The key milestones for breast cancer treatment were endocrine therapy in the 1960s and polychemotherapy in the 1970s. In recent decades, the development of effective targeted therapies has been another significant milestone in breast cancer treatment [1]. As an adjuvant chemotherapy regimen, cyclophosphamide, methotrexate and 5-fluorouracil (CMF) combination chemotherapy has achieved similar excellent efficacy as in the salvage setting [2]. Anthracyclines appeared in 1972, followed by taxanes in the 1990s. Anthracycline- and taxane-based polychemotherapy regimens have achieved better efficacy than any previous chemotherapy regimen, so they have long been recommended as standard adjuvant regimens in the main breast cancer treatment guidelines, such as the National Comprehensive Cancer Network (NCCN) guidelines and St Gallen Consensus. Based on data from large and well-controlled clinical studies, gemcitabine, vinorelbine and capecitabine have also entered clinical care as salvage treatment for metastatic breast cancer. Unfortunately, some chemotherapy drugs such as vinorelbine and gemcitabine have failed to show superior efficacy in the adjuvant treatment, as the Finland Herceptin Trial and tAnGo trial showed [3], [4].
Capecitabine is a fluoropyrimidine carbamate, which is supplied for oral administration as a systemic prodrug of 5′-deoxy-5-fluorouridine (5′-DFUR). 5′-DFUR is converted to 5-fluorouracil by sequential enzyme activity. The enzyme responsible for the final step is thymidine phosphorylase (TP), which is overexpressed in breast cancer [5]. Some cytotoxic drugs such as docetaxel show synergistic effects with capecitabine, because the former can increase the TP level in tumors [6]. Furthermore, capecitabine combined with docetaxel has improved time to progression and overall survival (OS) in some pivotal phase III trials [7]. For this reason, the NCCN Breast Cancer Guidelines Committee decided to incorporate docetaxel/capecitabine as a preferred combined chemotherapy regimen for recurrent or metastatic breast cancer [8].
Capecitabine is effective and indicated for the salvage treatment of metastatic breast cancer. Therefore, it is essential to evaluate the efficacy of capecitabine-containing regimens in the adjuvant setting. To date, there have been two large randomized, open-label, multicenter phase III studies to determine whether patients with high-risk early breast cancer benefit from the addition of capecitabine to standard chemotherapy, but they have yielded inconsistent results. The Finxx trial suggested that addition of capecitabine to a taxane–anthracycline regimen did not significantly improve recurrence-free survival (HR = 0.79, 95% CI: 0.60–1.04, P = 0.087) or OS (HR = 0.73, 95% CI: 0.52–1.04, P = 0.08) as compared to the taxane–anthracycline regimen. The USON 01062 trial found significant improvement in OS (HR = 0.68, 95% CI: 0.51–0.92, P = 0.011) rather than in disease-free survival (DFS) (HR = 0.84, 95% CI: 0.67–1.05, P = 0.125) in favor of capecitabine combined with a taxane–anthracycline regimen [9], [10], [11].
For the above reasons, we sought to undertake a meta-analysis to evaluate the efficacy of capecitabine when combined with standard treatment in the adjuvant setting for early breast cancer. We compared the outcomes of OS, DFS, local recurrence, distant recurrence and breast-cancer-specific survival because these are the main endpoints used in clinical trials. Besides, we performed subgroup analyses according to hormone receptor and HER2 status as well as triple negativity.
Methods
Identification of randomized studies
Two investigators (YW Jiang and WJ Yin) independently obtained relevant English language articles through searches of PubMed, EBSCO and Web of Science databases, conference proceedings (American Society of Clinical Oncology, San Antonio Breast Cancer Symposium and European Society for Medical Oncology) and scanned the reference lists of key trials and review articles from 1998 (based on the first reported trial of capecitabine efficacy in humans) to the end of November 2011. PubMed, EBSCO and Web of Science databases were searched using terms ‘capecitabine’, ‘Xeloda’, and the exploded MeSH term ‘breast neoplasms’. We searched conference proceedings through online websites at www.asco.org, www.esmo.org and www.sabcs.org. We included randomized, open-label, phase III trials in early breast cancer. We excluded trials if they compared the efficacy of capecitabine-based regimens in the salvage and neoadjuvant setting, as well as those to test the efficacy of single capecitabine in the adjuvant setting.
