Abstract
During gastrulation, cells in the dorsal marginal zone polarize, elongate, align and intercalate to establish the physical body axis of the developing embryo. Here we demonstrate that the bifunctional channel-kinase TRPM7 is specifically required for vertebrate gastrulation. TRPM7 is temporally expressed maternally and throughout development, and is spatially enriched in tissues undergoing convergent extension during gastrulation. Functional studies reveal that TRPM7’s ion channel, but not its kinase, specifically affects cell polarity and convergent extension movements during gastrulation, independent of mesodermal specification. During gastrulation, the non-canonical Wnt pathway via Dishevelled (Dvl) orchestrates the activities of the GTPases Rho and Rac to control convergent extension movements. We find that TRPM7 functions synergistically with non-canonical Wnt signaling to regulate Rac activity. The phenotype caused by depletion of the Ca2+- and Mg2+-permeant TRPM7 is suppressed by expression of a dominant negative form of Rac, as well as by Mg2+ supplementation or by expression of the Mg2+ transporter SLC41A2. Together, these studies demonstrate an essential role for the ion channel TRPM7 and Mg2+ in Rac-dependent polarized cell movements during vertebrate gastrulation.
Introduction
Vertebrate gastrulation is comprised of a series of dynamic cell shape changes that results in part via modulation of the actin cytoskeleton (Keller, 2002). A key breakthrough in our understanding of the molecular mechanisms regulating gastrulation was the seminal findings that components of the non-canonical Wnt signaling pathway regulate this process (Wallingford et al., 2002; Yin et al., 2009). Understanding the molecular, cellular, and biochemical mechanisms that regulate gastrulation continues to remain a central focus of developmental studies. To date, a number of signaling pathways including Wnt, FGF, and BMP have been implicated in this process (Roszko et al., 2009).
Non-canonical Wnt signaling is currently loosely applied to a number of intracellular branches that function to regulate cell polarization and motility through modification of the actin cytoskeleton. This pathway appears to be independent of transcription and the well-elucidated canonical or β-catenin-dependent pathway (Semenov et al., 2007). The Wnt signal is mediated through the Frizzled (Fz) receptor and the LRP5/6 co-receptor, leading to activation of the cytoplasmic phosphoprotein Dishevelled (Dvl) (Wallingford and Habas, 2005). Of a number of branches downstream of Dvl, the activation of the small GTPases Rho and Rac plays central roles during gastrulation (Komiya and Habas, 2008). Activation of Rho occurs through the molecule Daam1, which complexes with Dvl in response to Wnt stimulation (Habas et al., 2001). This Rho pathway is coupled to the activation of the Rho associated kinase Rock that subsequently functions to regulate cytoskeletal changes through phosphorylation of myosin’s light chains (Marlow et al., 2002; Veeman et al., 2003; Wallingford et al., 2002). Activation of Rac occurs downstream of Dvl, which in turn stimulates JNK activity to also cause cytoskeletal changes (Habas et al., 2003; Li et al., 1999; Yamanaka et al., 2002). The mechanism of action of a host of identified factors including Strabismus, Prickle, and Diversin, among others, and their integration to subsequently govern cell polarization for directional cell migration remains unresolved (Semenov et al., 2007).
Interestingly, a number of studies have pointed to a non-canonical Wnt/Ca2+ pathway in which Wnt stimulation leads to an increase in intracellular Ca2+ levels (Kohn and Moon, 2005). This Ca2+ pathway has been shown to function during vertebrate gastrulation, and Ca2+ is a potent divalent second messenger that can further negatively influence canonical Wnt signaling (Slusarski and Pelegri, 2007). Indeed, while many studies have uncovered changes in Ca2+ levels in cells undergoing gastrulation, it appears that much of these intracellular fluxes are due to the release of Ca2+ from intracellular stores (Slusarski and Pelegri, 2007). The identity of calcium channels that may be expressed on the plasma membrane and functionally traffic extracellular Ca2+ into cells during early stages of development remains unknown. Moreover, whether other ion channels or divalent cations such as Mg2+ play any functional roles during gastrulation remains an understudied area of investigation.
The Transient Receptor Potential (TRP) superfamily is composed of cation-permeant ion channels that have varied, but poorly understood functions, ranging from thermosensation, chemosensation, and visual transduction (Clapham, 2003). Unique among ion channels, the ubiquitously expressed TRPM7 was discovered as the first ion channel to possess its own kinase domain (Nadler et al., 2001; Runnels et al., 2001). While most divalent cations block ion fluxes through Ca2+-permeable ion channels, TRPM7 exhibits high permeation of Ca2+ as well as other essential divalent cations, including Mg2+ and Zn2+ (Monteilh-Zoller et al., 2003). Consequently, an array of functions has been assigned to TRPM7, presumably owing to the diversity of cations permeated by the channel. Loss of TRPM7 has been reported to cause skeletal, pigmentation, and kidney defects in zebrafish (Elizondo et al., 2005). More recently, it was found that deletion of TRPM7 in mice disrupted embryonic development, resulting in lethality between E 6.5–7.5, although the reason for this lethality remains unknown (Dorovkov et al., 2005; Jin et al., 2008).
In this study, we report the cloning and functional analysis of TRPM7 in the Xenopus embryo. We show that XTRPM7 plays a previously undefined role in convergent extension movements during gastrulation. Importantly, our studies point to a key role of the channel, but not the kinase domain of this protein, and for the Mg2+ cation in regulating polarized cell movements during gastrulation, independent of mesodermal specification. Additionally, we show that XTRPM7 regulates gastrulation via its ability to modulate the activation levels of the small GTPase Rac and not Rho. These studies underscore a key role for this ion channel in regulating gastrulation, and furthermore uncover a central role for Mg2+ in this crucial embryonic process.
Materials and Methods
Embryo manipulations
Embryo manipulations and convergent extension assays in explants were performed as described (Khadka et al., 2009; Sato et al., 2006). Embryo injections were performed using in vitro transcribed RNAs, cDNAs, or Morpholino oligonucleotides (MO). Microinjection and microdissection of Xenopus embryos were performed as described (Khadka et al., 2009; Liu et al., 2008). Briefly, RNAs (500 pg) encoding GFP-CAAX and membrane-tethered Cherry were microinjected separately into dorsal blastomeres of four-cell stage embryos, alone or with RNAs encoding TRPM7 (2 ng) or XTRPM7 MOs (75 ng). “Shaved” Keller explants and analysis of cell shape and alignment were performed as described (Khadka et al., 2009; Liu et al., 2008). Detection of activated Rho and Rac was accomplished using a modified GST-pulldown purification assay (Habas and He, 2006). The relative level of activated GTPase was calculated by comparing the total amount of activated protein in each band to the total amount of the protein in the lysate, and then normalizing this number to the amount of activated protein captured in the GST-pulldown assay from uninjected embryos.
