Abstract
The M3 subtype of muscarinic acetylcholine receptors (M3-mAChR) plays a protective role in myocardial ischemia and microRNAs (miRNAs) participate in many cardiac pathophysiological processes, including ischemia-induced cardiac injury. However, the role of miRNAs in M3-mAChR mediated cardioprotection remains unexplored. The present study was designed to identify miRNAs that are involved in cardioprotective effects of M3-mAChR against myocardial ischemia and elucidate the underlying mechanisms. We established rat model of myocardial ischemia and performed miRNA microarray analysis to identify miRNAs involved in the cardioprotection of M3-mAChR. In H9c2 cells, the viability, intracellular free Ca2+ concentration ([Ca2+]i), intracellular reactive oxygen species (ROS), miR-376b-5p expression level, brain derived neurophic factor (BDNF) and nuclear factor kappa-B (NF-κB) levels were measured. Our results demonstrated that M3-mAChR protected myocardial ischemia injury. Microarray analysis and qRT-PCR revealed that miR-376b-5p was significantly up-regulated in ischemic heart tissue and the M3-mAChRs agonist choline reversed its up-regulation. In vitro, miR-376b-5p promoted H2O2-induced H9c2 cell injuries measured by cells viability, [Ca2+]i and ROS. Western blot and luciferase assay identified BDNF as a direct target of miR-376b-5p. M3-mAChR activated NF-κB and thereby inhibited miR-376b-5p expression. Our data show that a novel M3-mAChR/NF-κB/miR-376b-5p/BDNF axis plays an important role in modulating cardioprotection. MiR-376b-5p promotes myocardial ischemia injury possibly by inhibiting BDNF expression and M3-mAChR provides cardioprotection at least partially mediated by the downregulation of miR-376b-5p through NF-κB. These findings provide new insight into the potential mechanism by which M3-mAChR provides cardioprotection against myocardial ischemia injury.
Introduction
Ischemic heart disease is the leading cause of death in the industrialized world [1]. Myocardial ischemia could induce unstable angina, arrhythmia, myocardial infarction or its neopathy, leading to sudden cardiac death [2]–[4]. While many drugs failed to efficaciously treat cardiac death with myocardial ischemia and myocardial infarction [5], recent studies have shown that M3 subtype of muscarinic acetylcholine receptors (M3-mAChR) in the heart have cardioprotective effect against myocardial ischemia [6]–[10].
MicroRNAs (miRNAs) emerge as important regulator of gene expression and have been implicated in numerous cardiovascular pathological processes, including cardiac fibrosis, cardiac hypertrophy, heart failure and cardiac arrhythmia [11]–[14]. Specially, several miRNAs including miRNA-320 [15], miRNA-21 [16] and miRNA-494 [17] are involved in myocardial ischemia. However, the potential role of miRNAs in cardioprotection of M3-mAChR remains to be elucidated. Therefore, the present study aimed to explore the contribution of miRNAs to cardioprotective effects mediated by M3-mAChR against myocardial ischemia. Our results revealed for the first time that miR-376b-5p increased myocardial ischemia injury and was involved in M3-mAChR's cardioprotection.
Materials and Methods
Ethics Statement
All experimental protocols were pre-approved by the Experimental Animal Ethic Committee of Harbin Medical University, China (Animal Experimental Ethical Inspection Protocol No. 2009104). Use of animals was confirmed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypsin and other tissue culture reagents were obtained from Life Technologies, Inc (Carlsbad, CA, USA). The bicinchoninic acid (BCA) protein assay reagents were obtained from Pierce (Rockford, IL, USA). Sodium pentobarbital was obtained from Shanghai Chemicals (Shanghai, China). Choline chloride (choline, agonist of M3-mAChR), 4-diphenylacetoxy-N-methylpiperidine methiodide (4DAMP, antagonist of M3-mAChR) and lipopolysaccharide (LPS, an agonist of NF-κB) were obtained from Sigma (St. Louis, MO, USA). MiRNAs were from Shanghai GenePharma Co.,Ltd.(Shanghai, China). Lipofectamine 2000 and Alexa Fluor® 800 goat anti-mouse IgG or anti-rabbit IgG were purchased from Invitrogen (Carlsbad, CA,USA). Total RNA Purification Kit was from Norgen Biotek (Thorold, ON, Canada). The mirVanaTM qRT-PCR miRNA Detection Kit was from Ambion (Austin, TX, USA). Bio-Rad Protein Assay Kit was obtained from Bio-Rad, (Mississauga, ON, Canada). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Roche Diagnostics GmBH (Mannhein, Germany). 2′,7′- dichlorodihydrofluorescein diacetate (DCFH-DA) was from Molecular Probes (Eugene, USA). Anti-nuclear factor kappa-B (NF-κB) antibody and anti-GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-brain derived neurophic factor (BDNF) antibody was obtained from abcam (Lake Placid, NY, USA). Luciferase reporter assay kit was from Promega (Madison, WI, USA). All other chemicals were purchased from either Sigma. The purity of all reagents was at least analytical grade.
