Abstract
The laryngeal neuromuscular mechanisms for modulating glottal posture and fundamental frequency are of interest in understanding normal laryngeal physiology and treating vocal pathology. The intrinsic laryngeal muscles in an in vivo canine model were electrically activated in a graded fashion to investigate their effects on onset frequency, phonation onset pressure, vocal fold strain, and glottal distance at the vocal processes. Muscle activation plots for these laryngeal parameters were evaluated for the interaction of following pairs of muscle activation conditions: (1) cricothyroid (CT) versus all laryngeal adductors (TA/LCA/IA), (2) CT versus LCA/IA, (3) CT versus thyroarytenoid (TA) and, (4) TA versus LCA/IA (LCA: lateral cricoarytenoid muscle, IA: interarytenoid). Increases in onset frequency and strain were primarily affected by CT activation. Onset pressure correlated with activation of all adductors in activation condition 1, but primarily with CT activation in conditions 2 and 3. TA and CT were antagonistic for strain. LCA/IA activation primarily closed the cartilaginous glottis while TA activation closed the mid-membranous glottis.
INTRODUCTION
The ability to control the fundamental frequency (F0) of voice is critical to human communication, as F0 variations carry important linguistic information in speech and are also widely used in non-speech communication and singing. The control of F0 is a complex interplay between respiratory controls (i.e., subglottic pressure) and laryngeal controls (i.e., intrinsic laryngeal muscle activation affecting vocal fold posture). The role of subglottic pressure (Psub) on F0 regulation is considered to be secondary, with the primary role attributed to intrinsic laryngeal muscle activation (Ohala, 1970; Titze, 1988; Titze, 1994). However, control of F0 by intrinsic laryngeal muscles remains to be fully investigated.
The current framework for understanding F0 control by laryngeal muscle activation is the “body-cover” biomechanical model, which proposes that F0 is controlled through changes in length (strain) or tension (stress) of the vocal fold cover layer (Hirano, 1974). In this model the layered histology of the vocal fold is divided biomechanically into the “body” layer, consisting of the thyroarytenoid (TA) muscle and the adjacent deep collagen fibers, and the “cover” layer consisting of the superficial and intermediate lamina propria layer and the vocal fold epithelium. The body layer is able to actively change its stiffness and length by TA muscle activation, while the cover layer stress and strain depend on the relative activation levels of cricothyroid (CT) and TA muscles. Thus, in the body-cover model the TA and CT muscles control F0 by primarily affecting the strain of the vocal fold cover layer. CT activation elongates and stiffens the cover layer and increases F0. TA activation shortens and stiffens the body layer, creates a slack in the cover layer, and decreases F0. This paradigm is also the basis of many mathematical models of F0 control (Lowell and Story, 2006; Farley, 1994, 1996). Note that CT activation additionally elongates and stiffens the body layer. Therefore, CT activation and TA activation are expected to have antagonistic effects on vocal fold stress and strain.
The role of intrinsic laryngeal muscle activation in F0 control has been studied in humans and animals but has yet to be systematically investigated, in particular the role of the TA muscle and its interactions with other laryngeal adductors and the CT muscle. Ohala (1970) performed laryngeal electromyography (LEMG) during speech in five subjects and found CT muscle active only during increase in F0, while the lateral cricoarytenoid (LCA) and TA muscles were active immediately before the onset and throughout phonation. The LCA in particular showed an increase in activity during a rise in pitch, while the interarytenoid (IA) and posterior cricoarytenoid (PCA) muscles showed no correlated activity with pitch variations. However, subglottic pressure (Psub) was not concurrently measured. Atkinson (1978) also performed LEMG during speech in subjects, additionally measured concurrent Psub, and correlated F0 and activation levels of various intrinsic and extrinsic laryngeal muscles. CT activation showed the highest positive correlation with F0, followed by LCA, while TA played a minor role. Psub correlated negatively with F0. Kempster et al. (1988) stimulated the CT and TA muscles via bipolar LEMG electrodes while subjects attempted phonation of a sustained vowel at a constant F0. Stimulation of either CT or TA resulted in an increase in F0, with a mean change of 34% with CT stimulation alone and 9.5% with TA stimulation alone. However their study is limited by not measuring the concurrent Psub. Choi et al. (1993a) studied the role of TA in an in vivo canine model and found that increasing TA activation resulted in an increase in Psub. However, the effect on F0 varied from decreasing to increasing. SLN stimulation alone increased F0 significantly, and when combined with increasing TA stimulation at high SLN stimulation, abrupt F0 drops occurred for medium TA activation.
The role of CT in increasing F0 by vocal fold elongation has been firmly established, as it was apparent in excised larynx experiments that mechanical approximation of the cricoid and thyroid cartilages lengthened the vocal folds and increased F0 in the presence of sufficient airflow (Ohala, 1970). However, the role of TA in F0 control has not been systematically investigated and continues to be debated. Specifically, based on preliminary experiments it has been proposed that TA activation could either raise or lower F0 depending upon the amount of body layer effectively in vibration (Titze et al., 1988, 1989; Titze, 1994). To explore the role of TA, Titze et al. (1989) used LEMG to record activation levels of TA and CT muscles in four subjects who attempted to maintain sustained phonation of a vowel at a constant pitch. Loudness levels from soft to loud were tested, and TA versus CT muscle activation plots were developed for each phonation. The subjects often found it difficult to maintain a given pitch when loudness was increased and consistent EMG trends were not seen, but a consistent finding across subjects was that concurrently high CT and TA activation levels were avoided during sustained phonation. The study was also limited by lack of concurrent Psub measurements. In a second part of that study the TA muscle was stimulated using bipolar EMG electrodes in each subject (1 ms pulse duration at 2 Hz repetition rate) during sustained phonation at various pitch and loudness levels. Selective data from the study was combined with data from excised and in vivo canine larynges to propose a theory (Titze et al., 1988, 1989 ): when the cover is lax and the amplitude of vibration is sufficiently large so that a portion of the TA muscle is effectively in vibration with the cover layer, increasing TA activation would raise F0 due to increase in effective vocal fold vibratory stiffness. However, if the amplitude of vibration is very small such that none of the muscle is vibrating with the cover, increased TA activity would lower F0 by shortening and reducing the stiffness of the cover. Finally, when cover layer stiffness is high due to high levels of CT activation, activation of TA would lower F0 regardless of vibratory amplitude because the small gain in body tension is negated by the large decrease in cover tension due to vocal fold shortening from TA activation. While this was a powerful hypothesis with the potential to explain discrepancies in previous studies which reported either increases or decreases in F0 as a function of TA activation, unfortunately the results of the study were inconclusive. As acknowledged by the authors: “only one out of the four subjects showed…consistent changes in fundamental frequency with stimulation of the thyroarytenoid muscle” (Titze et al., 1989). Thus, future studies are still needed to evaluate, confirm and/or clarify this theory of F0 control.