Plasmids and oligonucleotides
To obtain the complete Xenopus TRPM7 (XTRPM7) sequence (accession number: GQ304750), we employed a PCR based approach to amplify and clone this cDNA from a stage 10.5 Xenopus library. Details are available upon request. pBS-SV40-TRPM7 containing full length Mus musculus TRPM7 was generated by subcloning a KpnI/NotI fragment containing the TRPM7 ORF from pTracerCMV2-TRP-PLIK (Runnels et al., 2001) into pBS-SV40. pBS-SV40 contains the SV40 polyadenylation sequence from pCS2+, which was introduced by PCR subcloning an amplified 220 bp PCR fragment into the NotI/SacII sites of pBS using the following primers: 5′-GCG GCC GCG TAG ATC CAG ACA TGA TAA GAT ACA TTG ATG AGT TTG G-3′ and 5′-CCG CGG AAT TAA AAA ACC TCC CAC ACC TCC CC-3′. To create the “kinase-inactive” mutant of TRPM7 containing G1618D, we employed QuikChange (Stratagene) using the primers previously described (Su et al., 2006). The pOG1 vector encoding human TRPM6α was kindly provided by Dr. Vladimir Chubanov (Philipps Universität Marburg) (Chubanov et al., 2007). The Mg2+ transporter SLC41A2 was subcloned into pCS2+ from the FLAG-SLC41A2/pcDNA4/TO vector generously donated by Dr. Andrew Scharenberg (University of Washington) (Sahni et al., 2007). pCS2+ containing C-Daam1, ΔDIX-Dvl, ΔDEP-Dvl, and Xdd1 have been previously described (Habas et al., 2001) as has pCS2+ containing Rho and Rac derived constructs (Habas et al., 2003).
Results
XTRPM7 is temporally and spatially expressed in embryonic tissues undergoing morphogenesis
To investigate the role of TRPM7 during early embryogenesis, we first cloned Xenopus laevis TRPM7 (XTRPM7), a protein that shares a sequence identity greater than 77 % with the human protein. XTRPM7 possesses a domain architecture similar to that of its vertebrate orthologs, exhibiting high sequence identity within its NH2-terminal TRPM homology region, its pore domain, and its COOH-terminal kinase domain (Supplementary Fig. 1A–C). We then examined the temporal and spatial expression patterns of XTRPM7 mRNA during Xenopus development. Two primer sets were designed to amplify fragments from the 5′ UTR and the 3′ UTR regions of the XTRPM7 mRNA, and RT-PCR analysis revealed expression of XTRPM7 maternally to the tadpole stage (Supplementary Fig. 2A). In situ hybridization demonstrated a relatively low level of XTRPM7 maternally through the gastrula stages of the developing embryo (Fig. 1A). During the gastrula stage, XTRPM7 RNA was most highly expressed in the dorsal ectodermal and mesodermal regions. By the neurula stage, XTRPM7 expression was higher in the neural plate, anterior neural fold, and neural hinge point regions. Later in development, XTRPM7 expression became restricted to the developing brain, kidney, heart, and notochord (Fig. 1A). This expression pattern is suggestive of a broad role for XTRPM7 during development.
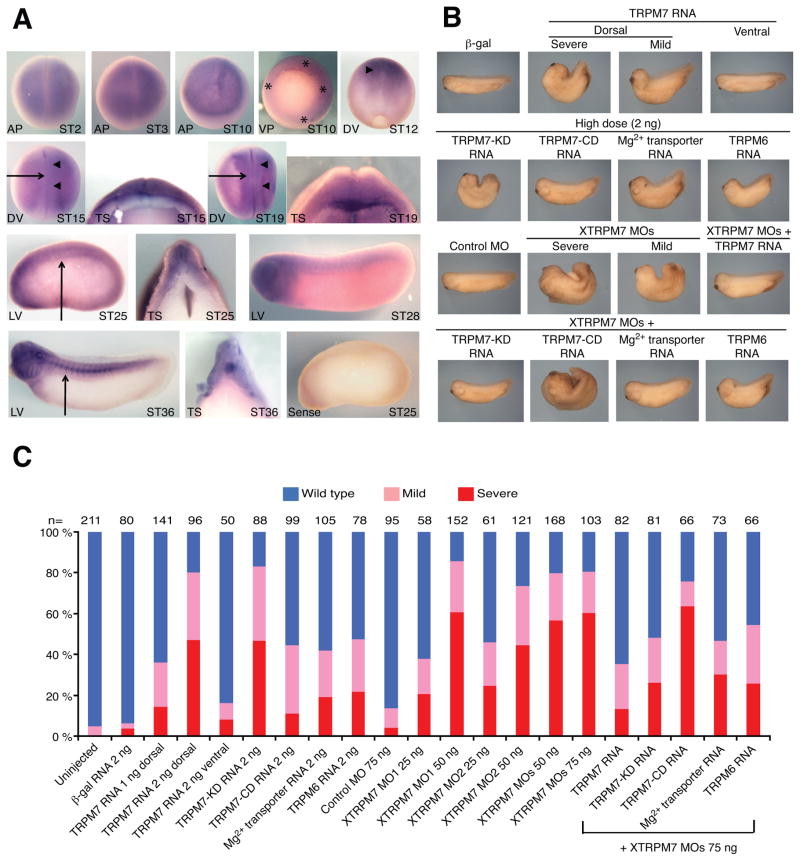
Fig. 1. XTRPM7 is required for gastrulation.
(A) Expression pattern of XTRPM7 at selected developmental stages as analyzed by whole-mount in situ hybridization using a TRPM7 anti-sense probe. A sense probe was used as a negative control. XTRPM7 is expressed at higher levels in the dorsal mesoderm and neural plate (stars and arrowheads, respectively), which is associated with dynamic morphogenesis movements. Arrows indicate the region where the embryo was vertically sectioned and examined. AP: Animal pole; VP: Vegetal pole; DV: dorsal view; TS: transverse section; LV: lateral view; ST: stage (B) Injection of TRPM7 RNA (2 ng) dorsally, but not ventrally, inhibited gastrulation resulting in embryos with curved axes, open neural folds, and reduced anterior structures. Dorsal injection of the kinase-dead TRPM7-G1618D (TRPM7-KD, 2 ng), but not the channel-dead TRPM7-E1047K (TRPM7-CD, 2 ng), produced a similar phenotype. In addition, dorsal injection of TRPM6 (2 ng) and the Mg2+ transporter SLC41A2 (2 ng) RNAs also produced gastrulation defects. Dorsal injection of XTRPM7 MOs (37.5 ng each) inhibited gastrulation, and this phenotype was rescued by co-injection of XTRPM7 MOs with TRPM7 RNA (400 pg), TRPM7-KD RNA (400 pg), and TRPM6 RNA (400 pg) as well as by SLC41A2 (Mg2+ transporter) RNA (400 pg). However, co-injection of TRPM7-CD RNA with the XTRPM7 MOs did not rescue the XTRPM7 MOs-induced gastrulation phenotypes. Injections were performed into the dorsal or ventral marginal zone of the four-cell embryo, and the phenotypes were scored at the tadpole stage. (C) Quantification of the phenotypic results from overexpression of TRPM7 or depletion of XTRPM7; the number of embryos examined is shown above each bar.
