Abstract
Relative to intravenous drug self-administration, locomotor activity is easier to measure with high throughput, particularly in mice. Therefore its potential to predict differences in self-administration between genotypes (e.g., targeted mutations, recombinant inbred strains) is appealing, but such predictive value is unverified. The main goal of this study was to evaluate the utility of the locomotor assay for accurately predicting differences in cocaine self-administration. A second goal was to evaluate any correlation between activity in a novel environment, and cocaine-induced hyperactivity, between strains. We evaluated locomotor activity in male and female Sprague-Dawley rats and 15 mouse strains (129S1/SvImJ, 129S6/SvEvTac, 129X1/SvJ, A/J, BALB/cByJ, BALB/cJ, C3H/HeJ, C57BL/6J, CAST/EiJ, DBA/2J, FVB/NJ, SJL/J, SPRET/EiJ, and outbred Swiss Webster and CD-1/ICR), as well as cocaine self-administration in BALB substrains. All but BALB/cJ mice showed locomotor habituation and significant cocaine-induced hyperactivity. BALB/cJ mice also failed to self-administer cocaine. BALB/cByJ mice showed modest locomotor habituation, cocaine-induced locomotion, and cocaine self-administration. As previously reported, female rats showed greater cocaine-induced locomotion than males, but this was only observed in one of fifteen mouse strains (FVB/NJ), and the reverse was observed in two strains (129X1/SvJ, BALB/cByJ). The intriguing phenotype of the BALB/cJ strain may indicate some correlation between all-or-none locomotion in a novel environment, and stimulant and reinforcing effects of cocaine. However, neither novelty- nor cocaine-induced activity offered a clear prediction of relative reinforcing effects among strains. Additionally, these results should aid in selecting mouse strains for future studies in which relative locomotor responsiveness to psychostimulants is a necessary consideration.
Keywords: cocaine, locomotor activity, self-administration, BALB/cJ, BALB/cByJ
INTRODUCTION
Numerous studies have suggested differences in the motor activating effects of psychomotor stimulants among inbred mouse strains. For example, BALB/cByJ mice were reported to exhibit little or no locomotor activation by cocaine (Deroche et al., 1997; Ruth, Ullman & Collins, 1988). Strains commonly used to generate targeted gene mutations (e.g., knockout mice) are of particular interest, in that the genetic background on which these mutations are expressed can influence the results (Kelly et al., 1998; Phillips, Hen & Crabbe, 1999). In particular, the common use of mixed genetic backgrounds (e.g., embryonic stem cells from a 129 substrain and breeding in the C57BL/6J strain) can obscure the attribution of a behavioral phenotype to the targeted mutation (Gerlai, 1996; Lathe, 1996). The C57BL/6J, substrains of 129 (particularly 129X1/SvJ and 129S6/SvEvTac), and the DBA/2J, are the most widely used strains in the generation of targeted mutations.
Mice of 129 substrains have typically been found less responsive to cocaine or d-amphetamine than C57BL/6J mice in locomotor activity assays (Kuzmin, Johansson, Fredholm & Ogren 2000; Mhyre et al., 2005; Miner, 1997; Schlussman, Ho, Zhou, Curtis & Kreek, 1998; Schlussman et al., 2003; Ralph, Paulus & Geyer, 2001). DBA/2 mice have been found more responsive to locomotor stimulation by cocaine relative to C57BL/6 mice under some conditions, but comparable activity levels were also reported between these two strains (Morse, Erwin & Jones, 1993; Orsini, Bonito-Oliva, Conversi & Cabib 2005; Phillips, Huson & McKinnon, 1998; Rocha et al., 1998; Tolliver & Carney, 1995; Womer, Jones & Erwin, 1994). However, those strains typically exhibited differences in drug-free activity levels as well. Mice of 129 substrains have consistently shown lower drug-free locomotor activity levels than C57BL/6J mice (Cook, Bolivar, McFadyen & Flaherty, 2002; Homanics, Quinlan & Firestone, 1999; Isles, Humby, Walters & Wilkinson, 2004; Kelly et al., 1998; Kuzmin, Johansson, Fredholm & Ogren, 2000; Miner, 1997; Paulus, Dulawa, Ralph & Mark, 1999; Ralph, Paulus & Geyer, 2001), while DBA/2 mice exhibited either lower or comparable activity levels relative to C57BL/6 mice (Anisman & Cygan, 1975; Downing, Rodd-Henricks, Marley & Dudek, 2003; Kafkafi et al., 2003; Mhyre et al., 2005; Morse, Erwin & Jones, 1993; Orsini, Buchini, Piazza, Puglisi-Allegra &, Cabib, 2004; Orsini, Bonito-Oliva, Conversi & Cabib, 2005; Phillips, Huson & McKinnon, 1998; Rocha et al., 1998; Ventura et al., 2004; Womer, Jones & Erwin, 1994). Further complicating the issue is the fact that investigations have varied widely in the extent to which animals were habituated to the test environment before drug administration. As strain differences in the degree and rate of habituation have also been observed, such differences in experimental design may be influential in drug tests. For example, DBA/2J failed to exhibit habituation under conditions in which C57BL/6J mice did so, while both strains showed habituation in another investigation (Bolivar, Caldarone, Reilly & Flaherty, 2000; Helmeste & Seeman, 1982). Thus varying periods of habituation could explain some discrepancies in comparisons of C57BL/6 and DBA mice between investigations.
Importantly, many studies also suggested differences in the abuse-related effects of cocaine between mouse strains using conditioned place preference (CPP) or self-administration procedures (Cabib, Orsini, Le Moal & Piazza, 2000; Carney, Landrum, Cheng & Seale 1991; Grahame & Cunningham, 1995; Kuzmin & Johansson, 2000; Rocha et al., 1998; Seale & Carney, 1991; Zhang, Mantsch, Schlussman, Ho & Kreek, 2002). For example, cocaine was found to engender little or no CPP and was self-administered at lower rates in 129X1/SvJ mice in direct comparisons to C57BL/6J mice (Miner, 1997; Thomsen & Caine, 2006). BALB/cByJ mice failed to self-administer cocaine under conditions that led to self-administration in C57BL/6JxSJL hybrid mice (Deroche et al., 1997). Of great interest is the possibility that the locomotor assay may be useful as a predictor of differences in the abuse-related effects of cocaine among mouse strains, e.g., as an efficient and accurate screen of ENU mutagenized mice (Orsini, Buchini, Piazza, Puglisi-Allegra & Cabib 2004; Wise & Bozarth, 1987). Researchers have also suggested that locomotor activity in a novel environment may predict the psychomotor, rewarding, or reinforcing effects of psychostimulants in rodents (Brabant, Quertemont & Tirelli, 2005; Hooks, Jones, Smith, Neill & Justice, 1991; Klebaur, Bevins, Segar & Bardo, 2001; Orsini, Buchini, Piazza, Puglisi-Allegra & Cabib, 2004; Piazza, Deminiere, Le Moal & Simon, 1989; Pierre & Vezina, 1997).