A significant limitation of previous investigations on neuromuscular control of F0 is lack of control of independent laryngeal muscle activation, thus it is difficult to attribute the change in F0 to a particular muscle or a group of muscles. Differentiating the roles of each intrinsic laryngeal muscle in F0 control is difficult in humans because multiple intrinsic laryngeal muscles are concurrently active in speech and phonation (Atkinson, 1978). In both human (Ohala, 1970; Atkinson, 1978; Kempster et al., 1988; Titze et al., 1989) and animal (Choi et al., 1993a,b ) studies, fine control of intrinsic laryngeal muscle activation was not performed. In those studies where neuromuscular stimulation was applied (Kempster et al., 1988; Titze et al., 1989; Choi et al., 1993a,b ), “on/off” or “low-medium-high” stimulation was used, and all except one (Choi et al., 1993a,b ) stimulated only one intrinsic laryngeal muscle pair (TA or CT). Thus, only a limited set of intrinsic laryngeal muscle activation conditions have been studied, limiting interpretation of the roles of the intrinsic laryngeal muscles. Additionally, many previous studies did not measure concurrent Psub, which is an important parameter controlling F0. Moreover, F0 was measured at a variety of time periods after the onset of phonation, thus introducing potential errors in measured parameters by not controlling for changes in the aerodynamics and glottal posture after the onset of phonation.
In the present study, the roles of intrinsic laryngeal muscles in F0 control will be investigated in the in vivo canine model. Using a previously described technique of graded neuromuscular stimulation as applied to the static larynx (Chhetri et al., 2010), fine control of individual muscular activation was achieved in the current dynamic phonatory investigation using graded stimulation of laryngeal nerve branches. Concurrent measurements of F0, subglottic pressure Psub, glottal strain ɛ, and glottal width at the vocal process Dvp were obtained at phonation onset at various activation levels of intrinsic laryngeal muscles. Phonation onset was selected as the point of measurements because it is a well-defined and consistent event for all phonatory conditions. Based on bifurcation theory (Hopf bifurcation), phonation onset occurs when minimum lung pressure required to initiate self-sustained oscillation is reached (Titze, 1988), and reveals the stress state of the vocal folds, as measured by onset frequency (F0), prior to further deformation of glottal posture by rising Psub after the onset of phonation. Our automated technique of graded stimulation allows multiple combinations of laryngeal muscle activation states to be evaluated rapidly, thus allowing for a detailed examination of the roles and interplay between the adductor and CT muscles in control of F0 and glottal posture.
METHODS
In vivo canine larynx preparation
The canine larynx is a close match to the human larynx in terms of its gross, microscopic, and histologic anatomy, and the utility of the in vivo canine model in voice research is well established (Berke et al., 1987; Garrett et al., 2000). This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the study protocol was approved by the Institutional Animal Research Committee (ARC) of the University of California, Los Angeles.
Mongrel canines (weight 20–25 kg) were used for this study. One animal was used for each experimental condition (described below). Surgical exposure of the larynx and the laryngeal nerves was as described previously (Chhetri et al., 2010; Choi et al., 1993a ). In brief, a low tracheotomy was performed for intraoperative ventilation. A suprahyoid pharyngotomy was performed to expose the larynx in the neck and a supraglottic laryngectomy was performed for improved laryngeal exposure for high-speed video recording. The recurrent laryngeal nerves (RLNs) were identified on the tracheoesophageal grooves bilaterally and followed distally towards the larynx. The posterior cricoarytenoid (PCA) nerve branch was identified and divided bilaterally to eliminate the effects of this muscle during neuromuscular stimulation of the RLNs. The superior laryngeal nerve (SLN) was identified and the sensory branch (internal SLN) was divided. The external branch of the SLN innervating the CT muscle was then followed distally near its insertion into the CT muscle.
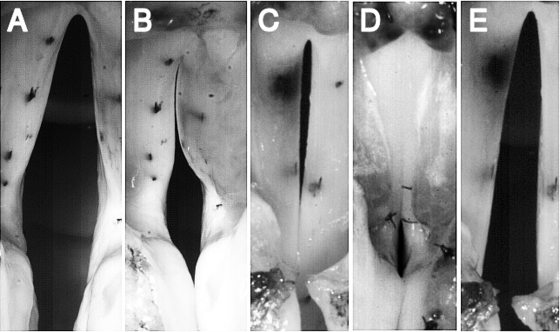
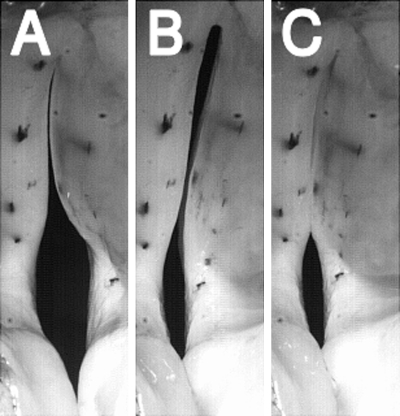
Appropriately sized bipolar or tripolar cuff electrodes (MicroProbes, Gaithersburg, MD, USA or Ardiem Medical, Indiana, PA, USA) were used for graded nerve stimulation. To activate the CT muscles, the electrodes were placed around the external SLN approximately 2–3 cm from the muscle. To activate all the laryngeal adductors (TA/LCA/IA), the RLN was stimulated by cuff electrodes around the RLN nerve trunk approximately 5 cm from the inferior border of the larynx. For selective activation of the adductors (TA or LCA/IA muscle complex), identification of the laryngeal adductor nerve branches from the RLN were performed (Choi et al., 1993a ) by following the RLN distally and dissecting the nerve under magnification until the TA and LCA branches were exposed. The TA branch was tied off with silk sutures, divided to separate from the RLN trunk, and a tripolar cuff electrode was placed around the TA nerve branch to activate the TA muscle. Once the TA nerve branch was separated from the RLN, the LCA and IA were activated as a single muscle complex via electrodes placed around the RLN nerve trunk. The LCA and IA were activated as a single complex because the IA branch is short and small and it is challenging to dissect and place electrodes to stimulate for prolonged periods, and because of the similar functional role of the LCA and IA in closing the posterior glottis. The accuracy of selective stimulation of the intrinsic laryngeal muscles was verified visually from changes in laryngeal configuration upon stimulation, as previously described (Choi et al., 1993a ,b; 1995), and also by the use of hooked wire electromyography. In particular, stimulation of the TA branch produced unique medial bulging of the membranous vocal fold, but the posterior commissure remained widely open [Fig. 1B]. Stimulation of the LCA/IA complex caused the vocal processes and the posterior commissure to adduct completely, but a glottal gap remained at the mid-membranous vocal fold [Fig. 1C]. Stimulation of all the adductors caused closure of the vocal folds at both the membranous and cartilaginous portions [Fig. 1D]. Finally, stimulation of the external branch of the SLN elongated the vocal folds [Fig. 1E].
Figure 1.