Establishment of rat myocardial ischemia model
Male Wistar rats (weight 280–300 g) were randomly divided into four groups: control, ischemia, choline (agonist of M3-mAChR), and choline+4DAMP (antagonist of M3-mAChR) groups. All treatments were administered via the lingual vein with doses of choline and 4DAMP as described previously [7]. Choline (10 mg/kg, i.v.) was administered 10 min before the occlusion. For choline+4DAMP group, 4DAMP (0.12 µg/kg) was administered 5 min before choline. Rat model of myocardial ischemia was established as previously described [8]. Briefly, the rats were anesthetized intraperitoneal injection with sodium pentobarbital (40 mg/kg) and the respiration of rats was controlled by the volume-controlled rodent ventilator. The ventilator setting and the level of anesthesia were adjusted to maintain the animal in an anaesthetize status without spontaneous breathing efforts. A left thoracotomy was performed at the 3rd–4th rib and a segment of saline-soaked 5-0 sutures was looped around the left anterior descending (LAD) coronary artery, near its origin from the left coronary artery. Successful occlusion was observed by electrocardiographic ST-segment elevation. The chest was closed before the rats were weaned from the ventilator and extubated. Rats were put into cage to breed with normal water and feeds after waking up. Mortality rate of the rats were calculated and compared after 24 h myocardial infarction. After 24 h ischemia, rats were reanesthetized and the hearts were removed for the measurement of myocardial infarct size and microRNA microarray analysis. Control group without drugs was handled in the same manner except that the coronary artery was not ligated.
Measurement of myocardial infarct size
Ventricular tissues were dissected from the animals after 24 h ischemia and kept overnight at −4°. Frozen ventricles were sliced into 2 mm thick sections, and then incubated in 1% triphenyltetrazolium chloride (TTC) at 37°C in 0.2 M Tris buffer (pH 7.4) for 30 min. The non-ischemia area was stained as red and infarct area grey white. Size of the infarcted area was estimated by weight as a percentage of the left ventricle [7].
MicroRNA microarray analysis
After 24 h ischemia, surrounding tissues of infarcted area from ventricular were departed for microRNA microarray analysis by using hybridization to μParaflo®microfluidics microarrays (LC Sciences) in the version of the Sanger miRBase database Release 10.1 (Release 12, http://microrna.sanger.ac.uk/). Each detection probe consisted of a chemically modified nucleotide-coding segment complementary to the target miRNA or other target and a spacer segment of polyethylene glycol to extend the coding segment away from the substrate. Each miRNA probe was represented 5 times on a single microarray, and the control probes were spiked into the RNA samples before the labeling. The detection probes were prepared by in situ synthesis using PGR (photogenerated) chemistry to allow highly sensitive and specific detection of miRNAs. The μParaflo® technology enabled on-chip synthesis, ensuring high probe quality and tight process control.
Cell culture
H9c2 myoblast cell line (the rat embryonic ventricular myocardial cell line) were cultured and maintained as monolayer in high glucose DMEM, supplemented with 10% heat inactivated FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, at 37°C in a humidified incubator with 5% CO2. H9c2 cells were plated at a density of 5000 cells/cm2 and allowed to proliferate in growth medium. Medium was changed every 3 days.
Transfection of miRNAs and miRNA inhibitor
Rat miR-376b-5p, miR-539, 2′-O-methyl-modified antisense oligoribonucleotides of miR-376b-5p (AMO-376b-5p, miR-376b-5p inhibitor) and miR-376b-5p negative control (miR-NC) were synthesized by Shanghai GenePharma. The sequence of AMO-376b-5p was antisense of the mature miRNA sequence (for rat: 5′-GUGGAUAUUCCUUCUAUGGUUA-3′) and miR-NC was a random sequence. AMO-376b-5p contained 2′-O-methyl modification at every base and a 3′C3-containing amino linker.
H9c2 cells were transfected with miR-NC (100 nM), miR-376b-5p (100 nM), miR-539 (100 nM), miR-376b-5p (100 nM) and AMO-376b-5p (100 nM) using lipofectamine 2000 according to the manufacturer's instruction and incubated at 37°C for 24 h. Subsequently, cells were maintained in the culture until analysis [11].
MTT assay
H9c2 cells were seeded into 96-well plates at the density of 2×104/well. After 24 h, MiR-539, miR-NC, miR-376b-5p and AMO-376b-5p were transfected and incubated for 24 h, followed by treatment with H2O2 (50 µM) for 12 h. Then 10 µl MTT reagents (0.5 mg/ml) was added and incubated for 4 h. After dissolving the formazine granulars with 150 µl dimethyl sulphoxide (DMSO), the absorbance at 570 nm was measured by using a microplate reader. The cell viability = (Acontrol group−Aexperimental group)/Acontrol group×100%.
Measurement of intracellular free Ca2+
H9c2 cells were seeded into 6-well plates. The cells were treated with H2O2 (50 µM) for 12 h before transfection with miRNAs. The [Ca2+]i levels were measured 24 h after transfection. Pluronic F-127 was used as a dispersing agent to facilitate the loading of cells with a final concentration of 0.012% Fluo-3/AM in the loading medium. Changes in the fluorescence intensity of Fluo-3/AM-loaded cells were detected by laser scanning confocal microscopy (FV-300; Olympus, Tokyo, Japan) at 488 nm excitation and 530 nm emission wavelengths. The acquisition rate was one frame at 10 s intervals and [Ca2+]i was monitored for 300 s.
Measurement of intracellular ROS
Intracellular reactive oxygen species (ROS) were stained using DCFH-DA. H9c2 cells were seeded into 6-well plates. The cells were treated with H2O2 (50 µM) for 12 h before transfection with miRNAs. The intracellular ROS levels were measured 24 h after transfection. The cells were washed with serum-free DMEM and incubated with 10 µmol/L DCFH-DA in the loading medium in 5% CO2/95% air at 37°C for 20 min. After DCFH-DA was removed, the cells were washed twice and the DCF fluorescence intensity was observed and photographed by laser scanning confocal microscopy (FV-300; Olympus, Tokyo, Japan) at 488 nm excitation and 525 nm emission wavelengths. The acquisition rate was one frame at 10 s intervals and ROS was monitored for 300 s.