In the present study we first evaluated the initial locomotor activity response to the test chamber (a novel environment) in 13 mouse strains, two outbred mouse stocks, and in Sprague Dawley rats. After habituation to the test chamber, we then obtained dose-effect functions for cocaine-induced locomotor activity in the same animals. A second test was performed to evaluate reproducibility of results within-subjects, and we also examined replicability between-subjects using a second cohort of the most commonly used parental strains for breeding mutant mice. We assessed qualitative (percent-wise distribution of behavior between repeated, consecutive, or elevated beam breaks) as well as quantitative differences (total beam breaks) in cocaine’s effects on motor activity between strains/stocks. A main purpose of the study was to appraise the utility of the locomotor assay for predicting differences in abuse-related effects of cocaine, viewing our findings in the light of published self-administration and CPP studies. In addition, based on the locomotor activity data, we selected the BALB/cJ and BALB/cByJ strains for evaluation in chronic intravenous cocaine self-administration, the hypothesis being that cocaine would act as a positive reinforcer in the BALB/cByJ, but not the BALB/cJ mice. Finally, we tested the hypothesis that rates of habituation to activity elicited by a novel environment and/or habituated baseline activity levels would consistently predict differences in cocaine’s effects in the locomotor activity assay.
METHODS
Animals
We characterized the following inbred strains, chosen by virtue of their ranking in the Mouse Phenome Database as priority strains, or because they are commonly used to generate knockout mice: 129S1/SvImJ, 129X1/SvJ, A/J, BALB/cByJ, BALB/cJ, C3H/HeJ, C57BL/6J, CAST/EiJ, DBA/2J, FVB/NJ, SJL/J, SPRET/EiJ (all Jackson Labs, Bar Harbor, ME), and 129S6/SvEvTac mice (Taconic, Germantown, NY). For further comparisons, we also selected outbred Swiss Webster (Taconic, Germantown, NY) and CD-1/ICR (Charles River, Wilmington, MA) mice, and outbred Sprague Dawley rats (Charles River). 8 female and 8 male animals were tested in the locomotor activity assay for each strain/stock, and 20 additional mice each of the BALB/cByJ and BALB/cJ strains (8 female, 12 male) were tested in operant procedures. All animals were group-housed 4 per cage (8.8 × 12.1 × 6.4 inches for mice, 11 × 22 × 8.5 inches for rats) in a climate-controlled animal facility. Each cage was fitted with a filter top through which HEPA-filtered air was introduced (40 changes per hour). Illumination was provided for 12 hr/day (starting at 7:00AM). Food (rodent diet 5001, PMI Feeds, Inc. St. Louis, MO) and tap water were available ad libitum outside test sessions, and various flavored treats (Bioserve, Frenchtown, NJ) were given weekly for enrichment. All behavioral testing started at approximately 8-10 weeks of age and occurred between 8:00 AM and 6:00 PM.
Vivarium conditions were maintained in accordance with the guidelines provided by the National Institutes of Health Committee on Laboratory Animal Resources. All experimental protocols were approved by the Institutional Animal Care and Use Committee. The health of the rodents was evaluated by research technicians on a daily basis and was also periodically monitored by consulting veterinarians.
Locomotor Activity
Clear plastic chambers 41.5 × 19 × 28 cm (l × w × h) loosely lined with pine shavings (to absorb urine), fitted with filter tops and placed within Photobeam Activity System monitoring frames (San Diego Instruments, San Diego, SA) were used to assess locomotor activity. Infrared beams were transmitted through the cage 2.7 cm above the floor for assessment of horizontal activity (repeated and consecutive beam breaks). Additional beams measured vertical activity (sometimes termed “rearing”), at 6.4 cm above the floor for mice, 13.9 cm above the floor for rats (“elevated beams”). The test sessions were 4 hours long and data were collected in 10-min bins. Animals were introduced to the test chambers for three daily habituation sessions (no injections), and data from the first session were used as a measure of locomotor activity in a novel environment. Subsequently, on each test day, the animals were allowed to acclimate to the test chambers for 1 hr; then removed for injection with vehicle or cocaine, and immediately returned to the test chamber for the remaining 3 hrs of the session. Test days were always separated by at least 2 days. For brevity and ease of presentation, total beam breaks (horizontal and vertical) were collapsed across time for the drug tests, and total activity counts for the 3 hours post-injection are presented.
In all groups, a second determination of the cocaine dose-effect function was made as described above, with the exception that only vehicle and 3, 10 and 32 mg/kg were included. In selected strains, C57BL/6J, 129X1/SvJ, and DBA/2J, we also evaluated a separate cohort of mice (between-subjects).
Cocaine self-administration
Operant conditioning chambers, training and evaluation of food-maintained behavior under a fixed-ratio (FR) schedule have been described in detail (Caine, Negus & Mello, 1999; Thomsen et al., 2005). Briefly, each chamber contained two nose-poke holes and a plate into which liquid food could be delivered. In all except the reversal procedure, responding in the right hole resulted in delivery of a reinforcer and illumination of the cue light for 20 sec during which no reinforcer could be earned. Responses in the left hole were counted but had no scheduled consequences. The house light was on during the session. Mice were allowed to acquire nose-poking behavior reinforced by vanilla-flavored Ensure® protein drink (liquid food) under a FR 1 schedule of reinforcement until at least 20 reinforcers per 2-hr session were earned for at least two sessions, with at least 70% responding in the reinforced hole. Mice were given access to liquid food for at least five sessions, and until acquisition criteria were met (all mice met criteria in 9 sessions or less). Water was then substituted until responding decreased to ≤60% of the acquisition level, after which indwelling jugular vein catheters were implanted.