Glottal posture changes induced by activation of the intrinsic laryngeal muscles: (a) the condition of no activation, (b) TA activation caused medial bulging and glottal closure at the mid-membranous glottis but a gap persists in the cartilaginous (posterior) glottis, (c) LCA/IA activation caused vocal fold adduction and complete closure at the cartilaginous glottis but a mid-membranous gap remains, (d) activation of all the adductors (TA/LCA/IA) causes glottal closure at both membranous and cartilaginous glottis, and (e) CT activation causes lengthening of the vocal fold.
Pressurized air was humidified to 100% relative humidity, warmed to 37 °C, and passed through a 50 liter expansion chamber downstream of the humidifier, which served as a reservoir of warm, moist air, and as an acoustic termination of the subglottic airflow tube. The larynx was connected downstream of the chamber with a 1.5 cm diameter tube. The length of the subglottal tube was 30 cm for condition 1 and 19 cm for conditions 2–4. The length of the subglottal tube was reduced for latter experiments to reduce the possible effects of a subglottal resonance. The airflow rate was varied as necessary to obtain phonation but kept within a range of 700–1200 ml/s.
Graded activation of intrinsic laryngeal muscles
Graded nerve stimulation was applied to the laryngeal nerves as described previously (Chhetri et al., 2010). A computer controlled graded nerve stimulation apparatus allowed for concurrent and independent stimulation of four separate nerves: Nerve stimulation pulse trains were generated with a C computer program that controlled two PCI bus-based AD/DA boards (PowerDAQ PD2-MFS-8-500/16, United Electronics Industries, Walpole, MA) to generate the stimulation pulse trains. The voltage pulse trains were transformed into current pulse trains with a constant current stimulus isolator (A-M Systems Analog Stimulus Isolator Model M 2200, A-M Systems, Sequim, WA). The digital timer of one AD/DA board (timer resolution of 1 ms) was used as a reference clock to simultaneously trigger the pulse train generation, analog signal acquisition, and high-speed digital camera recording.
With this graded nerve stimulation apparatus it was possible to concurrently activate four separate nerves. To provide graded stimulation, first the range of threshold muscle excitation current (I0) to maximal recruitment current (IMAX) was determined for each nerve. Once I0 and IMAX were established for each nerve, any level of graded stimulation could be performed within that range. For this investigation, all paired nerves (left and right TA branches, RLN trunk, and SLN) were stimulated at the same level of graded activation to achieve symmetric activation of respective muscles on either side of the larynx. Thus, interaction of paired muscles on laryngeal phonation parameters was studied (e.g., paired TA versus paired CT). Respective nerve branches were stimulated for 1500 ms with pulse trains composed of 0.1 ms long rectangular unipolar cathodic pulses at a repetition rate of 125 Hz for laryngeal adductors and 75 Hz for CT muscles. To allow muscle recovery, each 1500 ms stimulation pulse train was followed by a pause of 3.5 s prior to the next pulse train. Four pairs of muscle activation conditions were tested: (1) CT versus all adductors (LCA/IA/TA), (2) CT versus LCA/IA complex, (3) CT versus TA, and (4) TA versus LCA/IA.
Measurement of acoustic, aerodynamic, and glottal configuration
A high-speed digital video camera (Photron FASTCAM Ultima APX, Photron, San Diego, CA) recorded the postural changes of the laryngeal framework from a superior view. High speed imaging was done at 500 frames per second with a resolution of 512 × 512 pixels. The camera was triggered 2 ms after the stimulation pulse train started and recorded laryngeal deformations for 402 ms. Landmarks of the vocal folds were marked with 8-0 nylon sutures or India ink at several locations (anterior commissure, mid membranous vocal fold, vocal process, etc.). The distance from the high speed camera to the vocal folds did not vary during the experiment. To calculate postural changes, the distances between the anterior commissure marker and the vocal processes were measured manually (in pixel units) from the high speed image obtained around 80 ms to obtain the pre-phonatory posture. (The final posture was typically established within 200 ms, generally before onset of phonation occurred.) To calculate vocal fold strain ɛ, the vocal fold length between the anterior commissure and the vocal process during a stimulated condition (Li) was compared to the reference length (L0) measured at no stimulation: ɛ = (Li – L0)/L0. To calculate Dvp, the distance between the tips of vocal processes (junction of the membranous and cartilaginous glottis) at each experimental condition was measured and the relative distance calculated similarly to obtain a percentage glottal distance compared to the baseline non-stimulated condition.
A pressure transducer (MKS Baratron 220D) and a probe microphone (Bruel and Kjaer 4128) were mounted flush with the inner wall of the subglottic tube at right angles to the air stream. Each was placed at 120 angles to each other 5 cm below the inferior border of the glottis to record mean subglottal pressure and the acoustic pressure, respectively (sampling rate 125 kHz, 16 bit resolution, range of ±10 V). The subglottal acoustic pressure signal was used to determine the time instance when self-sustained vocal fold vibrations started (phonation onset). The corresponding mean subglottal pressure (Psub) at this instance represented the phonation onset pressure (Pth). The subglottal acoustic pressure signal was low-pass filtered with a running average filter (triangular filter window with filter length of 178 samples, cut-off frequency of about 700 Hz), and the local maxima were detected with a peak-picking algorithm with a peak-exclusion time window of 166 samples (maximum detectable frequency of 750 Hz). The low-pass filtered signal and the detected peaks were used to manually determine onset time and frequency by visually identifying the signal segments with growing and sustained acoustic fluctuations, suggesting phonation onset. Transient signal bursts and slowly decaying disturbances were not considered to indicate onset of sustained oscillation. Estimates of onset pressures and frequencies were confirmed with spectrograms of the subglottal acoustic pressure signal.
RESULTS
Phonation onset pressure and frequency
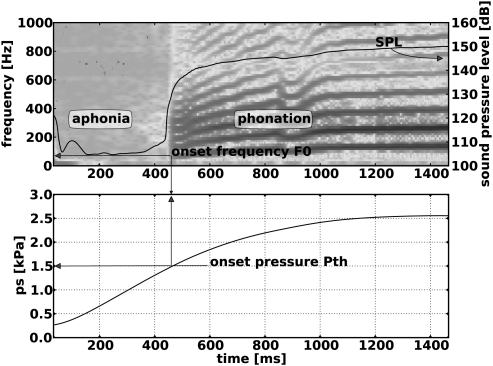
In this investigation, the laryngeal nerves were stimulated to obtain either nine (for condition 1) or eleven levels (for conditions 2–4) of graded activation of respective laryngeal muscles from threshold to maximal contraction. Laryngeal muscles were activated in various paired combinations (e.g., TA versus CT), while F0, ɛ, Pth, and Dvp, were measured. Each paired muscle activation lasted 1.5 s followed by a 3.5 s rest prior to the next stimulation. Therefore the maximum phonatory time for each tested condition was 182 s (121 paired stimulations × 1.5 s stimulation = 181.5 s) and the total duration of the phonatory experiments including rest time was about 10 min (121 paired stimulations per condition × 5 s per stimulation = 605 s). Prerun and postrun thresholds were checked to ensure that nerve stimulation thresholds remained the same. Figure 2 shows a spectrogram of the subglottal acoustic pressure (top) and the mean pressure Psub (bottom) over the entire stimulation duration (1500 ms) for one representative SLN/RLN activation condition. The compressibility in the subglottal air flow system due to the expansion chamber caused Psub to slowly rise after the beginning of nerve stimulation pulse trains. The acoustic spectrogram demonstrates that after an initial segment of aphonia, phonation onset occurs where harmonic frequency bands appear abruptly together with a large increase in the overall subglottal sound pressure level (SPL). After phonation onset both Psub and F0 typically continued to rise slowly. Phonation onset was not always reached in some conditions of low adductor/high CT activation levels, probably due to insufficient airflow and subglottal pressure.