Dorsal expression of TRPM7 disrupts Xenopus gastrulation
To determine the function of TRPM7 in vivo, we first expressed TRPM7 to elucidate its effect during Xenopus development. Injection of murine TRPM7 RNA into the ventral marginal zones of the four-cell embryo had no effect on Xenopus development. However, injection of TRPM7 RNA into the dorsal marginal zones of the four-cell embryo resulted in severe gastrulation defects in a dose-dependent manner, while injection of a similar dose of β–galactosidase (β-gal) RNA had no significant effect (Fig. 1B–C). In the TRPM7-injected embryos, the resulting gastrulation defect phenotypes were classified into two classes: severe and mild. In the severe class, anterior structures, including the head, eyes and cement glands, were reduced or absent; in addition, axial extension was impaired resulting in a severe dorsal-flexure in the embryos, and the blastopore and neural folds failed to close. In the mild class, there was a reduction in the size of the head, eyes and cement glands, and a slight dorsal-flexure of the axis; however, there was only a delay in blastopore and neural fold closure.
XTRPM7 is required for Xenopus gastrulation
To further delineate the role of XTRPM7 in early development, we designed two anti-sense Morpholino oligonucleotides (XTRPM7 MO1 & MO2) to deplete the endogenous XTRPM7 protein (Supplementary Fig. 2B). Both Morpholinos effectively inhibited translation of a Myc-tagged XTRPM7 construct when injected into Xenopus embryos (Supplementary Fig. 2C). Injection of the XTRPM7 MO1, XTRPM7 MO2 or a control MO at the highest dose of 75 ng into the ventral marginal zones of the four-cell embryo had little effect on Xenopus development (data not shown). Injection of the control MO into the dorsal cells also had little effect on development (Fig. 1B, C) or on Myc-XTRPM7 cDNA expression (Supplementary Fig. 2C). However, injection of either XTRPM7 MO1 or XTRPM7 MO2 into the dorsal marginal zones of the four-cell embryo dose-dependently produced severe gastrulation defects (Fig. 1B, C). The phenotype induced by injection of the XTRPM7 MO1 and MO2 combined (hereafter referred to as XTRPM7 MOs) was rescued by co-injection of murine TRPM7 RNA (Fig. 1B, C). Injection of this dose of TRPM7 RNA by itself produced no severe gastrulation defects (Supplementary Fig. 3).
TRPM7’s kinase domain is not required for gastrulation
To determine whether TRPM7’s channel or kinase was required for gastrulation, we injected a kinase-inactive mutant TRPM7-G1618D (Su et al., 2006) (TRPM7-KD) RNA into the dorsal blastomeres of the four-cell embryo and observed gastrulation defects similar to those obtained with the full-length TRPM7. Interestingly, co-injection of TRPM7-G1618D RNA with the XTRPM7 MOs rescued the XTRPM7 MOs-induced defects, indicating that TRPM7’s kinase activity is not required for the protein’s role during gastrulation (Fig. 1B, C). To confirm that TRPM7’s channel domain was required for the ability of TRPM7 to induce gastrulation defects, we injected a channel-inactive mutant TRPM7-E1047K (Li et al., 2007) (TRPM7-CD) RNA into the dorsal blastomeres of the four-cell embryo and observed lesser defects in gastrulation as compared to injection of the TRPM7 RNA or the kinase-inactive mutant TRPM7-G1618D (Fig. 1B, C). Likewise, co-injection of TRPM7-E1047K RNA with the XTRPM7 MOs failed to rescue the XTRPM7 MOs-induced defects, indicating that TRPM7’s channel activity was required for its effect on gastrulation (Fig. 1B, C). To determine whether TRPM7’s channel was unique in its ability to regulate gastrulation via the cations it conducts, we tested whether TRPM6 (Schlingmann et al., 2002), a channel closely related to TRPM7 and also selective for Ca2+ and Mg2+, could suppress the XTRPM7 MOs-induced phenotype. Co-injection of human TRPM6 RNA was observed to significantly rescue the XTRPM7 MOs-induced phenotypes, and expression of TRPM6 at higher doses did induce a gastrulation defects, though at a lower penetrance than TRPM7 RNA (Fig. 1B, C).
Mg2+ suppresses the XTRPM7 morpholinos-induced phenotype
TRPM6 RNA’s ability to suppress the XTRPM7 MOs-induced phenotype suggested that TRPM7’s ability to permeate Mg2+ and Ca2+ was central for the regulation of gastrulation. Elimination of TRPM7 expression from DT40 cells has been shown to arrest cell growth and proliferation, which can be overcome by growth of the cells in 10 mM extracellular Mg2+ or by expression of the Mg2+ transporter SLC41A2 (Sahni et al., 2007; Schmitz et al., 2003). The Mg2+ transporter SLC41A2 has been shown to specifically allow the passage of Mg2+ but not Ca2+ ions (Goytain and Quamme, 2005; Sahni et al., 2007). We therefore employed a similar approach and found that raising the concentration of Mg2+, but not Ca2+, in the buffer in which the embryos were cultured restored development of the embryos injected with the XTRPM7 MOs in a dose-dependent manner (Fig. 2A, B). Similarly, co-injection of SLC41A2 RNA with the XTRPM7 MOs also rescued the XTRPM7 morpholinos-induced phenotypes (Fig. 1B, C). Additionally, injection of SLC41A2 RNA at higher doses than those used to rescue the XTRPM7 MOs-induced phenotypes also inhibited gastrulation (Fig 1B, C and Supplementary Fig. 3). These results suggest that XTRPM7’s permeation of Mg2+ is required for gastrulation and that this critical developmental process is exquisitely sensitive to Mg2+ influx.
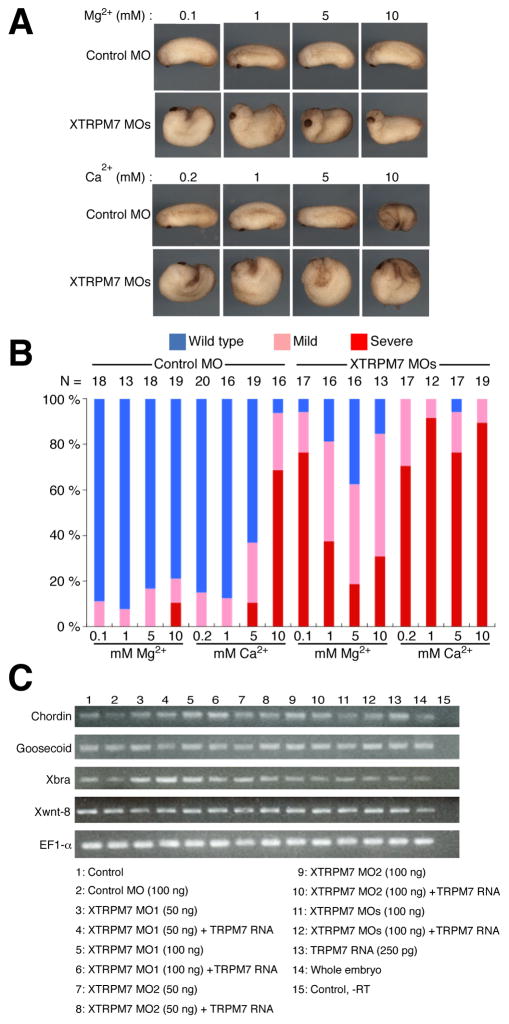
Fig. 2. Mg2+ is required for gastrulation.