Implantation and maintenance of the catheters, cocaine self-administration behavior under a FR 1 schedule of reinforcement including the reversal procedure have been described (Thomsen et al. 2005). All mice were allowed access to saline, 0.32, 1.0 or 1.8 mg/kg/infusion cocaine in daily 3-hr session, 5 consecutive sessions for each dose. Each dose was separated by one day of access to liquid food to reinstate nose poking to >50 responses per 2-hour session. Cocaine 1.0 mg/kg/infusion was presented first, then saline and cocaine doses were presented according to a Latin square design. Catheter patency was verified at the end of each 5-session period by infusion of a ketamine/midazolam and only data from mice with patent catheters (i.e., immobility in <5s.) were included for analysis and presentation.
Drug
Cocaine hydrochloride was supplied by NIDA/NIH and was dissolved in 0.9% saline and injected intraperitoneally (i.p.; 10 ml/kg for mice, 1 ml/kg for rats), or made available for intravenous self-administration (0.56 ml/kg/infusion). For locomotor activity, vehicle and 3, 10, 18 and 32 mg/kg cocaine were administered following a Latin-square design, while 56 mg/kg was always tested last (56 mg/kg was not tested in the rats). For self-administration, saline and 0.32, 1.0, 1.8 mg/kg/infusion cocaine were each presented for five consecutive days, following a Latin square design. Then a dose-effect function was determined by presenting saline, 0.1, 0.32 and 1.0 mg/kg/infusion in one daily session, following a Latin square design. The salt form was used in dose calculations.
Statistical Analyses
We had an a priori hypothesis that the groups of rodents would differ in baseline locomotor activity levels, and an ANOVA was performed with strain/stock as between-subjects factor, and confirmed this hypothesis. Consequently the effect of cocaine treatment was assessed in each strain/stock separately by repeated measures ANOVAs, with sex as between-subjects factor and dose as within-subjects factor. In the few cases where mice did not tolerate the high dose of drug, all data were excluded for these animals (i.e., a subject was removed from the study when seizures or clear pre-seizure symptoms were observed: the subject was then removed from the test chamber, “rescued” with a 5–10 mg/kg diazepam injection i.p., and returned to its home cage for observation and recovery). When there was a significant interaction between sex and drug treatment, data from female and male rodents were also analyzed separately. Distribution of activity by beam break type (i.e. consecutive, repeated, and vertical) was analyzed similarly, with break type and dose as within-subjects factors. A significant dose by break type interaction was followed by repeated measures ANOVA in each beam break type, with cocaine dose as factor. For comparison of the two determinations, dose and determination were within-subjects factors, strain/stock and sex between-subjects factors, followed by analysis within each strain. Significant main effects were followed by paired-samples t-test comparison of cocaine doses to saline. Significance level was set at p<0.05.
The mean doses estimated to elicit 50% of the maximal effect (A50 values) were calculated for each mouse by interpolation of the linear portion of the log dose-effect function. In a few mice, the lowest dose elicited more than 50% of the maximum effect; for effects above 55%, the A50 was estimated by extrapolation, in all other cases the animal was excluded from the A50 calculation. Strains/stocks in which 25% or more of the animals had to be excluded, no estimate was made. For each strain/stock, the group mean as well as 95% and 99% confidence limits (95%CL, 99%CL) were calculated. Non-overlapping confidence intervals indicated a significant difference in cocaine’s potency between strains.
For the habituation data, activity for the entire session was fitted by non-linear regression using the top to bottom decay equation [Y = (Top – Bottom)*e−K*X + Bottom] (Motulsky & Christopoulos, 2004), an exponentially decreasing function where Y is the activity in total beam breaks per 10-min bin, X is session time in minutes (per 10-min bin). Regression yielded estimates, with SEM, for Top (peak activity, at session start), Bottom (plateau level activity after habituation) and K (a constant determining the slope of the curve). Curve fitting was performed using GraphPad Prism 4 software for Mac, which was also used to determine the best fitting function (relative to one- or two-phase exponential decay and 4th order polynomial). In addition the linear portion of the curve was fitted by linear regression [Y = X*slope], yielding estimates and SEM for the slope of decrease in activity (not shown). The fitted values were then tested for correlation with peak effect (total beam breaks, % saline and difference score) and potencies for cocaine-induced hyperlocomotion using the Prism software.
For self-administration data, ANOVAs were performed on the number of reinforcers earned in the initial cocaine presentation with strain/stock, sex and cocaine dose as between-subjects factors (although cocaine doses were tested in the same mice in a randomized sequence, data were not obtained for all doses in each mouse due to attrition, thus precluding the use of repeated measures analysis). For the cocaine dose-effect function, numbers of reinforcers earned were analyzed by ANOVA with strain//stock and sex as between-subjects factor and cocaine dose as within-subjects factor. Significant effects of dose were followed by two-sided paired-sample t-test relative to saline. In the acquisition of food-maintained behavior, numbers of reinforcers earned were analyzed by ANOVA with strain and sex as between-subjects factor and session as within-subjects factor.