Figure 2.
(Color online) Subglottic acoustic spectrogram (top) and subglottic pressure Psub (bottom) over the entire stimulation duration (1500 ms) for one representative SLN/RLN activation condition, illustrating a typical experimental observation. Psub starts to slowly rise after the beginning of stimulation pulse train. After a period of aphonia a large increase in subglottal sound pressure level (SPL) occurs at phonation onset, after which both Psub and F0 continue to rise slowly.
Muscle activation condition 1: All laryngeal adductors (TA/LCA/IA) versus CT activation
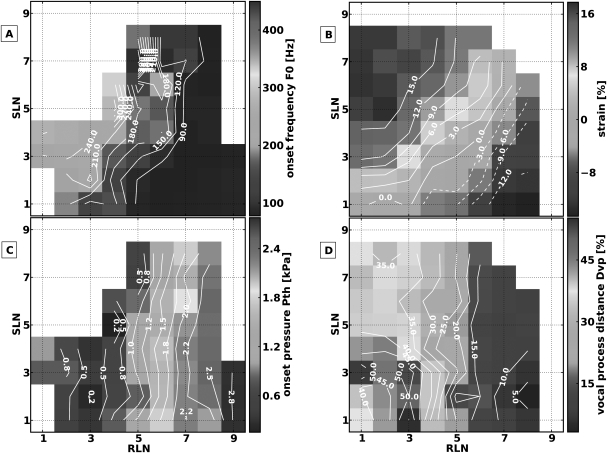
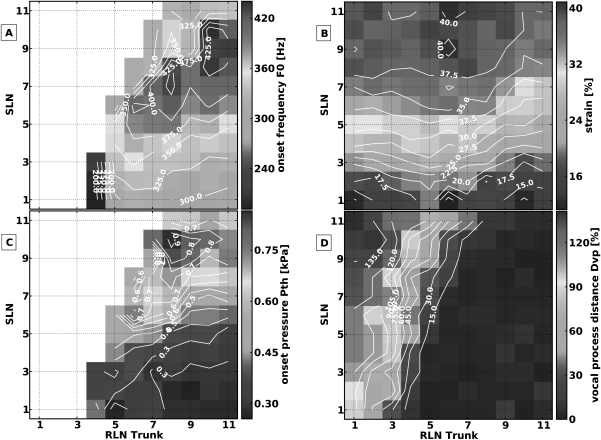
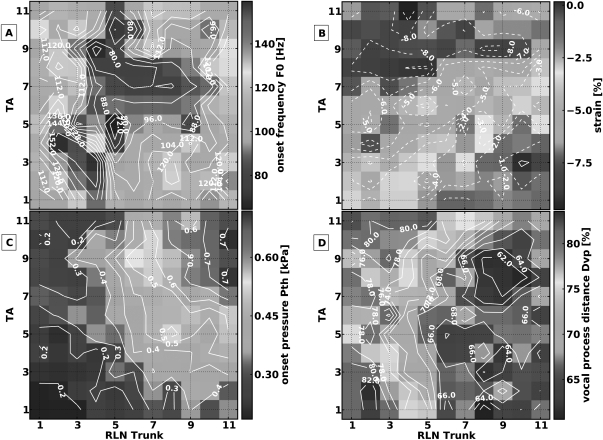
In this muscle activation condition, the RLNs were stimulated to symmetrically activate bilateral laryngeal adductors from threshold to maximal activation (TA/LCA/IA), and the SLNs were stimulated to symmetrically activate bilateral CT muscles from threshold to maximal activation. Approximately eight levels of SLN and nine levels of RLN activation were tested for each muscle (a total of 67 distinct pairs of muscle activation levels). Figure 3 shows the muscle activation plots for the measured parameters. In this muscle activation condition phonation onset was not reached in the area of low RLN/high SLN activation [left upper area Fig. 3a]. Where phonation onset was reached, increasing CT activation always increased F0 while increasing RLN activation always decreased F0, except at the highest levels of RLN activation where there was no further increase in F0 with further CT activation. Higher levels of onset F0 were seen at the low activation levels of adductors and CT [left lower area in Fig. 3a]. These observations show that a way to increase F0 was to increase CT activation and decrease RLN activation. To maintain F0 with increasing muscle activation, concomitant activation of both muscles groups (adductors and CT) would be required, as demonstrated by the approximately diagonal contour lines for F0 [Fig. 3a] and ɛ [Fig. 3b]. Laryngeal adductors activation always shortened the vocal folds while SLN activation always lengthened them. Strain ranged from +18% at low RLN/high SLN activation, to −14% at high RLN/low SLN activation. The vertical contour lines for Pth [Fig. 3c] and Dvp [Fig. 3d] demonstrate that these were primarily controlled by the adductors. High speed images of the glottal configuration at the transition from phonation to aphonia (e.g., constant RLN activation level 3, while increasing SLN activation level) demonstrated increasing glottal area due to increased ɛ and reduction of medial bulging due to CT activation (Fig. 4).
Figure 3.
(Color online) Muscle activation plots for muscle activation condition 1: CT versus all laryngeal adductors (TA/LCA/IA). (A) onset F0, (B) strain (ɛ), (C) onset subglottic pressure (Pth), and (D) glottal distance between the vocal processes (Dvp). Graded stimulation was applied to the SLN and RLN (after division of PCA branches) to activate the CT muscle and laryngeal adductors respectively.
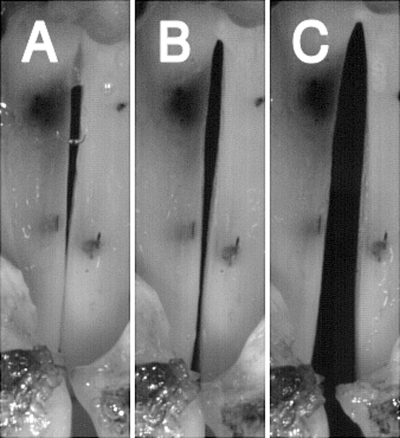
Figure 4.