RT-PCR
Total RNA was isolated from H9c2 cells or rat myocardium with Total RNA Purification Kit. qRT-PCR for miR-376b-5p, miR-539 was performed on a GeneAmp 5700 thermocycler using mirVanaTM miRNA Detection Kit following the manufacturer's instructions. U6 was used as an internal control. The primers were as follows: U6 primers q5′-CTCCGATAGATCTGCCCTCTTGAA-3′ (forward), 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse). miR-376b-5p primers 5′-GGGGGTGGATATTCCTTCT-3′ (forward), 5′-CAGTGCGTGTCGTGGAGT-3′ (reverse). miR-539 primers 5′-GGGGGAGAAATTATCCTT-3′ (forward), 5′-TGCGTGTCGTGGAGTC-3′ (reverse).
Western blot analysis
Whole cell extract and nuclear extract were extracted from H9c2 cells. The protein content was determined with Bio-Rad Protein Assay Kit using bovine serum albumin as the standard. Protein sample (50 µg) was fractionated by 7.5–10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in 5% milk PBS-Tween20 (PBST) for 3 h and incubated overnight at 4°C with the following primary antibodies: BDNF, NF-κB or GAPDH as internal controls, followed by incubation with Alexa Fluor® 800 goat anti-mouse IgG or anti-rabbit IgG (1∶10000) for 1 h. The images were captured on the Odyssey Infrared Imaging System (LI-COR Bioscience, Lincoln, NE, USA) and band intensity was quantified using Odyssey v1.2 software.
Luciferase activity assay
A fragment of the 3′-untranslated region (3′-UTR) of BDNF containing the putative miR-376b-5p binding sequence was cloned into psiCHECK-2 (Renilla luciferase vector) and the construct (0.5 µg) was transfected into HEK 293T cells with miR-NC, miR-376b-5p, AMO-376b-5p or AMO-376b-5p negative control (AMO-NC). After 24 h, luciferase activities were measured with a dual luciferase reporter assay kit on a luminometer (Lumat LB9507) (EG&G Berthold, Bad Wildbad, Germany).
Statistical analysis
Average data were presented as mean ± standard error and were analyzed by analysis of variance (ANOVA) followed by Bonferroni's post-hoc test. P<0.05 was considered statistically significant.
Results
MicroRNAs are involved in M3-mAChR mediated cardioprotection against myocardial ischemia in rat
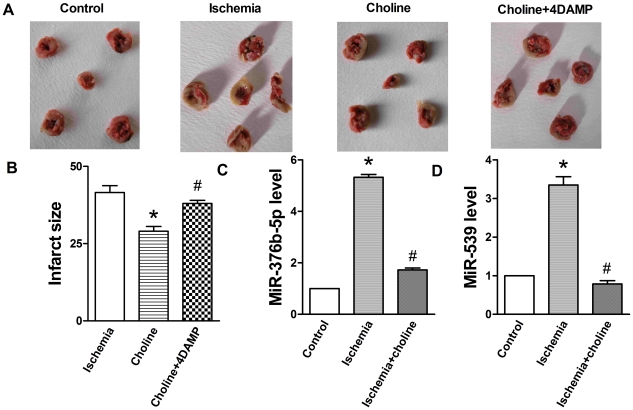
We established rat model of myocardial ischemia and examined mortality rate and myocardial infarct size. Choline significantly reduced the infarct size, which was reversed by 4DAMP (Figure 1A and B). As expected, choline group showed an obvious decrease in mortality rate (8.33%, n = 12, 1 died) compared to control group subjected to the same procedure (28.6%, n = 14, 4 died), and 4DAMP increased mortality rate (14.3%, n = 14, 2 died) compared to choline group. Taken together, these results confirmed that M3-mAChR provides cardioprotection against myocardial ischemia.
Figure 1. MiRNA is involved in M3-mAChR mediated cardioprotection against myocardial ischemia in rat.
Choline (10 mg/kg, i.v.) was administrated 10 min before occlusion. 4DAMP (0.12 µg/kg) was administered 5 min before choline. (A) Representative infarct photographs. (B) Infarct size. Values were expressed as mean ± SEM; n = 8; *P<0.05 vs. Control; # P<0.05 vs. Choline. (C) Choline reversed ischemia-induced miR-376b-5p up-regulation. (D) Choline reversed ischemia-induced miR-539 up-regulation. Values were expressed as mean ± SEM; n = 3; *P<0.05 vs. Control, # P<0.05 vs. Ischemia.
To determine whether miRNAs are involved in M3-mAChR mediated cardioprotection, we performed miRNA microarray analysis and identified 12 differentially upregulated miRNAs upon myocardial ischemia, among which 10 miRNAs were differentially downregulated by M3-mAChR agonist choline (Table 1). While miR-199a, miR-376b-5p, miR-539 and miR-106 were all significantly upregulated (more than 3 times compared to the control), only miR-376b-5p and miR-539 were downregulated to the level similar to the control group after treatment with choline.