(A) Dorsal injection of XTRPM7 MOs (75 ng) in embryos cultured in 0.1X MMR (0.1 mM Mg2+) inhibited gastrulation. Supplementation of Mg2+, but not Ca2+, into the culture media at 5 mM at stage 10.5 rescued the gastrulation phenotype. (B) Quantification of the effects of Mg2+ and Ca2+ supplementation on embryos injected with XTRPM7 MOs; the number of embryos is shown above each bar. (C) Embryos injected dorsally with XTRPM7 MOs or TRPM7 RNA had no defects in expression of mesodermal marker genes Chordin, Goosecoid, Xbra and XWnt8 as monitored by RT-PCR analysis; EF1-α was used as a loading control.
XTRPM7 is required for convergent extension movements
During embryogenesis, a failure of mesodermal specification can produce gastrulation defects, and therefore we examined whether XTRPM7 was required for mesodermal specification. We assayed the dorsal mesodermal markers Chordin and Goosecoid, the pan-mesodermal marker Brachyury (Xbra), and the ventral mesodermal marker Wnt8 (Xwnt8) by RT-PCR. Injection of XTRPM7 MO1 and MO2, either singly or combined, had no effect on the expression levels of these molecular markers (Fig. 2C). Together, these studies indicate that XTRPM7 regulates gastrulation independently of mesodermal cell fate specification.
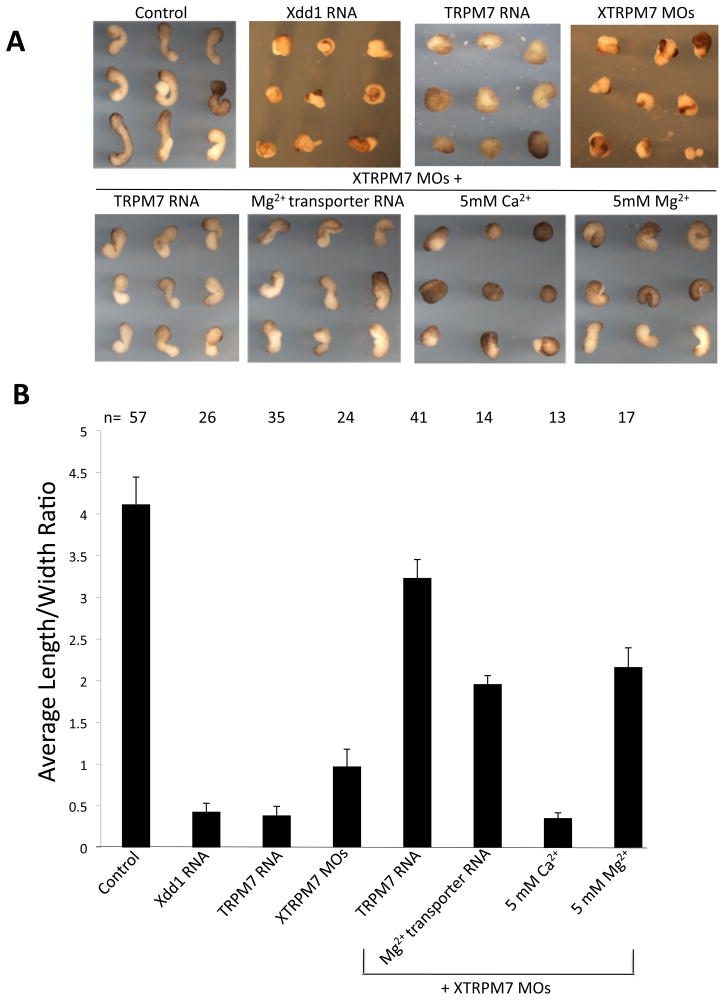
A major feature of gastrulation is convergent extension in which a series of highly orchestrated polarized cell movements allows the establishment of the physical body axis of the embryo. To further delineate how TRPM7 regulates gastrulation, we examined its effects on convergent extension movements using Keller explant assays. In control, uninjected embryos, we observed normal elongation of the explanted dorsal tissues, while embryos injected with TRPM7 RNA produced dramatically suppressed elongation of the explants, similar to injection of dominant negative Dishevelled (Xdd1) RNA (Fig. 3A, B). Injection of XTRPM7 MOs also strongly suppressed elongation of the explants, which could be rescued by co-injection of TRPM7 RNA (Fig. 3A, B). Similar to the results obtained using whole embryos, injection of the Mg2+ transporter SLC41A2 RNA as well as Mg2+ supplementation of the explant culture buffer restored the elongation of the explants while supplementation of the buffer with additional Ca2+ did not restore extension of the explants derived from the XTRPM7 MOs-injected embryos (Fig. 3A, B).
Fig. 3. XTRPM7 is required for convergent extension movements.
(A) Dorsal marginal zone (Keller) explant assay revealed that overexpression of TRPM7 (2 ng) and injection of XTRPM7 MOs inhibit convergent extension movements. Dominant negative Dishevelled (Xdd1, 2 ng) was used as a positive control. Inhibition of convergent extension produced by depletion of XTRPM7 was rescued by co-injection of XTRPM7 MOs with TRPM7 RNA (400 pg) and the Mg2+ transporter SLC41A2 (400 pg), as well as supplementation of the buffer with 5 mM Mg2+ but not 5 mM Ca2+. (B) Quantification of Keller explant assay; bars indicate the average length to width ratio of Keller explants and the number of explants analyzed is shown above each bar.
To better understand XTRPM7’s regulation of convergent extension movements during gastrulation, we examined the polarization and cell shape changes of dorsal mesodermal cells. During convergent extension, these cells adopt an elongated and polarized shape with a long axis oriented towards the midline (Shih and Keller, 1992). For these studies, we examined the parameters of length-to-width ratio and of medial axial alignment of explanted dorsal mesodermal cells; both of which are dramatically affected by components regulating convergent extension (Liu et al., 2008; Wallingford et al., 2000). We observed that injection of TRPM7 RNA dramatically altered the length-to-width and medial axial alignment to levels similar to dominant negative Dishevelled (Xdd1) RNA (Fig. 4A–D). Injection of XTRPM7 MOs also significantly affected the length-to-width ratio and medial axial alignment of the explanted cells, and these effects were rescued by co-injection of the XTRPM7 MOs with TRPM7 RNA (Fig. 4A–D). These data reveal that XTRPM7 regulates polarization during convergent extension movements.