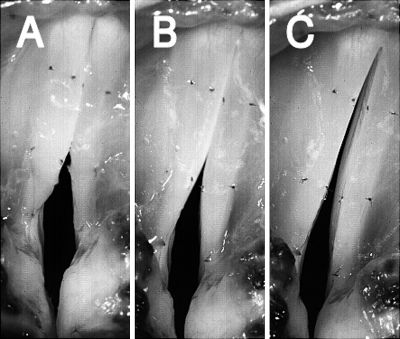
High-speed images of glottal configuration in muscle activation condition 1 (CT versus [TA/LCA/IA] activation), demonstrating the changes in prephonatory posture in regions of transition from phonation to aphonia. In the figure, RLN stimulation is kept constant (activation level 3) while CT activation (SLN stimulation) is increased from threshold to maximal. Images correspond to activation levels in shown in Fig. 3. (A) RLN level 3, CT level 1, (B) RLN 3, CT 4, and (C) RLN 3, CT 8.
Muscle activation condition 2: LCA/IA versus CT activation
In this muscle activation condition the RLN Trunks were stimulated after division of the TA and PCA branches to symmetrically activate bilateral LCA/IA muscle complexes, and the SLNs were stimulated to symmetrically activate bilateral CT muscles. Eleven graded levels of muscle activation were tested for each muscle pair for a total of 121 distinct muscle activation conditions. Figure 5 shows muscle activation plots for measured parameters. As in stimulation condition 1, areas of aphonia existed in the left upper and lower region [Fig. 5a] which corresponded to Dvp > 15% of baseline [Fig. 5d]. Where phonation onset was reached, increasing CT activation increased F0 whereas increasing LCA/IA activation had little impact on F0 [Fig. 5a]. CT activation primarily controlled both ɛ [Fig. 5b] and Pth [Fig. 5c]. ɛ ranged from 15 to 40%. CT activation always elongated the vocal folds and LCA/IA activation had no major effect on ɛ except at the very high activation levels for both muscle groups, where there was some shortening with LCA/IA activation for constant CT activation. Phonation onset occurred at low levels of Pth [Fig. 5c], and the range and magnitude of Pth (0.3–0.8 kPa) was lower compared to activation condition 1 [where RLN stimulation activated all the adductors, Fig. 3a]. The contour lines for Dvp demonstrate that this parameter was primarily controlled by LCA/IA activation, although higher levels of CT activation slightly widened the cartilaginous glottis, particularly at lower LCA/IA activation levels [Fig. 5d].
Figure 5.
(Color online) Muscle activation plots for muscle activation condition 2 (CT versus LCA/IA). (A) Onset F0, (B) strain (ɛ), (C) onset subglottic pressure (Pth), and (D) glottal distance between the vocal processes (Dvp). Graded stimulation was applied to the SLN and RLN trunk (after division of PCA and TA branches) to activate the CT muscle and LCA/IA muscle complex, respectively.
The approximately horizontal contour lines in Figs. 5a, 5b indicate that F0 and ɛ were affected primarily by CT activation. Without TA activation the antagonistic roles of RLN and SLN stimulation seen in Fig. 3a, 3b disappeared. Further examination of muscle activation plots in Fig. 5 reveals that phonation onset was typically reached when the cartilaginous glottis was nearly closed [Figs. 5a, 5d]. High speed images of the glottal configuration at the transition from phonation to aphonia (e.g., constant LCA/IA activation level 5, while increasing CT activation level) showed increasing glottal area together with increases in both ɛ and Dvp from CT activation (Fig. 6).
Figure 6.
High-speed images of glottal configuration in muscle activation condition 2 (CT versus LCA/IA), demonstrating the changes in prephonatory posture in regions of transition from phonation to aphonia. LCA/IA activation (via RLN Trunk stimulation) is kept constant (activation level 5) while CT activation (SLN stimulation) is increased from threshold to maximal. Images correspond to activation levels shown in Fig. 5. (A) LCA/IA level 5, CT level 1, (B) LCA/IA 5, CT 6, (C) LCA/IA 5 CT 11.
At high CT activation levels [right upper region in Fig. 5a] F0 lowered with increasing CT activation. In this region, further LCA/IA activation was required to increase F0. This scenario contrasts with the findings in condition 1 [Fig. 3a], where increasing RLN activation always resulted in a decreasing F0. These findings demonstrate that in muscle activation condition 2 phonation onset occurs after near approximation of the cartilaginous glottis, and that LCA/IA and CT may act synergistically to increase F0.
Muscle activation condition 3: TA versus CT activation
In this muscle activation condition the TA branches were stimulated after division from the main RLN trunk to symmetrically activate the TA muscles, and the SLNs were stimulated to symmetrically activate the CT muscles. Eleven graded levels of muscle activation were tested for each muscle pair for 121 pairs of distinct muscle activation conditions. Figure 7 shows muscle activation plots for the measured parameters. Significant areas of aphonia were present in this activation condition [left area of high CT activation in Fig. 7a]. Where phonation onset was reached, increasing CT activation increased F0 whereas increasing TA activation had minimal effect on F0. The contour lines for ɛ [Fig. 7b] indicate that TA and CT acted antagonistically for vocal fold strain: CT activation always elongated the vocal folds and TA activation shortened them. Strain ranged from −8% +14 %. Onset pressure [Fig. 7c] was in the range of 0.8–1.7 KPa and was controlled primarily by CT activation [Fig. 7c]. Figure 7d shows muscle activation plots for Dvp. The posterior glottis remained open throughout the muscle activation range and TA activation tended to decrease Dvp. These results demonstrate that without concomitant LCA/IA activation, TA activation was antagonistic with CT activation only for ɛ, similar to when all adductors were activated (Fig. 3). High speed images of the glottal configuration at the transition from phonation to aphonia (e.g., constant TA activation level 7, while increasing CT activation level) showed an increasing glottal area together with increases in ɛ, Dvp, and reduction of medial bulging due to CT activation (Fig. 8).
Figure 7.
(Color online) Muscle activation plots for muscle activation condition 3 (CT versus TA). (a) onset F0, (b) strain (ɛ), (c) onset subglottic pressure (Pth), and (d) glottal distance between the vocal processes (Dvp).
Figure 8.
High-speed images of glottal configuration in muscle activation condition 3 (CT versus TA), demonstrating the changes in pre-phonatory posture in regions of transition from phonation to aphonia. TA activation is kept constant (activation level 7) while CT activation is increased from threshold to maximal. Images correspond to activation levels shown in Fig. 7. (a) TA level 7, CT level 1, (b) TA 7, CT 6, (c) TA 7, CT 11.