Table 1. The levels of differentially expressed miRNAs in the rat sufferred from myocardial ischemia or administrated with choline after ischemia.
| Probe_ID | Control | Ischemia | Ischemia-choline |
| rno-miR-199a | 1 | 12.7 | 2.30 |
| rno-miR-449 | 1 | 1.80 | 1.20 |
| rno-miR-376b-5p | 1 | 9.70 | 1.87 |
| rno-miR-539 | 1 | 4.00 | 0.81 |
| rno-miR-106 | 1 | 3.15 | 0.15 |
| rno-miR-92 | 1 | 2.52 | 1.11 |
| rno-miR-135b | 1 | 1.80 | 1.16 |
| rno-miR-349 | 1 | 1.58 | 0.41 |
| rno-miR-122a | 1 | 1.65 | 1.10 |
| rno-miR-18 | 1 | 2.00 | 1.03 |
Male Wistar rats were divided into control, ischemia and choline chloride (choline, agonist of M3-mAChR) groups. Myocardial ischemia model was induced for 24 h. Choline (10 mg/kg, i.v.) was administrated 10 min before occlusion. After 24 h ischemia, ventricular tissues were dissected for miRNA microarray analysis. MiRNAs levels were calculated based on the normalization to control group (set as 1).
To confirm the expression changes of miR-376b-5p and miR-539 in myocardial ischemia, next we performed qRT-PCR analysis and found that miR-376b-5p and miR-539 were significantly upregulated upon myocardial ischemia and down-regulated by choline (P<0.05, Figure 1C and D). These data demonstrate that miR-376b-5p and miR-539 are significantly upregulated in ischemic heart of the rats and M3-mAChR agonist choline could reverse their upregulation. Therefore, miR-376b-5p and miR-539 appear to be involved in M3-mAChR mediated cardioprotection against myocardial ischemia.
MiR-376b-5p promotes H2O2 induced H9c2 cells injury
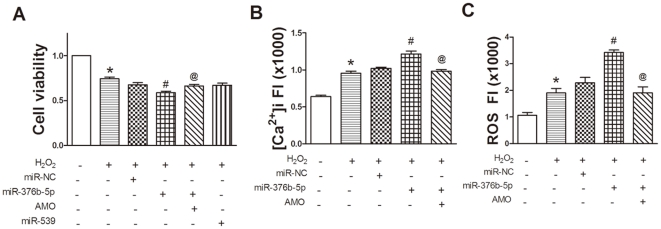
To determine the involvement of miR-376b-5p and miR-539 in myocardial ischemia, we used H9c2 cells as an in vitro model. MTT assay showed that miR-376b-5p significantly enhanced H2O2-induced inhibition of H9c2 cells viability (P<0.05), which was reversed by AMO-376b-5p. However, miR-539 had no significant effect on H2O2-induced inhibition of H9c2 cells viability (Figure 2A). These results suggest that miR-539 is unlikely involved in myocardial ischemia and we focused on miR-376b-5p in the following experiments.
Figure 2. MiR-376b-5p promotes H2O2-induced H9c2 cells injury.
H9c2 cells were pre-treated with H2O2 (50 µM) for 12 h and then transfected with miR-376b-5p negative control (miR-NC), miR-376b-5p, miR-539, and 2′-O-methyl-modified antisense oligoribonucleotides of miR-376b-5p (AMO-376b-5p, miR-376b-5p inhibitor) for 24 h. (A) MTT assay. MiR-376b-5p, not miR-539, reduced cell viability in H2O2-treated H9c2 cells. n = 19 values from 5 independent experiments. (B) Intracellular Ca2+ concentration ([Ca2+]i) fluorescence intensity. MiR-376b-5p significantly increased [Ca2+]i and this effect was reversed by AMO-376b-5p (AMO). n = 29 cells from 5 independent experiments. (C) Reactive oxygen species (ROS) fluorescence intensity. MiR-376b-5p significantly increased relative fluoresence intensity of ROS. n = 12 cells from 3 independent experiments. Values were expressed as mean ± SEM; *P<0.05 vs. Control, # P<0.05 vs. H2O2+miR-NC, @ P<0.05 vs. H2O2+miR-376b-5p. FI: fluorescence intensity.
The overload of intracellular free Ca2+ concentration ([Ca2+]i) is an important cause of cell injury [7], [9]. Therefore, we examined [Ca2+]i in H2O2-treated H9c2 cells and found that miR-376b-5p significantly increased the overload of [Ca2+]i (P<0.05) while AMO-376b-5p reversed this increase (Figure 2B).
Moreover, miR-376b-5p significantly increased the intracellular ROS in H2O2-treated H9c2 cells (P<0.05) and this effect was reversed by AMO-376b-5p (Figure 2C). Taken together, these results suggest that miR-376b-5p promotes H2O2-induced H9c2 cells injury.
M3-mAChR inhibits H2O2 induced upregulation of miR-376b-5p in H9c2 cells
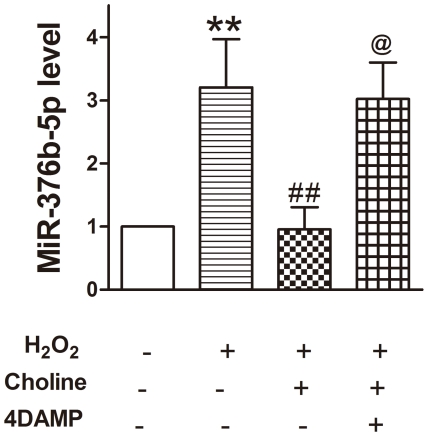
To examine further miR-376b-5p expression in ischemic myocardial cells and the effects of M3-mAChR on miR-376b-5p expression in vitro, H9c2 cells were co-incubated with H2O2 (50 µM), choline (1 mM) or 4DAMP (5 nM) for 12 h. The results showed that H2O2 significantly upregulated the miR-376b-5p expression (P<0.01) and this effect was antagonized by M3-mAChR agonist choline (Figure 3). Furthermore, M3-mAChR antagonist 4DAMP reversed the effect of choline on miR-376b-5p. These results are consistent with the results of gene array analysis verified by qRT-PCR (Figure 1C) and suggest that M3-mAChR inhibits the up-regulation of miR-376b-5p stimulated by H2O2 in H9c2 cells.