RESULTS
I. Locomotor activity
I.A Quantitative effects of cocaine: efficacy and potency of locomotor stimulation
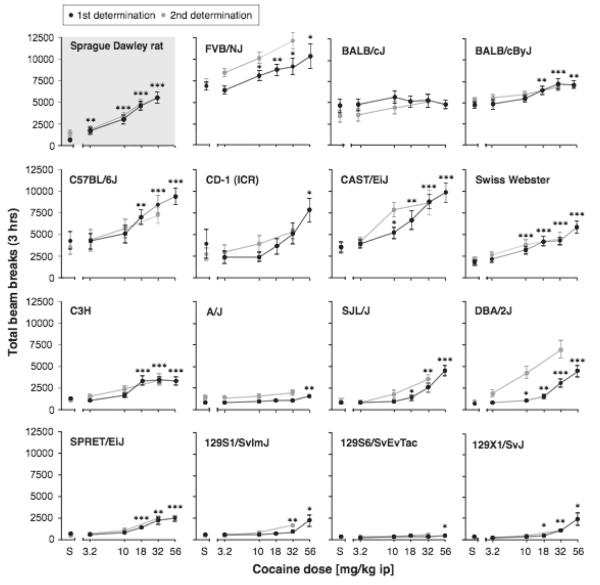
A comparison of total beam breaks after saline injection (“baseline”) showed a significant effect of strain or stock [F(1,14)=11.4, p<0.001], i.e., the baseline activity level was not homogeneous across strains/stocks. Because of these baseline differences, separate ANOVAs were conducted for each inbred strain and outbred stock for cocaine. Figure 1 shows locomotor activity as total beam breaks over 3 hours following the i.p. cocaine injection, for each strain/stock. Sprague-Dawley rats are shown in the upper left corner, and mouse strains/stocks are then ranked by decreasing baseline activity levels. Both determinations of the dose-effect function are shown, but all statistical analyses were performed on the first determination because fewer doses were included in the second determination. Cocaine increased locomotor activity above saline levels in all groups with the exception of the BALB/cJ. Table I shows the significance of the dose effect for each strain/stock (first column), and significant doses relative to saline can be seen from Figure 1.
Figure 1.
Locomotor activity stimulation by cocaine in Sprague-Dawley rats, 13 strains of mice, and two outbred mouse stocks. Abscissa: cocaine dose in mg/kg, i.p.; ordinate: total beam breaks per 3-hour session. Data are group means, bars represent one SEM. “V” indicated vehicle. Group size N=16 (8 males, 8 females) for all but the CAST/EiJ strain (N=12; 4 males, 8 females) and the SPRET/EiJ strain (N=14; 7 males, 7 females). Rats are shown in top left. Thereafter, mouse strains/stocks are ranked from top-left to bottom-right by decreasing activity levels after saline injection. Black symbols represent the first determination, grey symbols the second determination. For clarity and ease of presentation, statistical significance is indicated for male and female rodents combined, and for the first determination only. *p<0.05, **p<0.01, ***p<0.001 paired sample t-test vs. vehicle.
Table I.
Summary of the statistics of cocaine-induced hyperactivity per strain
| Strain | Main Dose | Sex | A50 |
|
|---|---|---|---|---|
| Max. breaks | Diff. score | |||
| RAT (SD) | p < 0.001 | F | 9.9 [7.3 - 13.4] | 10.4 [7.6 - 14.1] |
|
| ||||
| 129S6/SvEvTac | p = 0.001 | M (no int) | 8.0 [5.9 - 11.0] | 11.6 [ 7.9 - 17.1] |
| A/J | p < 0.001 | ns | 9.4 [7.1 - 12.5] | 15.7 [11.2 - 22.0] |
| Swiss Webster | p < 0.001 | ns | 9.7 [6.9 - 13.5] | 13.6 [10.5 - 17.5] |
| C57BL/6J | p < 0.001 | ns | 9.9 [6.9 - 14.2] | 16.6 [12.6 - 22.0] |
| C3H | p < 0.001 | M (no int) | 10.3 [8.2 - 13.0] | 13.3 [10.3 - 17.0] |
| CAST/EiJ | p < 0.001 | ns | 10.9 [7.2 - 16.5] | 16.7 [11.8 - 23.6] |
| CD-1 (ICR) | p < 0.001 | ns | 18.2 [12.8 - 25.9] | 22.8 [18.5 - 27.9] |
| SPRET/EiJ | p < 0.001 | ns | 18.5 [14.1 - 24.2] | 22.4 [18.2 - 27.5] |
| 129S1/SvImJ | p < 0.001 | ns | 19.3 [15.2 - 24.6] | 26.2 [20.5 - 33.4] |
| SJL | p < 0.001 | ns | 22.9 [18.6 - 28.2] | 26.9 [23.4 - 30.8] |
| DBA/2J | p < 0.001 | ns | 24.0 [20.0 - 29.0] | 25.3 [20.6 - 31.1] |
| 129X1/SvJ | p < 0.001 | M | 25.6 [21.4 - 30.7] | 29.3 [24.8 - 34.7] |
| FVB | p = 0.001 | F | nc | 13.9 [10.6 - 19.8] |
| BALB/cByJ | p < 0.001 | M | nc | 14.2 [11.6 - 17.4] |
| BALB/cJ | ns | F (no int) | nc | nc |
Dose: significance level of the main effect of dose (repeated measures ANOVA). Sex: significant dose by sex interactions, F = female>male effect; M = male>female effect; no int: “no interaction”, i.e., main effect of sex, but no dose by sex interaction. A50 values are group means and 95% confidence intervals, calculated from the individual values for each rodent, in mg/kg. “Max breaks”: estimated dose that produced 50% of the animal’s maximum beam breaks; “Diff score”: estimated dose that produced 50% of the animal’s maximum increase in beam breaks above baseline. ns = not significant; nc = not calculated.
There were significant effects of sex or dose by sex interactions in several strains as well as in the rats. Table II shows activity as total beam breaks for the females and the males of each strain/stock, by cocaine dose. In Sprague-Dawley rats, there was both a significant main effect of sex [F(1,14)=21.6, p<0.0001] and a significant drug by sex interaction, [F(4,56)=2.9, p<0.05]. Female rats had higher activity levels including at baseline, but the curve also appeared shifted to the left in the females relative to the males, and all cocaine doses reached significance in the females while the lowest dose did not increase activity in the males. In FVB and 129X1/SvJ mice, there was a significant dose by sex interaction [F(5,70)=3.7, p<0.01 and F(5,70)=2.8, p=0.01, respectively] but no main effect of sex. In the FVB strain the interaction reflected a higher effect in the female mice of the highest cocaine dose, as the male mice showed a shallow inverted-U shaped curve while the females showed a monotonic increase of activity with increasing cocaine dose. In 129X1/SvJ mice however, the interaction reflected a higher effect in the male mice of the highest cocaine dose only, with comparable dose-effect function between sexes at lower doses. There was a significant main effect of sex in the BALB/cJ [F(1,14)=6.1, p<0.05], C3H [F(1,14)=7.3, p<0.05], and 129S6/SvEvTac [F(1,14)=8.1, p<0.05) mice but no cocaine by sex interaction. In the BALB/cJ mice, the sex effect reflected higher overall activity levels in the female mice relative to the males, including at baseline. In contrast, in the C3H and 129S6/SvEvTac mice, the sex effect reflected higher overall activity levels in the male mice relative to females, including at baseline. Thus there was no consistent trend among the rodents in either sex differences in activity levels, or in cocaine’s effects between sexes. These sex effects are also summarized in Table I.