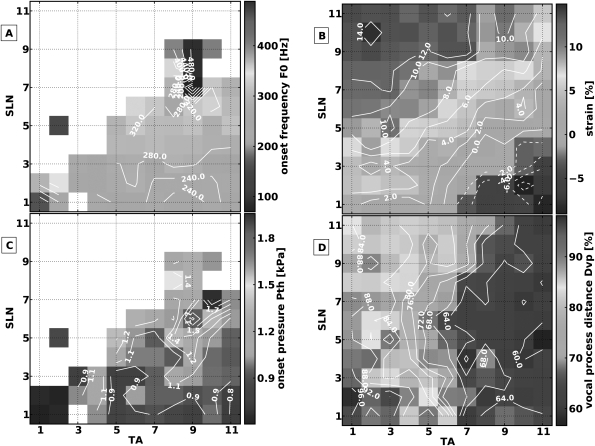
Muscle activation condition 4: TA versus LCA/IA activation
In this muscle activation condition the TA branches were stimulated symmetrically after division from RLN trunk to activate the TA muscles, and the RLN nerve trunk was stimulated after division of the TA and PCA branches to symmetrically activate the LCA/IA complexes. Eleven graded levels of muscle activation were tested for each muscle pair for a total of 121 pairs of distinct muscle activation conditions. Figure 9 shows the muscle activation plots for the measured laryngeal parameters. In this muscle activation condition, phonation was achieved in ALL muscle activation conditions [Fig. 9a]. The contour lines for F0 show no simple pattern for F0 control. F0 ranged between 80 and 152 Hz, and there was an area of higher F0 for the low activation levels of both pairs of muscles (left lower region) therefore register shifts may be present in this condition. Contour lines for ɛ reveal that TA shortened and LCA/IA slightly elongated the vocal folds, especially at high LCA/IA activation levels [Fig. 9b]. Strain ranged from −8 to 0 %. TA and LCA/IA were synergistic for Pth (range 0.2 to 0.7 KPa) [Fig. 9c] and Dvp was controlled primarily by the LCA/IA complexes [Fig. 9d]. High speed images of the glottal configuration (Fig. 10) for three extreme activation conditions (A: RLN trunk level 1, TA level 11; B: RLN trunk level 11, TA level 1; C: RLN trunk level 11, TA level 11) show the interaction between the membranous and cartilaginous adduction.
Figure 9.
(Color online) Muscle activation plots for muscle activation condition 4 (TA versus LCA/IA). (a) onset F0, (b) strain (ɛ), (c) onset subglottic pressure (Pth), and (d) glottal distance between the vocal processes (Dvp).
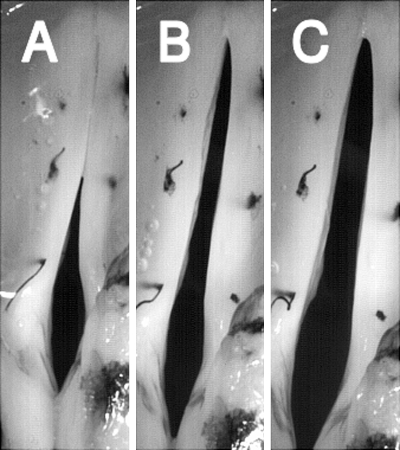
Figure 10.
High-speed images of glottal configuration in muscle activation condition 4 (TA versus LCA/IA), demonstrating the interactions between the membranous adduction due to TA activation and cartilaginous adduction due to LCA/IA activation. Images correspond to activation levels shown in Fig. 9. (A) TA level 11, (LCA/IA) level 1, (B) TA 1, (LCA/IA) 11, (C) TA 11, (LCA/IA) 11.
DISCUSSION
The body-cover model proposes that the cover tension is primarily responsible for F0 control, and that TA and CT are antagonistic in F0 control. Previously, we used the method of graded stimulation of laryngeal muscles to demonstrate the antagonistic effects of CT and laryngeal adductor muscle activation on vocal fold strain for the pre-phonatory static condition (Chhetri et al., 2010). In the present study, we have extended this technique to dynamic phonatory conditions, and examined the control of F0 and glottal posture at phonation onset as a function of CT and laryngeal adductor muscle activations.
Our results demonstrate the complex interplay between the intrinsic laryngeal muscles in controlling F0 and glottal posture. CT activation elongated and TA activation shortened the vocal folds, therefore the resulting glottal strain was a balance between these two antagonistic muscles, as shown by the diagonal isocontour lines [Figs. 3b, 7b]. In addition, particularly at high levels of activity CT activation resulted in slight abduction of the vocal processes (Figs. 68), leading to areas of transition from phonation to aphonia unless accompanied by a concomitant increase in adductor muscle activity [Fig. 5a, 7a]. The role of LCA/IA was to close the posterior cartilaginous glottis [Figs. 1c, 5d] while TA closed the mid-membranous glottis [Fig. 1b, 10]. CT activation always increased F0 [Figs. 3a, 5a, 7a], whereas activation of the laryngeal adductors had a more complex effect on F0. RLN stimulation to activate all the adductors always decreased F0 and ɛ until zero strain was reached, at which point further RLN activation did not reduce F0 further [Figs. 3a, 3b]. When only the LCA/IAs, or only the TAs, were activated against CT activation, F0 control was dependent upon CT activation alone. Finally, in the muscle plots with TA versus LCA/IA activation, slightly higher F0 was seen at lower activation levels for both [left lower area Fig. 9a], but this did not correlate with strain [Fig. 9d].
Theoretical models have predicted that F0 could increase, remain unchanged, or decrease, with TA activation depending on the effective depth of vibration (Titze et al., 1988; Titze et al., 1989). In this study, an increase in F0 was not seen with activation of all adductors together, TA activation alone, or LCA/IA activation alone. However, Titze et al. (1989) acknowledged that if the amplitude of vibration were small, such as in soft phonation, F0 could only decrease with increased TA activity. Because the present study measured F0 at phonation onset (which is typically a softest phonation condition) for each neuromuscular condition, our results must be interpreted within the framework of small amplitude vibrations. Thus, results of this study are consistent with Titze et al. (1989). A systematic study of the effects of vocal intensity and vibratory amplitude on F0 as a function of intrinsic laryngeal muscle activation level remains to be done.
The effect of TA activation is medial bulging of the glottis, whereas CT activation elongates the vocal fold cover and the TA muscle, counteracting the medial bulging effects of the TA muscle and thus changing the glottal posture. In addition, slight abduction of the vocal processes was seen at high levels of CT activation. These CT effects would be expected to have significant impact on phonation onset pressure and glottal dynamics. In fact, in this study, we found that increasing CT activation at low adductor activation levels resulted in transitions from regions of phonation onset to aphonia [Figs. 3a, 5a, 7a]. To re-establish phonation in these areas, further activation of the adductor muscles was required. CT activation appears to induce transitions to aphonia in several ways. First, CT activation stretched and stiffened the vocal fold. This can be appreciated in Figs. 4a, 4b, 4c in the case of CT versus all adductor activation. Here, although the cartilaginous glottis distance remained constant, aphonia resulted. The increased stiffness probably resulted in a corresponding increase in Pth. Secondly, when increased CT activation occurred with no corresponding increase in the activation of the adductors, the cartilaginous glottal width increased, in addition to the increase in vocal fold elongation, so the increased glottal width may have also played a role in elevating Pth. Finally, CT activation may lead to postural changes of the medial vocal fold which could not be imaged in this study. This will be a focus of future studies. F0 increase in humans is associated with concurrent increases in both CT and TA activation levels (Ohala, 1970; Atkinson, 1978; Kempster et al., 1988). Figures 3a, 5a, 7a suggest that this may be necessary to avoid transitioning into regions of aphonia with CT activation alone. Indeed, a concomitant increase in both CT and TA may also be useful in maintaining a desired F0.