Figure 3. M3-mAChR inhibits the up-regulation of miR-376b-5p stimulated by H2O2 in H9c2 cells.
H9c2 cells were co-incubated with H2O2 (50 µM), choline (1 mM) and 4DAMP (5 nM) for 12 h. MiR-376b-5p expression was determined by qRT-PCR. U6 served as an internal control for the normalization. Values were expressed as mean ± SEM; n = 4; **P<0.01 vs. Control, ## P<0.01 vs. H2O2+choline, @ P<0.05 vs. H2O2+choline+4DAMP.
BDNF is a target gene of miR-376b-5p in cardiac myocytes
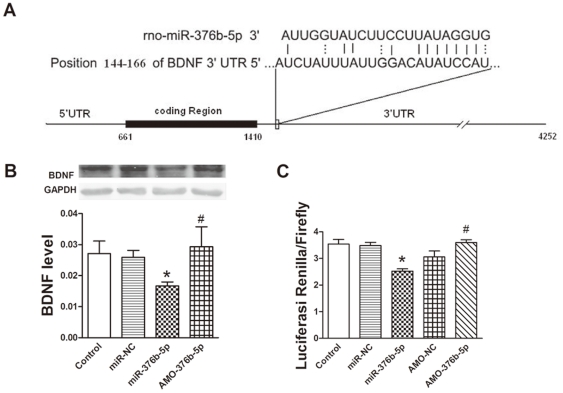
To further explore the role of miR-376b-5p in myocardial ischemia, we predicted the targets of miR-376b-5p by targetScan Release 5.1 online (www.targetscan.org) and focused on BDNF (Figure 4A). Next we determined the expression of BDNF at the protein level in H9c2 cells and found that miR-376b-5p significantly reduced the expression of BDNF (P<0.05), and this reduction was reversed by AMO-376b-5p (Figure 4B). These results suggest that BDNF is a target gene of miR-376b-5p.
Figure 4. BDNF is a target gene of miR-376b-5p in cardiac myocytes.
(A) MiR-376b-5p binding site in 3′-untranslated region (3′-UTR) of BDNF gene. (B) Inhibitory effects of miR-376b-5p on BDNF expression at the protein level. BDNF level was determined by Western blot after H9c2 cells were transfected with miR-376b-5p negative control (miR-NC), miR-376b-5p and 2′-O-methyl-modified antisense oligoribonucleotides of miR-376b-5p (AMO-376b-5p, miR-376b-5p inhibitor) for 24 h. GAPDH served as loading control. Top, representative Western blots; bottom, quantitation as mean ± SEM. Note: n = 3; *P<0.05 vs. miR-NC, # P<0.05 vs. miR-376b-5p. (C) Luciferase assay for direct inhibitory effects of miR-376b-5p on BDNF expression. The reporter construct was transfected into HEK 293T cells with miR-NC, miR-376b-5p, AMO-376b-5p or AMO-376b-5p negative control (AMO-NC). Luciferase activity was normalized by Firefly luciferase signal in HEK 293T cells. Following transfection for 24 h, luciferase activities were measured on a luminometer. Values were expressed as mean ± SEM; n = 3; *P<0.05 vs. miR-NC, # P<0.05 vs. AMO-NC.
To confirm that miR-376b-5p could directly bind to BDNF gene and inhibit its expression, a reporter construct with the insertion of a fragment of the 3′-UTR of BDNF mRNA containing the predicted miR-376b-5p binding site was transfected into HEK 293T cells. Luciferase assay showed that miR-376b-5p significantly reduced the luciferase activity (P<0.05) while AMO-376b-5p significantly increased the luciferase activity (P<0.05) (Figure 4C). Taken together, these data prove that miR-376b-5p directly inhibits BDNF expression.
NF-κB mediates the inhibitory effect of M3-mAChR on miR-376b-5p expression in H9c2 cells
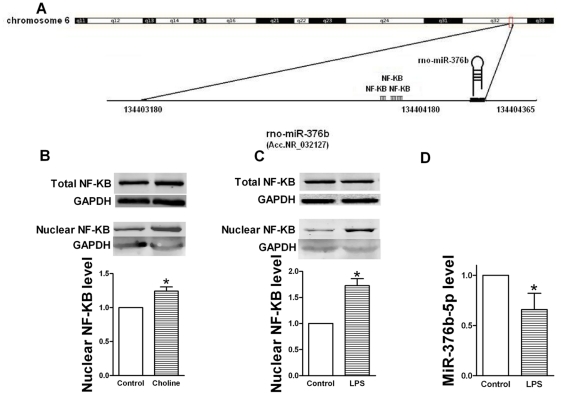
Previous studies demonstrated that M3-mAChR could activate NF-κB [18]–[20]. Moreover, 3 binding sites of NF-κB were predicted on the promoter of miR-376b-5p gene by TFSEARCH (Figure 5A). Therefore, we postulated that NF-κB possibly mediates the inhibitory effects of M3-mAChR on miR-376b-5p expression.
Figure 5. NF-κB mediates the inhibitory effect of M3-mAChR on miR-376b-5p expression.