Study endpoints
In this meta-analysis, the primary outcome was DFS, defined as time from randomization to the first occurrence of disease progression or death from any cause without documentation of a cancer-related event. Secondary outcomes included overall survival (death from any cause), time to distant recurrence, breast-cancer-specific survival (death from breast cancer) and time to death from other causes.
Data extraction
From each eligible trial, two independent reviewers (YW Jiang and WJ Yin) extracted data, including authors' names, journal, year of publication, trial design, patient eligibility, baseline patient characteristics, dosing regimens, duration of follow-up, and treatment changes due to toxicity. For trials that compared different types of treatment, we derived the number of patients with any recurrence or any death and the total number of patients in each treatment arm. We also derived HRs and 95% CIs for the outcomes OS and DFS, or evaluated the logarithm of the HR for death. All of the outcomes were based on the intention-to-treat (ITT) analysis. If the trial results were reported in multiple publications, we extracted the most recently reported endpoints.
Statistical analysis
HR was used to evaluate the efficacy of a taxane–anthracycline regimen and a taxane–anthracycline–capecitabine regimen in early breast cancer. For each study, the between-study heterogeneity was assessed by the χ2 based Q statistics and I2 test. Heterogeneity was considered as either P<0.50 or I2>50%. All of the data from each study used either fixed effects (Mantel–Haenszel's method) or random-effects (DerSimonian and Laird's method) models according to the heterogeneity result. If there was no between-study heterogeneity, the two methods provided similar results. Funnel plots and Begger's test were used to test the possible publication bias. Sensitivity analyses were performed to evaluate the influence of individual studies on the summary effect. In the subgroup analysis, statistical analysis was performed in different hormone receptor status, HER2 status and triple negative status. All of the analyses were performed by Stata 10.0 software (Stata Corporation, College Station, TX, USA), using two-sided P values.
Results
Eligible studies
Based on the search strategy, two studies were selected. The two trials enrolled 4107 breast cancer patients, of whom 2058 received a taxane–anthracycline–capecitabine-containing regimen and 2049 received a taxane–anthracycline-based regimen. All of the patients were histologically confirmed as having invasive breast cancer. These two studies had study population and trial design in common. The study details are shown in Table 1.
Table 1. Characteristics of studies included for the meta-analysis.
| study | patients | treatment | Cycles | Duration of follow-up |
| per treatment arm | ||||
| USO | 1304 | Epirubicin+Cyclophosphamide q3w | 4 | 5 years |
| Docetaxel q3w | 4 | |||
| 1307 | Epirubicin+Cyclophosphamide q3w | 4 | ||
| Docetaxel+Capecitabine q3w | 4 | |||
| FinXX | 745 | Docetaxel q3w | 3 | 5 years |
| +Epirubicin+Cyclophosphamide+5- fluorouracil q3w | 3 | |||
| 751 | Docetaxel++Capecitabine q3w | 3 | ||
| +Epirubicin+Cyclophosphamide+5- fluorouracil+Capecitabine q3w | 3 |
Meta-analysis of the primary endpoint
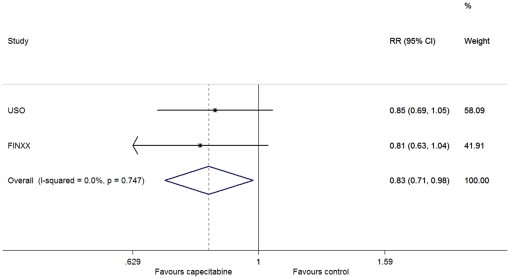
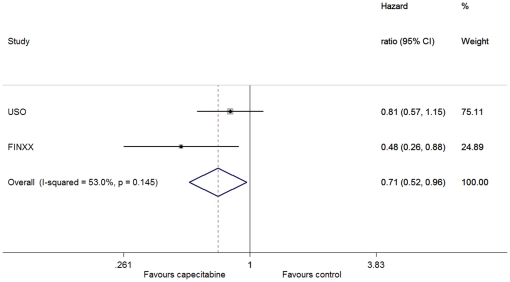
There was no between-study heterogeneity in DFS (heterogeneity χ2 = 0.10 (d.f. = 1), I2 = 0.0%, P = 0.747). Therefore, we used the fixed-effect model to analyze the data and found that DFS was significantly improved in the capecitabine arm versus the controls (HR = 0.83, 95% CI: 0.71–0.98; P = 0.027) (figure 1).