Table II.
Cocaine-induced hyperactivity in male and female rats and mice
| Sex | Saline | 3.2 mg/kg | 10 mg/kg | 18 mg/kg | 32 mg/kg | 56 mg/kg | |
|---|---|---|---|---|---|---|---|
| RAT (SD) | M | 436.8 ± 87.1 | 659.4 ± 103.6 | 1619.9 ± 346.7 | 2892.3 ± 480.0 | 4852.9 ± 885.1 | not determined |
| F | 1027.3 ± 151.8 | 2765.5 ± 480.7 | 4559.9 ± 737.1 | 6339.3 ± 455.7 | 6229.5 ± 821.6 | not determined | |
|
| |||||||
| FVB | M | 6710.1 ± 610.7 | 6069.6 ± 512.2 | 8441.9 ± 861.4 | 8951.1 ± 1091.9 | 9069.8 ± 1390.3 | 7221.5 ± 1132.0 |
| F | 7180.8 ± 742.6 | 6791.0 ± 834.9 | 7818.1 ± 757.6 | 8634.6 ± 795.6 | 9314.5 ± 1249.7 | 13547.5 ± 1999.8 | |
| BALB/cJ | M | 2868.5 ± 1231.1 | 3454.0 ± 1075.7 | 4277.8 ± 1128.7 | 3744.4 ± 1016.3 | 3707.4 ± 1205.4 | 4359.0 ± 948.5 |
| F | 6422.6 ± 652.2 | 6138.0 ± 537.6 | 7112.9 ± 684.2 | 6255.5 ± 584.0 | 6838.9 ± 814.3 | 5206.5 ± 384.8 | |
| BALB/cByJ | M | 5173.9 ± 344.7 | 6205.3 ± 342.5 | 6514.4 ± 298.7 | 7591.3 ± 412.9 | 8490.8 ± 589.5 | 6755.6 ± 361.3 |
| F | 4256.8 ± 695.7 | 3415.4 ± 664.4 | 4445.5 ± 557.5 | 5314.8 ± 718.0 | 5915.5 ± 852.3 | 7485.8 ± 812.9 | |
| C57BL/6J | M | 2152.8 ± 463.2 | 2374.0 ± 613.7 | 3675.5 ± 1230.3 | 5764.9 ± 1457.9 | 7761.8 ± 1207.8 | 9454.1 ± 1378.6 |
| F | 6571.4 ± 1572.7 | 6151.4 ± 1340.0 | 6563.5 ± 1472.7 | 8301.3 ± 733.2 | 9296.1 ± 1569.1 | 9351.9 ± 1323.2 | |
| CD-1 (ICR) | M | 2792.8 ± 1348.3 | 2838.0 ± 1316.7 | 2265.0 ± 798.2 | 3792.8 ± 1525.1 | 5710.3 ± 2207.7 | 8940.4 ± 2076.1 |
| F | 5084.1 ± 3072.0 | 1869.8 ± 587.0 | 2560.6 ± 775.5 | 3539.4 ± 945.6 | 4511.0 ± 1123.2 | 6744.1 ± 1888.9 | |
| CAST/EiJ | M | 3116.8 ± 1070.9 | 3694.8 ± 1183.4 | 5139.0 ± 1038.1 | 9213.3 ± 2531.9 | 9976.0 ± 1464.0 | 9912.8 ± 1450.1 |
| F | 3797.0 ± 746.7 | 4082.9 ± 463.6 | 5245.1 ± 802.3 | 5460.1 ± 870.2 | 8231.6 ± 1000.3 | 9829.0 ± 1553.1 | |
| Swiss Webster | M | 1441.4 ± 328.0 | 1967.5 ± 360.1 | 2736.9 ± 619.4 | 3612.5 ± 539.5 | 3538.6 ± 621.1 | 4948.4 ± 1148.9 |
| F | 2123.4 ± 394.6 | 2285.5 ± 353.5 | 3676.5 ± 493.1 | 4851.0 ± 851.2 | 5082.9 ± 436.9 | 6787.4 ± 696.1 | |
| C3H | M | 1729.6 ± 279.1 | 1605.9 ± 317.5 | 2007.3 ± 466.1 | 3996.8 ± 684.2 | 3963.4 ± 635.3 | 4558.6 ± 563.5 |
| F | 869.6 ± 110.2 | 723.4 ± 101.7 | 1440.3 ± 240.8 | 2737.4 ± 735.7 | 2935.3 ± 314.6 | 2153.1 ± 524.2 | |
| A/J | M | 963.4 ± 202.8 | 999.0 ± 220.2 | 1199.0 ± 241.4 | 911.6 ± 151.7 | 1087.5 ± 158.0 | 1527.1 ± 272.6 |
| F | 806.4 ± 144.8 | 749.0 ± 137.8 | 811.8 ± 150.7 | 1294.4 ± 294.2 | 1123.9 ± 259.2 | 1639.5 ± 342.9 | |
| SJL | M | 502.4 ± 36.8 | 622.4 ± 108.8 | 734.9 ± 137.1 | 1520.3 ± 274.1 | 2865.9 ± 785.2 | 3953.6 ± 827.1 |
| F | 1115.6 ± 243.1 | 1058.0 ± 302.8 | 1267.9 ± 333.4 | 1345.0 ± 531.1 | 2392.5 ± 722.5 | 5162.4 ± 814.0 | |
| DBA/2J | M | 669.3 ± 190.0 | 1029.8 ± 261.3 | 1180.6 ± 171.6 | 1535.3 ± 273.1 | 3610.6 ± 697.1 | 5164.8 ± 1218.5 |
| F | 775.4 ± 143.3 | 834.0 ± 287.2 | 947.6 ± 111.2 | 1766.1 ± 429.5 | 2737.9 ± 676.1 | 3876.6 ± 446.3 | |
| SPRET/EiJ | M | 696.7 ± 147.1 | 531.0 ± 93.4 | 954.0 ± 225.1 | 1443.4 ± 335.7 | 2416.1 ± 690.6 | 2545.3 ± 483.4 |
| F | 667.9 ± 109.1 | 541.0 ± 166.1 | 690.6 ± 155.2 | 1215.6 ± 267.5 | 1911.4 ± 402.6 | 2266.9 ± 367.7 | |
| 129S1/SvImJ | M | 763.9 ± 89.0 | 804.3 ± 72.3 | 759.1 ± 132.0 | 872.9 ± 159.6 | 1164.8 ± 166.8 | 3155.4 ± 1276.3 |
| F | 417.5 ± 43.1 | 457.6 ± 62.3 | 603.4 ± 93.1 | 687.4 ± 45.2 | 728.6 ± 68.6 | 1457.5 ± 193.0 | |
| 129S6/SvEvTac | M | 418.4 ± 53.9 | 372.3 ± 83.7 | 508.8 ± 36.2 | 632.9 ± 92.8 | 531.9 ± 57.1 | 637.8 ± 80.8 |
| F | 308.1 ± 48.7 | 266.8 ± 55.6 | 360.3 ± 67.5 | 287.1 ± 55.9 | 351.8 ± 55.3 | 494.0 ± 76.4 | |
| 129X1/SvJ | M | 298.8 ± 75.2 | 205.3 ± 35.7 | 300.6 ± 35.5 | 507.9 ± 112.6 | 1153.9 ± 338.2 | 3677.6 ± 1426.1 |
| F | 284.4 ± 70.0 | 263.0 ± 44.1 | 308.4 ± 56.9 | 398.0 ± 73.8 | 937.4 ± 121.1 | 1215.5 ± 194.3 | |
Values are total beam breaks (3 hrs), group means ± one SEM, from the first cocaine dose-effect determination.