GAPDH as an internal control normalized NF-κB band for western blot. U6 as an internal control normalized miR-376b-5p for quantitative real-time RT-PCR (qRT-PCR). (A) Chromosomal organization of the miRNA-376b gene locus on rat chromosome 6q32 indicating three NF-κB binding sites in the pre-miR-376b gene promoter. (B) Choline activated NF-κB in H9c2 cells. Top, representative Western blots of total NF-κB; Middle, representative Western blots of nuclear NF-κB; bottom, quantitation of nuclear NF-κB as mean ±SEM Note: n = 4; *P<0.05 versus Control. (C) LPS activated NF-κB in H9c2 cells. Top, representative Western blots of total NF-κB; Middle, representative Western blots of nuclear NF-κB; bottom, quantitation of nuclear NF-κB as mean ±SEM. Note: n = 6; *P<0.05 versus Control. (D) LPS inhibited miR-376b-5p expression in H9c2 cells. MiR-376b-5p expression was determined by qRT-PCR. Values were expressed as mean ± SEM; n = 5; *P<0.05 vs. Control.
To verify that M3-mAChR could activate NF-κB, we determined nuclear NF-κB protein level in H9c2 cells. Western blot analysis showed that nuclear NF-κB level was significantly increased (P<0.05, Figure 5B) after treatment with choline with no significant variance of total NF-κB level, suggesting that M3-mAChR could activate NF-κB.
Next we measured miR-376b-5p expression by qRT-PCR in H9c2 cells treated with LPS (100 ng/ml), which is known to activate NF-κB in many previous studies [21]–[23]. As expected, LPS significantly activated NF-κB (P<0.05, Figure 5C) with no significant variance of total NF-κB level. Furthermore, LPS significantly reduced miR-376b-5p expression (P<0.05, Figure 5D). Collectively, these data indicate that NF-κB mediates the inhibitory effect of M3-mAChR on miR-376b-5p expression.
Discussion
In the present study, for the first time, we found that miR-376b-5p inhibited the expression of BDNF and miR-376b-5p promoted H2O2-induced H9c2 cells injury, indicating that miR-376b-5p promoted myocardial ischemia injury possibly by inhibiting the expression of BDNF. Moreover, we found that M3-mAChR inhibited miR-376b-5p expression by activating NF-κB. These findings provide new insight into the potential mechanism of cardioprotection of M3-mAChR against myocardial ischemia.
First, we observed that M3-mAChR decreased infarct size and mortality rate in rat models, consistent with previous studies showing that M3-mAChR has the protective effect against myocardial ischemia [6]–[10]. Next, to explore the underlying mechanisms responsible for our observation, we focused on miRNAs, given their emerging role in the pathogenesis of the cardiovascular system including myocardial ischemia [11]–[17]. Based on microarray analysis and further validation by qRT-PCR, we postulated that the upregulation of miR-376b-5p and miR-539 is involved in myocardial ischemia and M3-mAChR may protect against myocardial ischemia via the downregulation of miR-376b-5p and miR-539 expression.
H9c2 cells were treated with H2O2 for 12 h to simulate myocardial ischemia injury, which was employed as in vitro model to test our hypothesis. In our experiment, the miRNA transfection efficiency was measured by real-time PCR. MiR-376b-5p level was upregulated more than 3 times after transfection (Figure S1), demonstrating the efficient transfection. Notably, miR-376b-5p but not miR-539 was implicated in reduced H9c2 cells viability induced by H2O2. Therefore, miR-376b-5p was chosen for further characterization.
TargetScan Release 5.1 was used to predict the target genes of miR-376b-5p. Although 1134 potential targets of miR-376b-5p were predicted, only a few of them have been reported to be involved in myocardial ischemia, including insulin-like growth factor 2 mRNA binding protein 2 [24], [25], ryanodine receptor 2 [26]–[28] and BDNF [29]–[35]. Given recent studies demonstrating that BDNF was also expressed in the heart where it protected against myocardial ischemia injury [29]–[35], we proposed that BDNF is one potential target of miR-376b-5p in the heart. Our Western blot analysis and luciferase assay experiments demonstrated that miR-376b-5p could directly bind to BDNF gene and inhibit its expression, providing support for our hypothesis that miR-376b-5p promotes myocardial ischemia injury possibly via the downregulation of BDNF.
To determine the potential mechanism by which M3-mAChR inhibited miR-376b-5p expression, TFSEARCH was used to identify transcription factors that bind to the promoter of miR-376b-5p gene. Interestingly, we found 3 binding sites of NF-κB on the promoter, with percentage scores as 85, 85.8 and 91.4, respectively. NF-κB has been shown to provide myocardial protection by diverse mechanisms including increase of cardiac Bcl-2 [36], [37], induction of manganese superoxide disumutase [38], and inhibition of leukocyte adhesion, cytokines, and chemokines [39]–[42]. In the preconditioning episodes, NF-κB is activated and pharmacological inhibition of NF-κB abolishes its cardioprotective effect [36], [43], [44]. Moreover, several studies have demonstrated that M3-mAChR activated NF-κB [18]–[20], which may be mediated by intracellular Ca2+ and PKC-dependent signaling pathways [19], [45]. In the present study, we demonstrated that NF-κB was activated when M3-mAChR was activated by M3-mAChR agonist choline in H9c2 cells. Furthermore, LPS, NF-κB activator, significantly reduced miR-376b-5p expression. Collectively, these data strongly suggest that M3-mAChR inhibits miR-376b-5p expression via the activation of NF-κB.