Hirano et al. (1969) found (using LEMG) that in chest voice the activity of all three muscles (CT, LCA, and TA) varied proportionately with fundamental frequency. In falsetto voice, especially in extremely high falsetto, the activity of CT often did not vary very much but LCA activity increased with increasing F0 while the activity of TA was much lower in falsetto than chest voice. Our results shown in Fig. 5a seem to support Hirano’s findings.
Titze et al. (1989) have suggested that the high RLN/low SLN region is avoided in human phonation, as it represents a hyper-adducted activation state. The results of this investigation also support this notion that certain muscle activation regions are likely avoided in phonation [Fig. 3a]. The first region is the low RLN/high CT activation states where phonation onset was not reached at the airflow rates used as it likely required very large airflow rates to produce a possibly low phonation onset pressure. The second region is the high RLN/low SLN activation state where no change in F0 was seen with increasing muscle activation [Fig. 3a]. As there was no change in F0 in this region with further activation of adductors or CT muscles but onset pressure continued to increase, it would be reasonable to assume that phonation at high RLN/low SLN activation levels would be highly inefficient in terms of F0 control. Clinically, this region might resemble the conditions encountered in hyperfunctional laryngeal voice disorders such as spasmodic dysphonia and muscle tension dysphonia.
Recently it has been demonstrated that separate control of the membranous and cartilaginous glottis is possible in trained singers (Herbst et al., 2011). If LCA/IA and TA were controlled separately then the possibilities for F0 control increase [Figs. 5a, 7a]. Our study looked at F0 at onset of phonation. Some previous EMG studies have shown that both CT and TA activity increase with F0 increase (e.g., Kempster et al., 1988). However, such studies were performed at subglottal pressures higher than the phonation threshold pressure. Thus, with increased amplitudes of vibration, increased TA activity was likely necessary to counter the effects of CT on glottal shape and to maintain glottal closure. The current study reports phonatory output measured at phonation threshold pressure, and thus cannot be directly compared with studies for which higher subglottal pressures were considered. However, our results do support the notion that both CT and TA are used in F0 control and that muscle activation patterns which follow a diagonal line in the muscle activation plots [from low CT/low RLN levels towards high CT/high RLN levels, Fig. 3a] are preferred (Titze et al., 1989). With regard to register changes, the steepest changes in the F0 contour lines were seen at high CT and mid to high adductor activation levels [Figs. 3a, 5a, 7a]. Although the study of neuromuscular control of register changes was not the primary focus of this study, our methodology of graded stimulation can be used to study these regions of steep F0 change in finer detail.
Although this study represents an initial investigation into phonatory control as a result of stimulation of individual laryngeal muscles, additional systematic, comprehensive investigations of this type are needed. For example, in the present study, the CT versus LCA/IA parameter space was only studied for the condition of zero TA stimulation, and the CT versus TA parameter space was studied only for the condition of zero LCA/IA stimulation. Note that the CT versus LCA/IA condition corresponds to the condition of an excised larynx in which various levels of vocal fold elongation and posterior glottal adduction are imposed mechanically. However, mechanical TA activation to induce vocal fold bulging is not possible in an excised larynx experiment. The CT versus TA condition, which resulted in a relatively large posterior glottal gap, clearly failed to reveal the full range of possible CT versus TA phonatory interactions as a function of Dvp. Thus, to gain a more comprehensive understanding of the role of the intrinsic laryngeal muscles in regulating F0, Pth, and prephonatory vocal fold posturing, it is necessary to study the CT-LCA/IA parameter space at several levels of TA activation and the CT-TA parameter space at several levels of LCA/IA activation.
Another limitation of this study is that the four muscle activation conditions were performed in four separate animals. Therefore, the interaction of each of these muscles in the same larynx was not investigated. In addition, only four muscles (two pairs) could be activated at the same time. In the future, we plan to expand the number of muscles concurrently activated. For example, to delineate the effect of TA on register change we will keep the posterior glottis closed with high levels of LCA/IA stimulation, then traverse the graded levels of TA versus CT activation. We also did not study the effects of PCA muscles; in fact we eliminated their effects by cutting the PCA branches. Previous studies have implicated a role for the PCA in glottal control as a brace against the anterior pull of the adductor and CT muscles during phonation (Choi et al., 1992; Mu and Yang, 1991), or an active role in breathy phonation or production of voiceless segments in speech (Hirose, 1976). Nevertheless, the total number of conditions studied (434 unique muscle pair activation conditions) and the use of automated, graded stimulation to obtain systematic, comprehensive muscle activation plots (from which isofrequency, isopressure, isostrain, and isoadduction contours can be derived) provides phonatory data from an intact neuromuscular model of phonation heretofore not available. Previous attempts at developing such muscle activation plots have relied on selective combination of data from multiple, unrelated, cross-species experiments to generate isofrequency contours (Titze et al., 1988, 1989). We used the in vivo canine model to measure the relationships between fundamental frequency, subglottic pressure, glottal adduction and vocal fold strain as a function of laryngeal nerve stimulation at phonation onset, and to substantiate certain aspects of longstanding hypotheses regarding fundamental frequency control (Hirano, 1974; Titze et al., 1989).
Finally, another potential limitation of this study is that a canine larynx model was used, yielding possible differences in F0 control, as compared to a human larynx. For ethical reasons, these invasive studies could not be performed on an in vivo human larynx. However, the canine larynx closely resembles the human larynx in neuromuscular anatomy, overall dimensions of the vocal fold, and microanatomy. Arguably, the canine larynx may possess a less developed vocal ligament (intermediate and deep layers of the lamina propria layer), which hypothetically allows the larynx to handle more longitudinal stress, thus facilitating the production of higher fundamental frequencies in the upper vocal registers of the singing voice (Titze and Hunter, 2004). However, the literature is not consistent regarding the functional role of the vocal ligament, and the microanatomy of canine vocal ligament. For example, while Kurita et al. (1983) described a poorly developed vocal ligament in the canine larynx, Garrett et al. (2000) found a trilaminar LP layer that resembled the human larynx. In particular, Garrett et al. (2000) found similar intermediate layers in both human and canine species (dense ground substance over dense elastin), while the deep LP layer differed only in that the canine had more ground substance over collagen as compared to the human larynx, which had mostly collagen. In this study, the canine F0 ranged from 80 to 420 Hz, which covers the human speaking range very well. Therefore, for investigations of F0 control within the speaking range, the similarities between the human and canine larynx in terms of physical dimensions, neuromuscular control, and the trilaminar structure of the vocal folds make the in vivo canine larynx a useful model of the intact human larynx.