To assess cocaine’s potency in each strain/stock, we calculated A50 values, as the mean dose estimated to increase activity to 50% of the maximal effect in each strain/stock, both calculated as 50% of the total beam breaks, and as 50% increase in beam breaks over baseline level (difference score). For the FVB and BALB/cByJ mouse strains, an A50 value could not be accurately estimated as total beam breaks (see Methods). As can be seen from Table I, the two measures yielded very comparable ranking of the strains/stocks in terms of potency. Interestingly, the rodents appeared to fall in two groups, the CD-1, SPRET/EiJ, 129S1/SvImJ, SJL, DBA/2J and 129X1/SvJ mice showing A50 values approximately two-fold higher than the remaining mouse strains, the outbred Swiss-Webster, and the Sprague Dawley rats (18.2 to 25.6 versus 8.0 to 10.9 mg/kg i.p. as maximum beam breaks or 22.4 to 29.3 versus 10.4 to 16.7 mg/kg i.p. as beam breaks above baseline). The apparent segregation in two groups was supported by the statistical comparison of potencies, as non-overlapping 95% and 99% confidence intervals of the A50 values (see Table III). No strains/stocks differed significantly from each other within the low potency range or within the high potency range (including the rats), whereas most of the “low potency strains” reached significance relative to the “high potency strains”.
Table III.
Potency comparisons between strains.
| SD rat | 129S6 | FVB | BALB/cBy | A/J | SW | C57 | C3H | CAST | CD-1 | SPRET | 129S1 | SJL | DBA | 129X1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD RAT | X | - | n/a | n/a | - | - | - | - | - | - | * | * | ** | ** | ** |
| 129S6 | - | X | n/a | n/a | - | - | - | - | - | * | ** | ** | ** | ** | ** |
| FVB | - | - | X | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| BALB/cBy | - | - | - | X | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| A/J | - | - | - | - | X | - | - | - | - | * | * | ** | ** | ** | ** |
| SW | - | - | - | - | - | X | - | - | - | - | * | * | ** | ** | ** |
| C57 | - | - | - | - | - | - | X | - | - | - | - | * | ** | ** | ** |
| C3H | - | - | - | - | - | - | - | X | - | - | * | ** | ** | ** | ** |
| CAST | - | - | - | - | - | - | - | - | X | - | - | - | * | ** | ** |
| CD-1 | ** | * | * | - | - | * | - | * | - | X | - | - | - | - | - |
| SPRET | ** | * | * | - | - | * | - | * | - | - | X | - | - | - | - |
| 129S1 | ** | * | * | ** | ** | - | * | - | - | - | X | - | - | - | |
| SJL | ** | ** | ** | ** | * | ** | * | * | - | - | - | - | X | - | - |
| DBA | ** | * | * | ** | - | ** | - | * | - | - | - | - | - | X | - |
| 129X1 | ** | ** | ** | ** | * | ** | * | * | * | - | - | - | - | - | X |
Upper/right half: Comparisons of A50 values calculated as maximum beam breaks; lower/left half: comparisons of A50 values calculated as difference score. - overlapping 95% confidence intervals, * non-overlapping 95% confidence intervals, **, non-overlapping 99% confidence intervals. n/a: not applicable (A50 value could not be estimated)
I.B Re-determination of cocaine-induced hyperactivity within and between cohorts
We made two determinations of cocaine’s dose-effect function in the same cohort of each strain/stock. The second determination was made primarily to assess within-laboratory replicability and increase our confidence that we can reliably test and compare cocaine-modulated locomotor activity between strain/stock. Figure 1 shows that the two determinations paralleled each other very closely, although there was a significant effect of determination [F(1,200)=34.64, p<0.0001]. A second goal of the replication was to evaluate whether repeated cocaine administration would produce higher activity levels than the first determination, and whether this change would be strain-dependent. Indeed, there was a significant effect of strain/stock [F(14,200)=36.25, p<0.0001], as well as significant strain/stock by determination [F(14,200)=5.87, p<0.0001] and strain/stock by dose by determination interaction [F(42,600)=2.18, p<0.0001]. There was no significant effect of sex or any significant interactions involving sex and determination, suggesting that any altered sensitivity to cocaine was not affected by sex. Because of the determination by strain/stock interaction, the effect of determination was analyzed within each strain/stock.