In conclusion, our results help establish that a novel M3-mAChR/NF-κB/miR-376b-5p/BDNF axis plays an important role in modulating cardioprotection. To our knowledge, this is the first report to demonstrate that miR-376b-5p promotes myocardial ischemia injury and that the cardioprotection of M3-mAChR against myocardial ischemia is at least partly mediated by the downregulation of miR-376b-5p. The present observations expand our understanding of the cardioprotection of M3-mAChR associated with ischemic heart disease and may lead to rational target selection for therapeutic intervention.
Supporting Information
MiRNA transfection efficiency. (A) Representative H9c2 cell photomicrographs transfected with miRNA with fluorescence. (B) MiR-376b-5p level was significantly up-regulated after transfection with miR-376b-5p. H9c2 cells were transfected with miR-376b-5p for 24 h. MiR-376b-5p level was determined by quantitative real-time RT-PCR (qRT-PCR). Note: Values are expressed as mean ± SEM; n = 3 independent experiments; *P<0.05 versus Control.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the National Natural Science Foundation of China (no. 30973531 and 81072639). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R, Fisher DJ, Taylor AA, Young JB, Miller RR. Effect of alpha-adrenergic blockade on arrhythmias induced by acute myocardial ischemia and reperfusion in the dog. J Mol Cell Cardiol. 1984;16:1101–1117. doi: 10.1016/s0022-2828(84)80037-1. [DOI] [PubMed] [Google Scholar]
- 3.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira M, Santos-Silva PR, de Abreu LC, Valenti VE, Crispim V, et al. Sudden cardiac death athletes: a systematic review. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:19. doi: 10.1186/1758-2555-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circulation. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 6.Shi H, Wang H, Wang Z. Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts. Mol Pharmacol. 1999;55:497–507. [PubMed] [Google Scholar]
- 7.Yang B, Lin H, Xu C, Liu Y, Wang H, et al. Choline produces cytoprotective effects against ischemic myocardial injuries: evidence for the role of cardiac M3 subtype muscarinic acetylcholine receptors. Cell Physiol Biochem. 2005;16:163–174. doi: 10.1159/000089842. [DOI] [PubMed] [Google Scholar]
- 8.Yue P, Zhang Y, Du Z, Xiao J, Pan Z, et al. Ischemia impairs the association between connexin 43 and M3 subtype of acetylcholine muscarinic receptor (M3-mAChR) in ventricular myocytes. Cell Physiol Biochem. 2006;17:129–136. doi: 10.1159/000092074. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Sun HL, Li DL, Wang LY, Gao Y, et al. Choline produces antiarrhythmic actions in animal models by cardiac M3 receptors: improvement of intracellular Ca2+ handling as a common mechanism. Can J Physiol Pharmacol. 2008;86:860–865. doi: 10.1139/Y08-094. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Du J, Gao Y, Zhang Y, Cai BZ, et al. Role of M3 receptor in aconitine/barium-chloride-induced preconditioning against arrhythmias in rats. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:511–515. doi: 10.1007/s00210-008-0376-6. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Lin H, Xiao J, Lu Y, Luo X, et al. The muscle-specific microRNAmiR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Song XW, Zou J, Wang GK, Kremneva E, et al. Attenuation of microRNA-1 derepresses the cytoskeleton regulatory protein twinfilin-1 to provoke cardiac hypertrophy. J Cell Sci. 2010;123:2444–2452. doi: 10.1242/jcs.067165. [DOI] [PubMed] [Google Scholar]
- 13.Kumarswamy R, Anker SD, Thum T. MicroRNAs as circulating biomarkers for heart failure: questions about MiR-423-5p. Circ Res. 2010;106:e8. doi: 10.1161/CIRCRESAHA.110.220616. [DOI] [PubMed] [Google Scholar]
- 14.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren XP, Wu J, Wang X, Sartor MA, Qian J, et al. MicroRNA-320 Is Involved in the Regulation of Cardiac Ischemia/Reperfusion Injury by Targeting Heat-Shock Protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y, Zhu P, Yang J, Liu X, Dong S, et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87:431–439. doi: 10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Zhang X, Ren XP, Chen J, Liu H, et al. MicroRNA-494 Targeting Both Proapoptotic and Antiapoptotic Proteins Protects Against Ischemia/Reperfusion-Induced Cardiac Injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujino H, Uehara T, Murayama T, Okuma Y, Ariga H, et al. Extracellular signal regulated protein kinase and c-jun N-terminal kinase are involved in ml muscarinic receptor-enhanced interleukin-2 production pathway in Jurkat cells. J Biol Pharm Bull. 2000;23:1198–1205. doi: 10.1248/bpb.23.1198. [DOI] [PubMed] [Google Scholar]
- 19.Siehler S, Wang Y, Fan X, Windh RT, Manning DR. Sphingosine 1-phosphate activates nuclear factor-kappa B through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J Biol Chem. 2001;276:48733–48739. doi: 10.1074/jbc.M011072200. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H, Chen D, Zhang J, Tian Y. Involvement of M3 cholinergic receptor signal transduction pathway in regulation of the expression of chemokine MOB-1, MCP-1 genes in pancreatic acinar cells. J Huazhong Univ Sci Technolog Med Sci. 2004;24:140–143, 157. doi: 10.1007/BF02885413. [DOI] [PubMed] [Google Scholar]