Fig. 4. XTRPM7 is required for dorsal mesodermal cell polarization, elongation and alignment.
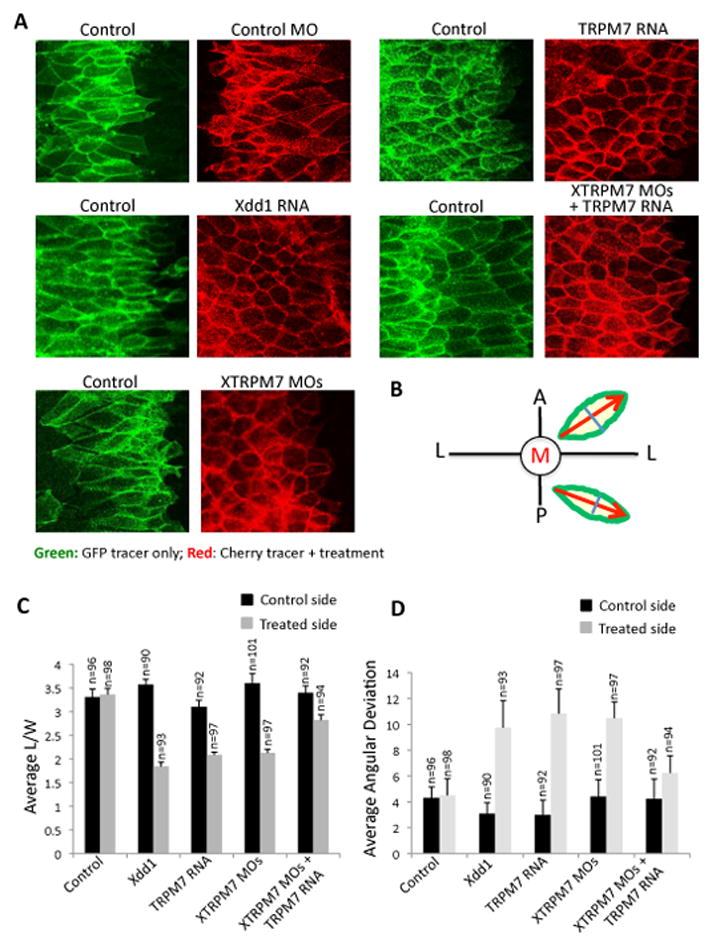
(A) Confocal imaging analysis revealed that injection of TRPM7 RNA and XTRPM7 MOs inhibits dorsal mesodermal cell polarization and elongation. Control cells were labelled with EGFP-CAAX (green, 500 pg) and membrane-tethered Cherry (red, 500 pg) was co-injected with either TRPM7 RNA (2 ng), dominant negative Dishevelled (Xdd1) RNA (2 ng), XTRPM7 MOs (75 ng), or with XTRPM7 MOs (75 ng) and TRPM7 RNA (400 pg). (B) Illustration of calculations of average length to width ratio and average angular deviation. Length to width ratio was measured by taking the length of the cell (red arrow) divided by the width of the cell (blue line). Angular orientation of the cell was measured by taking the absolute value of the angle of the red arrow in reference to the lateral axis (L) and was used for calculations of average angular deviation. (C) Injection of Xdd1 RNA and TRPM7 RNA or injection of the XTRPM7 MOs disrupted dorsal mesodermal cell elongation as quantified by the length/width ratio of cells. The error bars represent the standard deviation (s.d.) from at least three independent experiments and the number of cells examined is shown at the top of each bar. (D) Injection of Xdd1 RNA and TRPM7 RNA or injection of the XTRPM7 MOs disrupted alignment of dorsal mesodermal cells undergoing convergent extension. The error bars represent the s.d. from at least three independent experiments and the number of cells examined is shown at the top of each bar.
XTRPM7 synergizes with Dishevelled to regulate gastrulation through the Rac GTPase
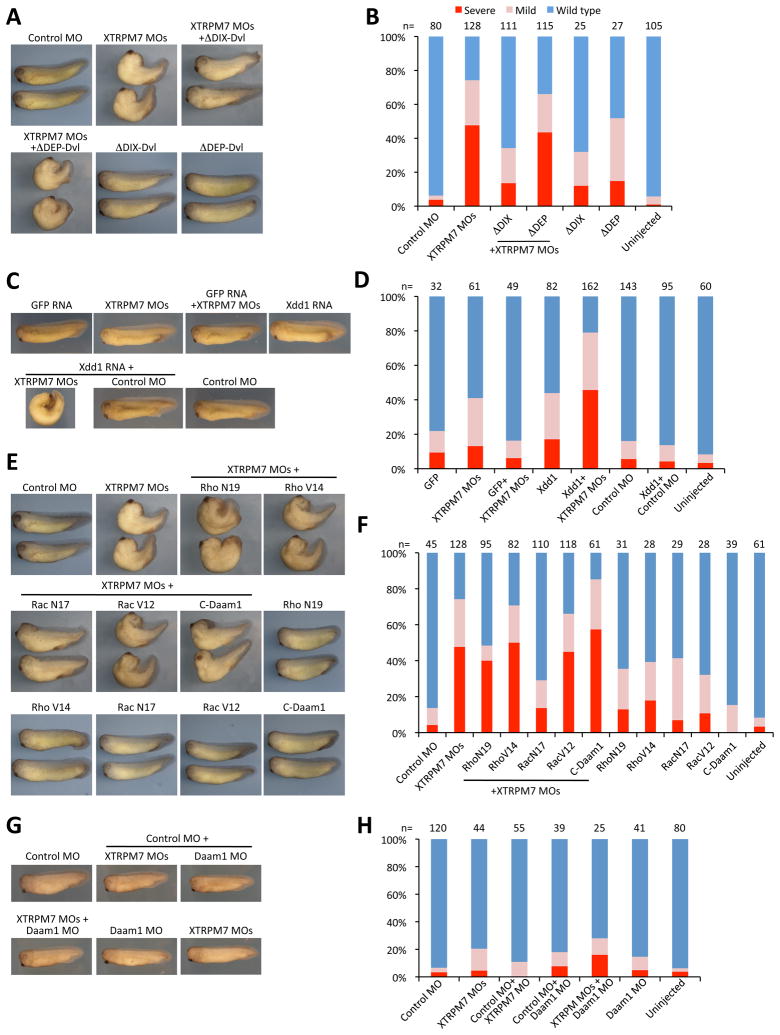
As the non-canonical Wnt pathway regulates convergent extension movements through the cytoplasmic phosphoprotein Dishevelled (Dvl), we tested whether TRPM7’s effect on convergent extension movements could be modulated by non-canonical Wnt signaling. We injected ΔDIX-Dvl, an activated form of Dvl that can activate Rho and Rac (Habas et al., 2003; Wallingford and Habas, 2005) together with the XTRPM7 MOs and observed that ΔDIX-Dvl strongly suppressed the XTRPM7 MOs-induced phenotype (Fig. 5A, B). However, injection of ΔDEP-Dvl, which cannot activate the Rac arm of the planar cell polarity (PCP pathway), did not have any effect (Habas et al., 2003). To further examine a possible connection between TRPM7 and non-canonical Wnt signaling, embryos were injected with sub-threshold levels of dominant negative Dishevelled (Xdd1) RNA (250 pg) or XTRPM7 MOs (25 ng), which individually produced little or no phenotypes in the injected embryos (Fig. 5C, D). However, when Xdd1 RNA and XTRPM7 MOs were co-injected, the number of affected embryos increased substantially (Fig. 5C, D), which would be expected if the two factors functioned in the same pathway. These results prompted us to investigate whether XTRPM7 may be involved in the regulation of the activity levels of Rho and Rac during gastrulation. We therefore examined the role of Rac and Rho in mediating XTRPM7 MOs-induced effects on gastrulation using dominant interfering mutants of Rac and Rho (RacN17 and RhoN19) and constitutively active mutants of these proteins (RacV12 and RhoV14). Co-injection of either dominant interfering or constitutively active Rho with the XTRPM7 MOs did not rescue the XTRPM7 MOs-induced phenotype (Fig. 5E, F). Likewise, co-injection of C-Daam1, a constitutively active form of Daam1, which functions in Rho activation during non-canonical Wnt signaling, did not rescue the XTRPM7 MOs-induced phenotype. However, co-injection of dominant interfering RacN17, but not constitutively activated RacV12, with the XTRPM7 MOs did suppress the gastrulation defects produced by the XTRPM7 MOs (Fig. 5E, F). To further rule out any role of TRPM7 in modulating Rho activation levels, we tested for synergy between depletion of Daam1 and TRPM7. A simultaneous depletion of Daam1 and TRPM7 resulted in no synergistic effects on gastrulation, again ruling out possible Rho involvement (Fig. 5G, H). These findings indicate that XTRPM7 may regulate gastrulation by modulating Rac activity during development.