CONCLUSION
Graded electrical stimulation of the RLN and SLN in an in vivo canine model of phonation were performed to activate laryngeal adductors and CT muscles and evaluate the interactions of these muscles in regulating F0, phonation threshold pressure, and prephonatory vocal fold posturing. At phonation onset, CT activation played a primary role in regulating F0, and the laryngeal adductors played an antagonistic, secondary role. Phonation onset pressure was primarily controlled by RLN activation. Glottal width and onset pressure were highly correlated, as were vocal fold strain and F0 at phonation onset. The results support the body-cover theory of F0 control, especially with regard to the antagonistic interaction between the laryngeal adductors and the CT muscle. More systematic, comprehensive studies of this type are needed to help resolve the complex interactions between both antagonistic and synergistic muscles, which enable the laryngeal structures to couple with the glottal airflow, and produce variations in phonatory output.
ACKNOWLEDGMENTS
This study was supported by Grant Nos. R01 DC011300, R01 DC003072, and R01 DC009229 from the National Institutes of Health.
References
- Atkinson, J. E. (1978). “Correlation analysis of the physiological factors controlling fundamental voice frequency,” J. Acoust. Soc. Am. 63, 211–222. 10.1121/1.381716 [DOI] [PubMed] [Google Scholar]
- Berke, G. S., Moore, D. M., Hantke, D. R., Hanson, D. G., Gerratt, B. R., and Burstein, F. (1987). “Laryngeal modeling: theoretical, in vitro, in vivo,” Laryngoscope 97, 871–881. 10.1288/00005537-198707000-00019 [DOI] [PubMed] [Google Scholar]
- Chhetri, D. K., Neubauer, J., and Berry, D. A. (2010). “Graded activation of the intrinsic laryngeal muscles for vocal fold posturing,” J. Acoust. Soc. Am. 127, EL127–EL133. 10.1121/1.3310274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H. S., Berke, G. S., Ye, M., and Kreiman, J. (1993a). “Function of the thyroarytenoid muscle in a canine laryngeal model,” Ann. Otol. Rhinol. Laryngol. 102, 769–776. [DOI] [PubMed] [Google Scholar]
- Choi, H. S., Berke, G. S., Ye, M., and Kreiman J. (1993b). “Function of the posterior cricoarytenoid muscle in phonation: In vivo laryngeal model,” Otolaryngol. Head Neck Surg. 109(6), 1043–1051. [DOI] [PubMed] [Google Scholar]
- Choi, H. S., Ye, M., and Berke, G. S. (1995). “Function of the interarytenoid(IA) muscle in phonation: In vivo laryngeal model,” Yonsei Med. J. 36, 58–67. [DOI] [PubMed] [Google Scholar]
- Farley, G. R. (1996). “A biomechanical laryngeal model of voice F0 and glottal width control,” J Acoust Soc Am. 100, 3794–3812. 10.1121/1.417218 [DOI] [PubMed] [Google Scholar]
- Farley, G. R. (1994). “A quantitative model of voice F0 control,” J. Acoust. Soc. Am. 95, 1017–1029. 10.1121/1.408465 [DOI] [PubMed] [Google Scholar]
- Garrett, C. G., Coleman, J. R., and Reinisch, L. (2000). “Comparative histology and vibration of the vocal folds: Implications for experimental studies in microlaryngeal surgery,” Laryngoscope 110, 814–824. 10.1097/00005537-200005000-00011 [DOI] [PubMed] [Google Scholar]
- Herbst, C. T., Qiu, Q., Schutte, H. K., Švec, J. G. (2011). “Membranous and cartilaginous vocal fold adduction in singing,” J. Acoust. Soc. Am. 129(4), 2253–2262. 10.1121/1.3552874 [DOI] [PubMed] [Google Scholar]
- Hirano, M., Ohala, J., and Vennard, W. (1969). “The function of laryngeal muscles in regulating fundamental frequency and intensity of phonation,” J. Speech Hear. Res. 12, 616–628. [DOI] [PubMed] [Google Scholar]
- Hirano, M. (1974). “Morphological structure of the vocal cord as a vibrator and its variations,” Folia Phoniatr (Basel). 26, 89–94. 10.1159/000263771 [DOI] [PubMed] [Google Scholar]
- Hirose, H. (1976). “Posterior cricoarytenoid as a speech muscle,” Ann. Otol. Rhinol. Laryngol. 85(3), 335–342. [PubMed] [Google Scholar]
- Kempster, G. B., Larson, C. R., and Kistler, M. K. (1988). “Effects of electrical stimulation of cricothyroid and thyroarytenoid muscles on voice fundamental frequency,” J. Voice. 2, 221–229. 10.1016/S0892-1997(88)80080-8 [DOI] [PubMed] [Google Scholar]
- Kurita S., Nagata K., and Hirano M. (1983). “A comparative study of the layer structure of the vocal fold,” in Vocal Fold Physiology: Contemporary Research and Clinical Issues, edited by D. M.Bless and Abbs J. H.(College-Hill Press, San Diego: ), pp. 3–21. [Google Scholar]
- Lowell, S. Y., and Story, B. H. (2006). “Simulated effects of cricothyroid and thyroarytenoid muscle activation on adult-male vocal fold vibration,” J. Acoust. Soc. Am. 120, 386–397. 10.1121/1.2204442 [DOI] [PubMed] [Google Scholar]
- Mu, L. C., and Yang, S. L. (1991). “The role of the posterior cricoarytenoid muscle in phonation: An electromyographic investigation in dogs,” Laryngoscope 101(8), 849–854. [DOI] [PubMed] [Google Scholar]
- Ohala J. (1970). “Aspects of the control and production of speech,” UCLA Working papers in phonetics No. 15 (http://escholarship.org/uc/item/1859f9tk; date last viewed 11/14/11).
- Titze, I. R., Jiang, J., and Drucker, D. G. (1988). “Preliminaries to the body-cover theory of pitch control,” J Voice. 4, 314–319. 10.1016/S0892-1997(88)80004-3 [DOI] [Google Scholar]
- Titze, I. R. (1988). “The physics of small-amplitude oscillation of the vocal folds,” J. Acoust. Soc. Am. 83, 1536–1552. 10.1121/1.395910 [DOI] [PubMed] [Google Scholar]
- Titze, I. R., Luschei, E. S., and Hirano, M. (1989). “Role of thyroarytenoid muscle in regulation of fundamental frequency,” J. Voice 3, 213–224. 10.1016/S0892-1997(89)80003-7 [DOI] [Google Scholar]
- Titze, I. R. (1989). “On the relation between subglottal pressure and fundamental frequency in phonation,” J. Acoust. Soc. Am. 85, 901–906. 10.1121/1.397562 [DOI] [PubMed] [Google Scholar]
- Titze, I. R., and Hunter, E. J. (2004). “Normal vibration frequencies of the vocal ligament,” J. Acoust. Soc. Am. 115, 2264–2269. 10.1121/1.1698832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze, I. R. (1994). Principles of Voice Production (Prentice Hall, New Jersey: ), pp. 1–354. [Google Scholar]