Figure 1. Forest plot of meta-analysis on the disease-free survival for the addition of capecitabine to standard treatment.
Meta-analysis of the secondary endpoints
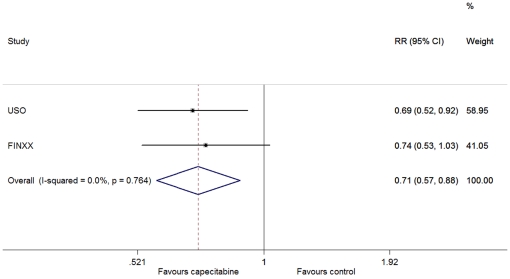
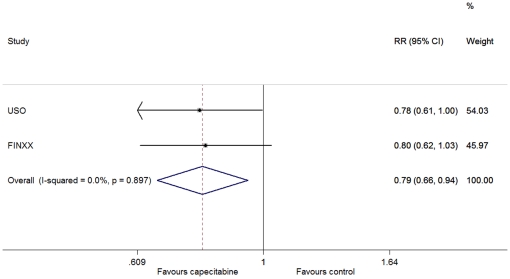
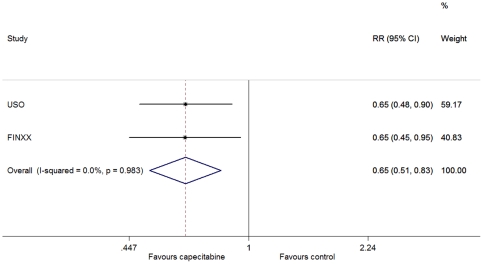
There was no between-study heterogeneity in HRs of the studies (heterogeneity χ2 = 0.09 (d.f. = 1), I2 = 0.0%, P = 0.764) and the addition of capecitabine to standard treatment showed improvement in OS (HR = 0.71, 95% CI: 0.57–0.88; P = 0.002) (figure 2). For the distant recurrence, through the fixed-effect model (heterogeneity χ2 = 0.02 (d.f. = 1), I2 = 0.0%, P = 0.897), we also observed a significant improvement in favor of the capecitabine-based arm (HR = 0.79, 95% CI: 0.66–0.94; P = 0.008) (figure 3). We used the fixed-effect model (heterogeneity χ2 = 0·00 (d.f. = 1), I2 = 0.0%, P = 0.983) to analyze the breast-cancer-specific survival, and found that there was a significant difference between the two arms (HR = 0.65, 95% CI: 0.51–0.83, P = 0.001) (figure 4). However, no difference was discerned in death from other causes between patients with and without the addition of capecitabine (heterogeneity χ2 = 0.31 (d.f. = 1), I2 = 0.0%, P = 0.576; HR = 1.07, 95% CI: 0.63–1.82, P = 0.798).
Figure 2. Forest plot of meta-analysis on the overall survival for the addition of capecitabine to standard treatment.
Figure 3. Forest plot of meta-analysis on the distant recurrence for the addition of capecitabine to standard treatment.
Figure 4. Forest plot of meta-analysis on the breast cancer specific survival for the addition of capecitabine to standard treatment.