There was a significant main effect of determination and a significant dose by determination interaction in the FVB/NJ strain [determination: F(1,14)=26.34, p<0.0001; interaction: F(3,42)=4.70, p<0.01], the DBA/2J strain [F(1,14)=25.59, p<0.0001; F(3,42)=6.28, p=0.001], the 129S6/SvEvTac strain [F(1,14)=5.85, p<0.05; F(3,42)=3.21, p<0.05], and the 129S1/SvImJ strain [F(1,14)=16.32, p<0.001; F(3,42)=17.73, p<0.0001]. In the FVB/NJ strain, all cocaine doses (but not saline) elicited significantly higher activity in the second determination than in the first (all p<0.01 post hoc), consistent with an upward or leftward shift in the dose-effect curve. The same was observed in the DBA/2J mice (post-hoc between determinations: 3 mg/kg, p<0.05, 10 and 32 mg/kg, p<0.01; saline no significant change). In the two 129 substrains, only the highest cocaine dose, 32 mg/kg, differed between determinations (129S1/SvImJ, p<0.001; 129S6/SvEvTac, p<0.01 post hoc), consistent with a leftward shift of the curve.
There was a significant dose by determination interaction with no significant main effect of determination in the CAST/EiJ strain [F(3,30)=3.16, p<0.05], in which only the 3 mg/kg dose reached significance (p<0.01 post-hoc between determinations). Finally, there was a main effect of determination with no dose by determination interaction in the A/J strain [F(1,14)=66.88, p<0.0001], in which all cocaine dose and saline elicited higher activity levels in the second determination relative to the first, consistent with an upward shift in the dose-effect curve (post-hoc: 3 mg/kg, p<0.001; other cocaine doses and saline, p<0.01). In all other mouse strains, the outbred mouse stocks, and in the rats, the cocaine dose-effect functions of locomotor activity were comparable between determinations.
In a further replication experiment, we tested separate cohorts of C57BL/6J, 129X1/SvJ and DBA/2J mice (N=16, data not shown). For all three strains, there was no significant dose by cohort interaction, and the same ranking of the three strains is apparent from each determination.
I.C Qualitative effects: distribution of beam breaks between consecutive, repeated, and elevated
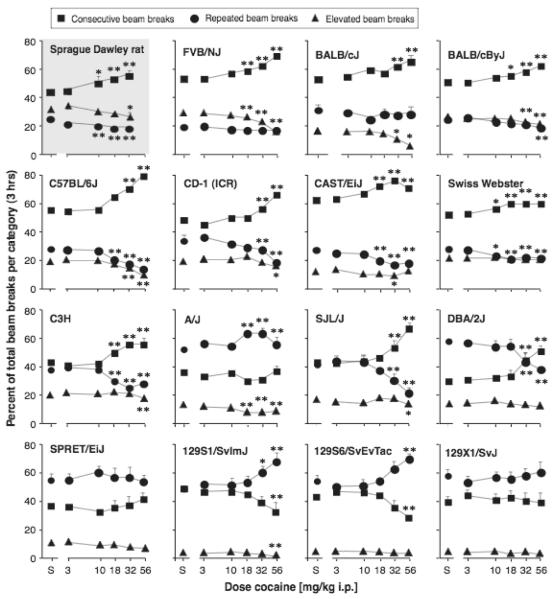
Activity distribution between consecutive, repeated and elevated beam breaks in the first cocaine dose-effect determination is shown in Figure 2, as percentage of total beam breaks, summed over 3 hours following the i.p. cocaine injection, for each strain/stock. The strain/stock are shown in the same order as in Figure 1. Comparison of beam break distribution after saline injection (“baseline”) showed a significant strain/stock by break type interaction [F(30,468)=20.91, p<0.0001]. Thus, the modulation of beam break distribution by cocaine was analyzed separately for each strain/stock. At baseline, breaks of the elevated beams typically accounted for less than one third of the total activity, consecutive breaks of the low beams accounted for most of the beam breaks in the rats and in about half of the mouse strains/stocks, while repeated breaks of a same beam (low beams) accounted for most of the beam breaks in the remaining mouse strains/stocks (see Figure 2). Interestingly, the strains/stocks with high baseline activity showed high percents of consecutive breaks (consistent with ambulation), while the lower baseline strains had higher percentages of repeated breaks (consistent with fine movements).
Figure 2.
Distribution of behavior defined by interruption of consecutive beams, repeated breaks of the same beam and breaks of elevated beams, as percent of total beam breaks, as a function of cocaine dose. Please note that data are percentages and therefore add to 100% at each dose, and that relative increases or decreases in percentage may not reflect similar increases or decreases in the actual beam break counts (see Results text for details). Abscissa: cocaine dose in mg/kg, i.p.; ordinate: % of total beam breaks over 3-hour session. “V” indicated vehicle. Data are group means from the fist cocaine dose-effect determination, bars represent one SEM. Group sizes as in Figure 1. *p<0.05, **p<0.01 paired sample t-test vs. vehicle within each break type.
Cocaine affected the distribution of beam breaks in most strains/stocks, reflected by a significant cocaine dose by break type interaction in all but the SPRET and 129X1/SvJ mice (all other strains and outbred stocks: p<0.001). Note that cocaine had a significant effect on break type distribution in the BALB/cJ mice, even though this strain did not shown any increase in total beam breaks. Analysis of cocaine’s effect within each break type showed this to result from a significant reduction in elevated beam breaks as a function of cocaine dose [F(5,75)=9.99, p<0.0001], while there was no significant effect of cocaine on consecutive or repeated breaks of the low beams (data not shown).
In strains/stocks that showed a cocaine by break type interaction, the proportion of consecutive breaks increased with dose in most cases: in the Sprague Dawley rats and in the FVB/NJ, BALB/cJ, BALB/cByJ, C57BL/6J, CD-1, CAST/EiJ, Swiss Webster, C3H, SJL/J and DBA/2J mice (see Figure 2 for doses significant from saline). The percent of consecutive breaks decreased with dose in two 129 mouse substrains: the 129S1/SvImJ and the 129S6/SvEvTac, and was not affected by cocaine in the A/J mice. The proportion of repeated and elevated beam breaks both decreased with cocaine dose in most strains/stocks where consecutive breaks increased, except in the BALB/cJ, in which only the percentage of elevated beam breaks decreased, and the DBA/2J in which only the percentage of repeated beam breaks decreased. Finally, in the three strains in which consecutive breaks decreased or was unaffected, the A/J, 129S1/SvImJ and 129S6/SvEvTac, the proportion of repeated breaks increased significantly with dose (Figure 2).