Fig. 5. TRPM7 is required for regulation of Rac and non-canonical Wnt signaling.
(A) Disruption of gastrulation produced by injection of XTRPM7 MOs (75 ng) can be suppressed by co-injection of dominant active Dishevelled (ΔDIX-Dvl) RNA (400 pg), but not by injection of Dishevelled lacking the DEP domain (ΔDEP-Dvl, 400 pg), whose deletion disrupts activation of the Rac component of the PCP pathway. (B) Quantification of the phenotypes observed with separate or co-injections of XTRPM7 MOs with ΔDIX-Dvl, and ΔDEP-Dvl RNAs. The number of embryos scored is indicated above each bar. (C) Co-injection of dominant negative Dishevelled (Xdd1) RNA (250 pg) and XTRPM7 MOs (25 ng) synergistically inhibit gastrulation but have little or no effect when injected separately. GFP RNA was injected at 250 pg. (D) Quantification of the phenotypes observed with separate or co-injections of XTRPM7 MOs with Xdd1. The number of embryos scored is indicated above each bar. (E) Co-injection of XTRPM7 MOs with dominant negative RacN17 RNA (500 pg), but not with C-Daam1 cDNA (250 pg), RNAs for dominant negative RhoN19 (500 pg) or with the dominant active mutants RacV12 (10 pg) and RhoV14 (10 pg), prevents the disruption of gastrulation caused by the XTRPM7 MOs. (F) Quantification of the phenotypes observed with separate or co-injections of XTRPM7 MOs with RacN17, RacV12, RhoN19 and RhoV14 RNAs and C-Daam1 cDNA. The number of embryos scored is indicated above each bar. (G) Co-injection of a low concentration of XTRPM7 MOs (25 ng) with control MO (25 ng) or with Daam1 MO (25 ng) did not produce synergistic gastrulation defects. (H) Quantification of the phenotypes observed with separate or co-injections of XTRPM7 MOs with control MO or Daam1 MO. The number of embryos scored is indicated above each bar.
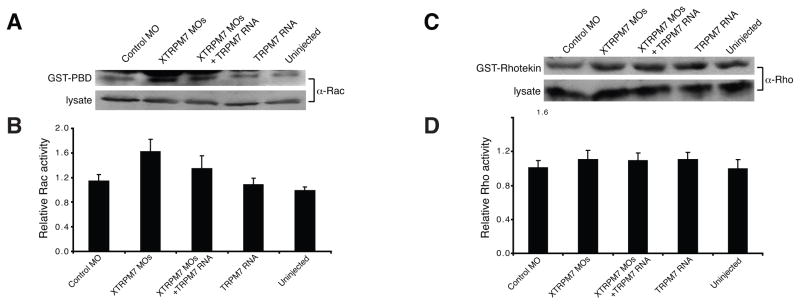
To further investigate the potential role of TRPM7 in modulating the activation levels of Rho and Rac, we tested whether XTRPM7 affected the endogenous activity levels of Rho and Rac during gastrulation. By employing pulldown assays (Habas and He, 2006), we detected higher levels of activated Rac in embryos injected with XTRPM7 MOs (Fig. 6A–B), but observed no effects of the TRPM7 MOs on Rho activation levels (Fig. 6C–D). Taken together, these studies reveal a critical role for TRPM7 and its ability to modulate Rac activity for vertebrate gastrulation.
Fig. 6. TRPM7 regulates Rac but not Rho activation levels.
(A) Western blot analysis and quantification of levels of activated Rac at stage 10.5 during gastrulation. Injection of 75 ng of XTRPM7 MOs increased levels of activated Rac, which could be prevented by co-injection of 500 pg of TRPM7 RNA. (B) Quantification of the results in (A), the error bars represent the s.d. from at least five independent experiments (C) Western blot analysis and quantification of levels of activated Rho at stage 10.5 during gastrulation. Levels of activated Rho were unaffected by injection of XTRPM7 MOs or TRPM7 RNA. (D) Quantification of the results in (C); error bars represent the s.d. from at least four independent experiments
Discussion
In this study, we have investigated the role of TRPM7 during Xenopus development and uncovered a central role for this bifunctional ion channel in regulating gastrulation. Using both gain-of-function and loss-of-function analysis, we show that TRPM7 perturbs cell polarization and alignment during convergent extension movements (Figs. 3 and 4) and that this effect is independent of mesodermal cell fate specification (Fig. 2). The phenotypes induced by overexpression or depletion of TRPM7 resembles those uncovered by other studies examining components of non-canonical Wnt signaling (Yin et al., 2009), revealing an important role for TRPM7 in modulating levels of activated Rac, but not Rho, in the developing embryo. Our results suggest a model in which the intracellular signaling cascade initiated by TRPM7 regulates levels of the Rac GTPase for balanced coordination of cell polarization and migration during convergent extension movements, underscoring a critical role for the bifunctional ion channel in regulating gastrulation.