Subgroup analysis
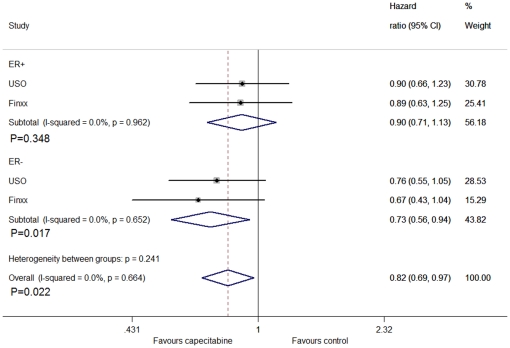
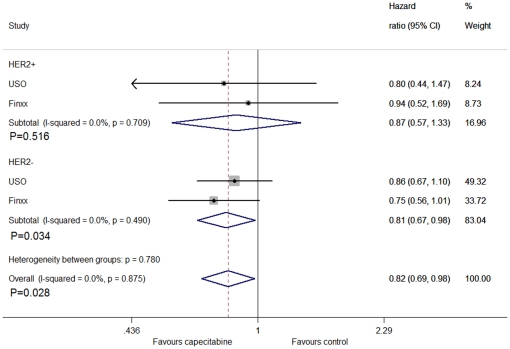
For the subgroup analysis, we divided them into triple negative breast cancer patients and non-triple negative breast cancer patients. From the analysis, we found that capecitabine improved DFS in triple negative patients (HR = 0.71, 95% CI: 0.53–0.96, P = 0.028), by fixed method (figure 5). For the different hormone receptor status, no significant difference was found between the groups in hormone receptor positive patients (HR = 0.90, CI: 0.71–1.13, P = 0.348), but we exactly found the difference between the groups in hormone receptor negative patients (HR = 0.73, CI: 0.56–0.94, P = 0.017) (figure 6).The association between DFS and HER2 status were not statistically significant in HER2 positive patients (HR = 0.87, CI: 0.57–1.33, P = 0.516), but revealed a significant difference in HER2 negative counterparts (HR = 0.81, CI: 0.67–0.98, P = 0.034) (figure 7).
Figure 5. Forest plot of meta-analysis on the disease-free survival in triple negative patients for the addition of capecitabine to standard treatment.
Figure 6. Forest plot of meta-analysis on the disease-free survival in hormone receptor positive and negative patients for the addition of capecitabine to standard treatment.
Figure 7. Forest plot of meta-analysis on the disease-free survival in HER2 positive and negative patients for the addition of capecitabine to standard treatment.
Publication bias and sensitivity analyses
We performed the funnel plots and Begger's test to assess the publication bias. As a result, there was no publication bias in each test (z = −1.00, P = 0.317) for the primary endpoint analysis and the secondary endpoint analysis (data not shown). The influence of individual studies on the summary effect estimate was performed by sensitivity analyses on the overall HR. No individual study affected the overall HR, because omission of any single study made no significant difference.
Discussion
This meta-analysis aimed to assess the efficacy of addition of capecitabine to anthracycline–taxane-based adjuvant therapy in high-risk early breast cancer for the first time. First, it was reasonable to merge these two large clinical trials because of their similar regimens for control (anthracycline–taxane-based polychemotherapy) and experiment arm (anthracycline–taxane–capecitabine-based polychemotherapy). Second, capecitabine in combination with docetaxel is synergic and effective and is indicated for treatment of metastatic breast cancer [12]. Therefore, it may also improve prognosis of adjuvant therapy. The present meta-analysis does demonstrate the clinical benefits of addition of capecitabine to polychemotherapy.
Many trials, such as PACS01 and NSABP B28, have demonstrated that taxane-containing regimens improve DFS and OS in an adjuvant treatment for breast cancer patients [13]. Capecitabine is a prodrug that is enzymatically metabolized in the liver to fluorouracil, and it eventually inhibits DNA synthesis and function. On the other hand, its synergistic effect with docetaxel and other cytotoxic drugs can be attributed to an increase in TP level in the tumors [14], [15]. The control regimens of the USON 01062 (AC-T) and Finxx (T-CEF) trials may be similar in efficacy, although the efficacy of the T-CEF regimen is still under investigation. These two large trials failed to provide a satisfactory outcome, but from the current meta-analysis, we found that capecitabine plus standard chemotherapy significantly improved OS, DFS, local recurrence, distant recurrence, and breast-cancer-specific survival. Due to the synergistic effect of taxane and capecitabine, taxane–anthracylcine-based regimens with capecitabine may effectively inhibit distant micrometastases, to further improve the efficacy, and could be a novel combination chemotherapy regimen. A previous study showed that adjuvant capecitabine monotherapy is not superior to CMF administered every 3 weeks. However, combination regimens with capecitabine may provide benefits over single-agent therapy because the combined regimen enhances the control of metastatic foci [16].