- 21.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 22.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akanji AO, Suresh CG, Al-Radwan R, Fatania HR. Insulin-like growth factor (IGF)-I, IGF-II and IGF-binding protein (IGFBP)-3 levels in Arab subjects with coronary heart disease. Scand J Clin Lab Invest. 2007;67:553–559. doi: 10.1080/00365510601173153. [DOI] [PubMed] [Google Scholar]
- 25.Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, et al. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Galphaq interaction and protein kinase C-alpha/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol. 2008;197:381–390. doi: 10.1677/JOE-07-0619. [DOI] [PubMed] [Google Scholar]
- 26.Guo ZY, Jiao Q, Wang ST, Xu MH, Gao FH. Effect of ryanodine receptor 2 gene silencing on ischemia-reperfusion injury of rat myocardial cells. Zhonghua Bing Li Xue Za Zhi. 2008;37:760–764. [PubMed] [Google Scholar]
- 27.Guo Z, Wang S, Jiao Q, Xu M, Gao F. RNAi targeting ryanodine receptor 2 protects rat cardiomyocytes from injury caused by simulated ischemia-reperfusion. Biomed Pharmacother. 2010;64:184–190. doi: 10.1016/j.biopha.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Fauconnier J, Pasquié JL, Bideaux P, Lacampagne A, Richard S. Cardiomyocytes hypertrophic status after myocardial infarction determines distinct types of arrhythmia: role of the ryanodine receptor. Prog Biophys Mol Biol. 2010;103:71–80. doi: 10.1016/j.pbiomolbio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Luo Z, Diaco M, Murohara T, Ferrara N, Isner JM, et al. Vascular endothelial growth factor attenuates myocardial ischemia–reperfusion injury. The Annals of thoracic surgery. 1997;64:993–998. doi: 10.1016/s0003-4975(97)00715-7. [DOI] [PubMed] [Google Scholar]
- 30.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 31.Hiltunen JO, Laurikainen A, Väkevä A, Meri S, Saarma M. Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J Pathol. 2001;194:247–253. doi: 10.1002/path.878. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, et al. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer research. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 33.Kreusser MM, Buss SJ, Krebs J, Kinscherf R, Metz J, et al. Differential expression of cardiac neurotrophic factors and sympathetic nerve ending abnormalities within the failing heart. J Mol Cell Cardiol. 2008;44:380–387. doi: 10.1016/j.yjmcc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Lorgis L, Amoureux S, Vergely C, Zeller M, Cottin Y, et al. Brain-derived neurotrophic factor (BDNF): role of this neurotrophin in cardiovascular physiopathology. Ann Cardiol Angeiol. 2009;58:99–103. doi: 10.1016/j.ancard.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Maulik N, Sato M, Price BD, Das DK. An essential role of NFkappaB in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischaemia. FEBS Lett. 1998;429:365–369. doi: 10.1016/s0014-5793(98)00632-2. [DOI] [PubMed] [Google Scholar]
- 37.Maulik N, Goswami S, Galang N, Das DK. Differential regulation of Bcl-2, AP-1, and NFkappaB on cardiomyocyte apoptosis during myocar-dial ischemic stress adaption. FEBS Lett. 1999;443:331–336. doi: 10.1016/s0014-5793(98)01719-0. [DOI] [PubMed] [Google Scholar]
- 38.Dana A, Jonassen AK, Yamashita N, Yellon DM. Adenosine A(1) receptor activation induces delayed preconditioning in rats mediated by manganese superoxide dismutase. Circulation. 2000;101:2841–2848. doi: 10.1161/01.cir.101.24.2841. [DOI] [PubMed] [Google Scholar]
- 39.Gumina RJ, Newman PJ, Kenny D, Warltier DC, Gross GJ. The eukocyte cell adhesion cascade and its role in myocardial ischemia-reperfusion injury. Basic Res Cardiol. 1997;92:201–213. doi: 10.1007/BF00788515. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu N, Yoshiyama M, Omura T, Hanatani A, Kim S, et al. Activation of mitogen-activated protein kinases and activator protein-1 in myocardial infarction in rats. Cardiovasc Res. 1998;38:116–124. doi: 10.1016/s0008-6363(97)00327-1. [DOI] [PubMed] [Google Scholar]
- 41.Cain BS, Harken AH, Meldrum DR. Therapeutic strategies to reduce TNF-alpha mediated cardiac contractile depression following ischemia and reperfusion. J Mol Cell Cardiol. 1999;31:931–947. doi: 10.1006/jmcc.1999.0924. [DOI] [PubMed] [Google Scholar]
- 42.Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, et al. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79:195–203. [PubMed] [Google Scholar]
- 43.Morgan EN, Boyle EM, Yun W, Griscavage-Ennis JM, Farr AL, et al. An essential role for NFkappaB in the cardioadaptive response to ischemia. Ann Thorac Surg. 1999;68:377–382. doi: 10.1016/s0003-4975(99)00646-3. [DOI] [PubMed] [Google Scholar]
- 44.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, et al. Nuclear factor-kB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 45.Todisco A, Ramamoorthy S, Pausawasdi N, Tacey K. Carbachol activates IkappaB kinase in isolated canine gastric parietal cells. J Biochem Biophys Res Commun. 1999;261:877–884. doi: 10.1006/bbrc.1999.1141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MiRNA transfection efficiency. (A) Representative H9c2 cell photomicrographs transfected with miRNA with fluorescence. (B) MiR-376b-5p level was significantly up-regulated after transfection with miR-376b-5p. H9c2 cells were transfected with miR-376b-5p for 24 h. MiR-376b-5p level was determined by quantitative real-time RT-PCR (qRT-PCR). Note: Values are expressed as mean ± SEM; n = 3 independent experiments; *P<0.05 versus Control.
(DOC)