What is the mechanism by which TRPM7 regulates gastrulation? The TRPM7 protein has a channel domain physically linked to a COOH-terminal cytoplasmic kinase domain. Between the two activities, the role of the kinase domain is less understood, as the identified substrates for the TRPM7 kinase thus far include annexin 1, myosin IIA, IIB, and IIC (Clark et al., 2006; Clark et al., 2008a; Clark et al., 2008b; Dorovkov and Ryazanov, 2004). Surprisingly, our results indicate that the kinase domain of TRPM7 is not required for the protein’s ability to regulate gastrulation, as an inactivating mutation within the kinase domain did not alter the ability of the overexpressed protein to interfere with gastrulation. Importantly, injection of kinase-inactive mutant RNA was able to rescue the gastrulation defects produced by injection of the XTRPM7 MOs to a similar level obtained when using wild type TRPM7 RNA, whereas injection of the channel-inactive mutant did not reverse the XTRPM7 MOs phenotype (Fig. 1B–C). These results strongly indicate that the ability of TRPM7 to regulate gastrulation is dependent on its channel domain. As TRPM7 has been shown to allow the passage of both Ca2+ and Mg2+ ions (Li et al., 2006; Monteilh-Zoller et al., 2003; Voets et al., 2004), it was possible that Ca2+ may be the cation responsible for its regulation of gastrulation, since Ca2+ was shown to play a central role in non-canonical Wnt signaling and gastrulation (Kohn and Moon, 2005; Slusarski and Pelegri, 2007). However, we show that elevating external Ca2+ failed to rescue the XTRPM7 MOs-induced phenotype, suggesting that disruption of Ca2+ signalling was likely not responsible for producing the XTRPM7 MOs-induced gastrulation defects (Figs. 2A–B and 3A–B). Surprisingly, we found that Mg2+ supplementation ameliorated the disruption of convergent extension movements caused by depletion of XTRPM7 (Figs. 2A–B and 3A–B). Additionally, co-expression of the Mg2+ transporter SLC41A2, a transporter that specifically allows the passage of Mg2+ ions (Goytain and Quamme, 2005; Sahni et al., 2007), in XTRPM7 MOs-injected embryos also suppressed the gastrulation defects induced by the XTRPM7 MOs (Figs. 2A–B and 3A–B). These studies suggest that the ability of the TRPM7 channel to passage Mg2+ is important for the ability of mesodermal cells to execute polarized cell movements during gastrulation. Therefore, these studies point to an important role for Mg2+ in regulating convergent extension movements, which to our knowledge was a previously undefined role for this divalent cation.
How does Mg2+ regulate cell behaviours including polarization and migration that are central for gastrulation? During gastrulation, the non-canonical Wnt pathway regulates convergent extension movements through activation of RhoA and Rac via the cytoplasmic phosphoprotein Dishevelled (Wallingford and Habas, 2005). Whereas RhoA is activated by Frizzled (Fz)/Dvl signaling through the Formin-homology protein Daam1 (Habas et al., 2001), Rac activation is independent of Wnt/Fz-triggered Rho activation, and mediates Wnt/Fz/Dvl activation of JNK (Habas et al., 2003). Indeed we show a strong synergy with a simultaneous depletion of XTRPM7 and expression of dominant-negative Dishevelled (Xdd1), suggesting a possible interplay between these two factors (Fig. 5C–D). Additionally, we observe that expression of a construct of Dishevelled, ΔDIX-Dvl that can rescue defects in convergent extension induced by a dominant negative form of Wnt11 (Tada and Smith, 2000; Wallingford and Habas, 2005), suppressed the gastrulation defects induced by the XTRPM7 Morpholinos (Fig. 5A–B). ΔDIX-Dvl harbours the PDZ domain that functions in the Wnt/Dvl/Rho pathway and also the DEP domain that functions in the Wnt/Dvl/Rac pathway suggesting that Rho and/or Rac may be affected downstream of TRPM7 (Habas et al., 2003; Habas et al., 2001).
To tease out a potential interplay between TRPM7, Rho and Rac, we find that TRPM7 functions synergistically with Dvl independent of Rho and Daam1 to regulate convergent extension movements (Fig. 5E–F). To further uncover the possible mechanism by which TRPM7 regulates Rho and Rac, we show that the gastrulation defects induced by a loss of XTRPM7 is suppressed by expression of a Rac dominant negative construct, but not by that of a Rho dominant negative construct (Fig. 5E–F). This ability of TRPM7 to modulate Rac, but not Rho, activation level was further verified using Rac and Rho pulldown activation assays from Xenopus embryo lysates (Fig. 6A–D). We find that a loss of XTRPM7 leads to an elevation in the levels of activated Rac levels, and this effect is rescued by co-expression of TRPM7 RNA (Figure 6A–B). However, it is important to note that while we observe that TRPM7 MOs injections cause an elevation in the levels of activated Rac, expression of ΔDIX-Dvl, which can activate both Rho and Rac in mammalian culture cells (Habas et al., 2003), suppressed the TRPM7 MOs-induced gastrulation in the embryo (Fig. 5A–B). As a complex inhibitory interplay exists between these two small GTPases (Pertz, 2010; Sander et al., 1999), it is possible that this may explain the ability of the ΔDIX-Dvl to rescue the TRPM7 MOs-induced gastrulation defects. Additional studies to address the precise spatiotemporal activation of the Rho family of small GTPases during gastrulation are needed to resolve this important question.
In our study, we go further to show that expression of the closely related TRPM6, the only other vertebrate ion channel to contain a kinase domain, can also rescue the XTRPM7 morpholinos-induced gastrulation defects (Fig. 1B–C). Interestingly, mice made deficient in TRPM6 were shown to result in embryonic lethality and neural tube closure defects, highlighting the role of these unique proteins in early development (Walder et al., 2009). It is worth noting that studies in humans as well as in animals have shown that Mg2+ deficiency adversely affects gestational outcomes, however, explanations for this have remained obscure (Almonte et al., 1999; Durlach, 2004). Our in vivo studies indicate that interfering with TRPM7’s regulation of Mg2+ during embryogenesis adversely affects processes in early development in which Mg2+-dependent directional cell motility plays an essential role. Our studies may offer an explanation for the embryonic lethality caused by deletion of TRPM7 in mice by revealing a crucial role for TRPM7 in regulating gastrulation (Dorovkov et al., 2005; Jin et al., 2008; Ryazanova et al., 2010).
In summary, our studies reveal a central role for TRPM7 and for Mg2+ cations in regulating gastrulation. Additional studies to further elucidate how Mg2+ ions regulate gastrulation are therefore warranted as such studies will shed light on previously undefined roles for the Mg2+ cation during early vertebrate development.
Supplementary Material
Domain organization of TRPM7 orthologues and domain alignment. (A) Schematic of TRPM7 with TRPM homology region, transmembrane (TM) domain regions and pore, coiled-coil region (CCR), and alpha-kinase domain (KIN). Indicated percent sequence identities within subdomains of TRPM7 mouse (m), zebrafish (z), Xenopus tropocalis (xt), and Xenopus laevis (x) orthologues are with respect to human (h) TRPM7. (B) Alignment of pore regions of TRPM7 orthologues. The two glutamates that are determinants of TRPM7’s divalent selectivity are indicated with astericks. (C) Alignment of alpha-kinase domains of TRPM7 orthologues.