Our meta-analysis provides the efficacy of capecitabine to some subtypes of early breast cancer patients, such as hormone receptor negative, HER2 negative and triple negative cancers. This was also showed in ABCSG-24 trial that addition of capecitabine to epirubicin plus docetaxel is associated with a greater chance of achieving pCR when the cancer is hormone receptor negative [17]. Confirmation of these findings require further research but interactions of capecitabine with other chemotherapy agents, especially in some biologic shugroups of early breast cancer. Triple negative patients have an absence of estrogen receptor, progesterone receptor and HER2/neu expression, and have worse prognosis [18], [19]. Furthermore, there is no standard adjuvant treatment for this category of patient [20]. Some trials have provided a clue for the striking effect of taxane-containing polychemotherapy in improving breast cancer in triple negative patients [21], [22], [23]. However, our meta-analysis suggests that addition of capecitabine to anthracycline–taxane-based polychemotherapy is better than anthracycline–taxane-based polychemotherapy, and therefore, might be one of the good regimens for adjuvant therapy of triple negative breast cancer.
Capecitabine is an oral, tumor-targeted drug. From previous trials, we have found fewer adverse reactions with docetaxel and capecitabine compared with anthracycline or paclitaxel [7], [24], [25]. To some extent, addition of capecitabine did not significantly increase the toxicity, except for diarrhea and hand–foot syndrome related to capecitabine, but it might have reduced neutropenia and febrile neutropenia, which could have been due to reduced dose of docetaxel [3].
There were some limitations to our meta-analysis. Only two trials were included in this analysis. Fortunately, all encouraging results were yielded in the ITT analysis, which provides powerful evidence to support capecitabine-based polychemotherapy as a good candidate regimen for adjuvant therapy of early breast cancer. We are looking forward for more clinical trials to perfect this hypothesis. However, the two trials were very similar, and therefore this meta-analysis might be highly persuasive.
Above all, the results of the current meta-analysis indicate that taxane-based regimens with capecitabine may be effective, convenient, and well-tolerated in patients with early breast cancer; especially in triple negative patients.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Arcamone F, Bernardi L, Patelli B, Giardino P, Di Marco A, et al. Synthesis and antitumour activity of new daunorubicin and adriamycin analogues. Experientia. 1978;34:1255–1257. doi: 10.1007/BF01981401. [DOI] [PubMed] [Google Scholar]
- 3.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, et al. Adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for breast cancer: an open-label, randomised controlled trial. Lancet Oncol. 2009;10:1145–1151. doi: 10.1016/S1470-2045(09)70307-9. [DOI] [PubMed] [Google Scholar]
- 4.Poole C. Adjuvant chemotherapy for early-stage breast cancer: the tAnGo trial. Oncology (Williston Park) 2004;18:23–26. [PubMed] [Google Scholar]
- 5.Kaye SB. New antimetabolites in cancer chemotherapy and their clinical impact. Br J Cancer. 1998;78(Suppl 3):1–7. doi: 10.1038/bjc.1998.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, et al. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res. 1998;4:1013–1019. [PubMed] [Google Scholar]
- 7.O'Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7:1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H, Kellokumpu-Lehtinen P-L, Huovinen R, Jukkola-Vuorinen A, Tanner M, et al. FinXX Final 5-Year Analysis: Results of the Randomised, Open-Label, Phase III Trial in Medium-to-High Risk Early Breast Cancer; 2010 December 8–12; San Antonio, TX. Cancer Res. Abstract nr S4-1. [Google Scholar]