Temporal and spatial expression pattern of XTRPM7 and characterization of the XTRPM7 morpholinos (MOs). (A) XTRPM7 is expressed maternally and throughout embryogenesis as monitored by RT-PCR analysis. Two independent primer sets were used to amplify a 5′-fragment and a 3′- fragment of XTRPM7. Ornithine decarboxylase (ODC) was used as a loading control. (B) Schematic diagram of two XTRPM7 morpholinos (MO1 & MO2) binding sites upstream and overlapping the start site of XTRPM7. (C) Western blot analysis shows that each of the XTRPM7 MOs can effectively inhibit translation of Myc-XTRPM7 5′UTR injected RNA (2 ng). Dose of injected MOs, including a non-targeting control morpholino, are shown.
Dorsal injection of a low concentration of TRPM7, TRPM7-KD, TRPM7-CD, TRPM6 and SLC41A2 RNAs does not produce severe gastrulation defects. (A) Dorsal injection of a low dose of β-gal RNA, TRPM7 RNA, the kinase-inactive TRPM7-G1618D (TRPM7-KD) RNA, the channel-inactive TRPM7-E1047K (TRPM7-CD) RNA, SLC41A2 (Mg2+ transporter) RNA, and TRPM6 RNA at concentrations (400 pg) used to rescue the phenotype produced by the XTRPM7 MOs did not produce severe gastrulation defects. (B) Quantification of the phenotypic results from injection of the above RNAs.
Acknowledgments
We thank Elizabeth Puccini and the Runnels and Habas laboratories for discussion and critical comments. We thank Drs. Jeffrey Miller, Chenbei Chang, Karen Symes, Sergei Sokol, Andrew Scharenberg, and Vladimir Chubanov for reagents. This work is supported by a grant from the National Institutes of Health (GM080753) to L.R., and R.H is supported by grants from the March of Dimes (#1-FY07-522) and the National Institutes of Health (GM078172).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almonte RA, Heath DL, Whitehall J, Russell MJ, Patole S, Vink R. Gestational magnesium deficiency is deleterious to fetal outcome. Biol Neonate. 1999;76:26–32. doi: 10.1159/000014128. [DOI] [PubMed] [Google Scholar]
- Chubanov V, Schlingmann KP, Waring J, Heinzinger J, Kaske S, Waldegger S, Mederos y Schnitzler M, Gudermann T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J Biol Chem. 2007;282:7656–67. doi: 10.1074/jbc.M611117200. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. Embo J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Middelbeek J, Dorovkov MV, Figdor CG, Ryazanov AG, Lasonder E, van Leeuwen FN. The alpha-kinases TRPM6 and TRPM7, but not eEF-2 kinase, phosphorylate the assembly domain of myosin IIA, IIB and IIC. FEBS Lett. 2008a;582:2993–7. doi: 10.1016/j.febslet.2008.07.043. [DOI] [PubMed] [Google Scholar]
- Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J Mol Biol. 2008b;378:790–803. doi: 10.1016/j.jmb.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem. 2004;279:50643–6. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- Dorovkov MV, Ryazanova LV, Nagele RG, Siu G, Ryazanov AG. TRPM7 channel-kinase: characterization of mouse knockout and identification of TRPM7 kinase substrates. Biophysical Journal. 2005;88:358A–358A. [Google Scholar]
- Durlach J. New data on the importance of gestational Mg deficiency. J Am Coll Nutr. 2004;23:694S–700S. doi: 10.1080/07315724.2004.10719411. [DOI] [PubMed] [Google Scholar]
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15:667–71. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Goytain A, Quamme GA. Functional characterization of the mouse [corrected] solute carrier, SLC41A2. Biochem Biophys Res Commun. 2005;330:701–5. doi: 10.1016/j.bbrc.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, He X. Activation of Rho and Rac by Wnt/frizzled signaling. Methods Enzymol. 2006;406:500–11. doi: 10.1016/S0076-6879(06)06038-1. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–60. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Khadka DK, Liu W, Habas R. Non-redundant roles for Profilin2 and Profilin1 during vertebrate gastrulation. Dev Biol. 2009;332:396–406. doi: 10.1016/j.ydbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem. 1999;274:129–34. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–30. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–37. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105:210–5. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–84. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler MJS, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kine JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci. 2010;123:1841–50. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–97. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J. 2007;401:505–13. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–22. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133:4219–31. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–70. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development. 1992;116:915–30. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol. 2007;307:1–13. doi: 10.1016/j.ydbio.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–70. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, Shi P, Searby CC, Husted RF, Sheffield VC. Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum Mol Genet. 2009;18:4367–75. doi: 10.1093/hmg/ddp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–36. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–5. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Ciruna B, Solnica-Krezel L. Convergence and extension movements during vertebrate gastrulation. Curr Top Dev Biol. 2009;89:163–92. doi: 10.1016/S0070-2153(09)89007-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Domain organization of TRPM7 orthologues and domain alignment. (A) Schematic of TRPM7 with TRPM homology region, transmembrane (TM) domain regions and pore, coiled-coil region (CCR), and alpha-kinase domain (KIN). Indicated percent sequence identities within subdomains of TRPM7 mouse (m), zebrafish (z), Xenopus tropocalis (xt), and Xenopus laevis (x) orthologues are with respect to human (h) TRPM7. (B) Alignment of pore regions of TRPM7 orthologues. The two glutamates that are determinants of TRPM7’s divalent selectivity are indicated with astericks. (C) Alignment of alpha-kinase domains of TRPM7 orthologues.
Temporal and spatial expression pattern of XTRPM7 and characterization of the XTRPM7 morpholinos (MOs). (A) XTRPM7 is expressed maternally and throughout embryogenesis as monitored by RT-PCR analysis. Two independent primer sets were used to amplify a 5′-fragment and a 3′- fragment of XTRPM7. Ornithine decarboxylase (ODC) was used as a loading control. (B) Schematic diagram of two XTRPM7 morpholinos (MO1 & MO2) binding sites upstream and overlapping the start site of XTRPM7. (C) Western blot analysis shows that each of the XTRPM7 MOs can effectively inhibit translation of Myc-XTRPM7 5′UTR injected RNA (2 ng). Dose of injected MOs, including a non-targeting control morpholino, are shown.
Dorsal injection of a low concentration of TRPM7, TRPM7-KD, TRPM7-CD, TRPM6 and SLC41A2 RNAs does not produce severe gastrulation defects. (A) Dorsal injection of a low dose of β-gal RNA, TRPM7 RNA, the kinase-inactive TRPM7-G1618D (TRPM7-KD) RNA, the channel-inactive TRPM7-E1047K (TRPM7-CD) RNA, SLC41A2 (Mg2+ transporter) RNA, and TRPM6 RNA at concentrations (400 pg) used to rescue the phenotype produced by the XTRPM7 MOs did not produce severe gastrulation defects. (B) Quantification of the phenotypic results from injection of the above RNAs.