- 10.O'Shaughnessy J, Paul D, Stokoe C, Pippen J, Jr, Blum JL, et al. First Efficacy Results of a Randomized, Open-Label, Phase III Study of Adjuvant Doxorubicin Plus Cyclophosphamide, Followed by Docetaxel with or without Capecitabine, in High-Risk Early Breast Cancer; 2010; San Antonio, TX. Cancer Res. Abstract nr S4-2. [Google Scholar]
- 11.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: final analysis of the randomized FinXX trial. J Clin Oncol. 2012;30:11–18. doi: 10.1200/JCO.2011.35.4639. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Aspitia A, Perez EA. Anthracycline- and/or taxane-resistant breast cancer: results of a literature review to determine the clinical challenges and current treatment trends. Clin Ther. 2009;31:1619–1640. doi: 10.1016/j.clinthera.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Campone M, Fumoleau P, Bourbouloux E, Kerbrat P, Roche H. Taxanes in adjuvant breast cancer setting: which standard in Europe? Crit Rev Oncol Hematol. 2005;55:167–175. doi: 10.1016/j.critrevonc.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Kurosumi M, Tabei T, Suemasu K, Inoue K, Kusawake T, et al. Enhancement of immunohistochemical reactivity for thymidine phosphorylase in breast carcinoma cells after administration of docetaxel as a neoadjuvant chemotherapy in advanced breast cancer patients. Oncol Rep. 2000;7:945–948. doi: 10.3892/or.7.5.945. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto-Ouchi K, Tanaka Y, Tominaga T. Schedule dependency of antitumor activity in combination therapy with capecitabine/5′-deoxy-5-fluorouridine and docetaxel in breast cancer models. Clin Cancer Res. 2001;7:1079–1086. [PubMed] [Google Scholar]
- 16.Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 17.Steger GG, Greil R, Jakesz R, Lang A, Mlineritsch B, et al. Pathologic complete response (pCR) in patient subgroups: An analysis of ABCSG-24, a phase III, randomized study of anthracycline- and taxane-based neoadjuvant therapy with or without capecitabine in early breast cancer (EBC); 2010 June 4–8; Chicago, Illinois. J Clin Oncol. abstr 530. [Google Scholar]
- 18.Lin Y, Yin W, Yan T, Zhou L, Di G, et al. Site-specific relapse pattern of the triple negative tumors in Chinese breast cancer patients. BMC Cancer. 2009;9:342. doi: 10.1186/1471-2407-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin WJ, Lu JS, Di GH, Lin YP, Zhou LH, et al. Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat. 2009;115:325–333. doi: 10.1007/s10549-008-0096-0. [DOI] [PubMed] [Google Scholar]
- 20.De Laurentiis M, Cianniello D, Caputo R, Stanzione B, Arpino G, et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer Treat Rev. 2010;36(Suppl 3):S80–86. doi: 10.1016/S0305-7372(10)70025-6. [DOI] [PubMed] [Google Scholar]
- 21.Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 22.Loesch D, Greco FA, Senzer NN, Burris HA, Hainsworth JD, et al. Phase III multicenter trial of doxorubicin plus cyclophosphamide followed by paclitaxel compared with doxorubicin plus paclitaxel followed by weekly paclitaxel as adjuvant therapy for women with high-risk breast cancer. J Clin Oncol. 2010;28:2958–2965. doi: 10.1200/JCO.2009.24.1000. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Segui MA, Anton A, Ruiz A, Ramos M, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 24.Morales S, Lorenzo A, Ramos M, Ballesteros P, Mendez M, et al. Docetaxel plus epirubicin is a highly active, well-tolerated, first-line chemotherapy for metastatic breast cancer: results of a large, multicentre phase II study. Cancer Chemother Pharmacol. 2004;53:75–81. doi: 10.1007/s00280-003-0690-0. [DOI] [PubMed] [Google Scholar]
- 25.Airoldi M, Cattel L, Pedani F, Marchionatti S, Tagini V, et al. Clinical and pharmacokinetic data of a docetaxel-epirubicin combination in metastatic breast cancer. Breast Cancer Res Treat. 2001;70:185–195. doi: 10.1023/a:1013070612986. [DOI] [PubMed] [Google Scholar]