In summary, cocaine-induced increases in locomotor activity was accompanied by a proportionately greater increase in consecutive beam breaks relative to repeated or elevated beam breaks in most, but not all, strains/stocks. The mice with lower baseline activity levels in particular fell outside this pattern.
I.D Predictive value of baseline activity level and locomotor activity in a novel environment for stimulant effects of cocaine
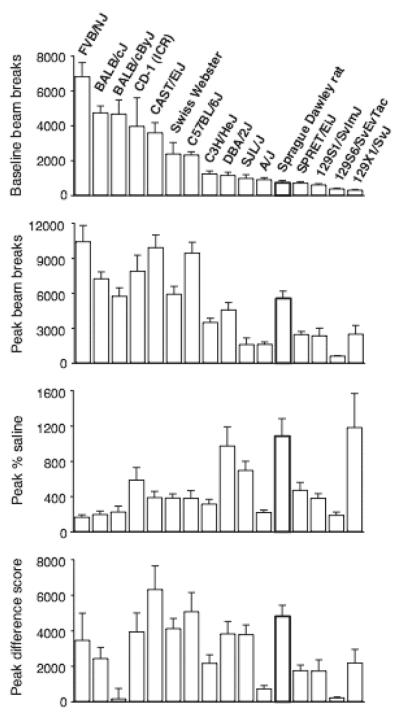
Figure 3 shows peak effects of cocaine as total beam breaks, as percent of baseline activity, and as a difference score (i.e., increase in activity above baseline, in beam breaks) as well as baseline activity. Baseline activity levels did not unequivocally predict the magnitude or potency of cocaine’s effect. For example, the Sprague Dawley rats ranked relatively low in baseline activity, but showed one of the greatest increases in activity as % increase or as a difference score (despite the fact that doses up to 56 mg/kg were tested in the mice, but only up to 32 mg/kg was tested in the rats). Conversely, the 129S6/SvEvTac strain, which had one of the lowest baseline levels, also had one of the lowest peak effects, whether as raw beam breaks, % increase or difference score. Statistical analysis confirmed that baseline activity correlated only with maximal activity levels as total beam breaks (r2=0.65, p<0.001), but not with cocaine-induced increase in activity above baseline either as percentage (r2=0.22, not significant) or as difference score (r2=0.04, not significant).
Figure 3.
Activity after administration of vehicle, and peak effects of cocaine as total beam breaks, as percent of vehicle activity, and as difference score from vehicle. Abscissa: rodent strain/stock arranged by decreasing baseline activity – the order is the same in each panel; ordinate: total beam breaks per 3-hour session (1st and 2nd panel), % of the strain’s or stock’s vehicle level (3rd panel), and difference score as the peak total beam breaks per 3-hr session minus the vehicle level (4th panel). Data are group means (vehicle or maximum mean activity count), bars represent one SEM. Group sizes as in Figure 1.
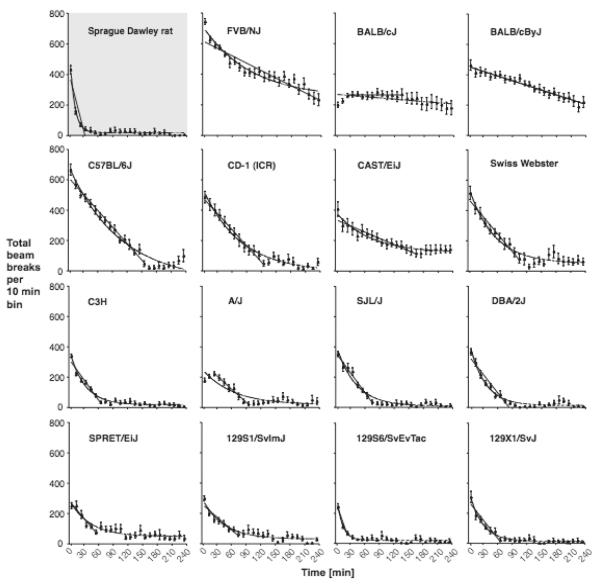
Locomotor activity during the first session the mice were exposed to the test chambers was used as a measure of locomotor response to a novel environment, and habituation thereto. Figure 4 shows the time courses of locomotor activity over the first 4-hour habituation session for each strain/stock, presented in the same order as in the previous figures. ANOVA confirmed a significant effect of strain/stock [F(15,5428)=82.1, p<0.0001] and a significant strain by time interaction [F(345,5428)=9.0, p<0.0001], and time course data were therefore fitted separately for each strain/stock. In addition to beam breaks per 10-bins, Figure 4 shows curve fit by non-linear regression (top to bottom decay). All strains/stocks except the BALB/cJ showed a decrease in activity as a function of time, indicating habituation to novelty-induced hyperactivity. Neither the slope of the linear portion nor the rate of decrease of the non-linear regression correlated with effects of cocaine, whether measured as peak effect or as potency. However, the peak activity in the novel environment correlated positively with the peak activity induced by cocaine (Top factor of non-linear fit: r2=0.54, P<0.01, Y intercept of linear fit: r2=0.66, p<0.001). Thus high baseline activity and high activity levels in a novel environment appeared to correlate with high peak cocaine-induced hyperactivity, but no factor of baseline or novelty-induced activity correlated with the potency or efficacy of cocaine in increasing locomotor activity relative to baseline.
Figure 4.
Locomotor activity in a novel test environment, without vehicle or drug administration, as a function of time. Abscissa: time in minutes; ordinate: total beam breaks per 10-minute bin. Data are group means, bars represent one SEM. Group sizes as in Figure 1. Solid lines show best fit of the total session (non-linear regression) and of the linear portion of the data (linear regression).
II. Cocaine self-administration
Because only one of 15 mouse strains/stocks, the BALB/cJ, showed no locomotor activating effect of cocaine, we tested this strain along with the closely related BALB/cByJ strain, in intravenous cocaine self-administration. The mice were first allowed to acquire nose-poking behavior reinforced by liquid food to assess the acquisition of cocaine self-administration under permissive conditions.
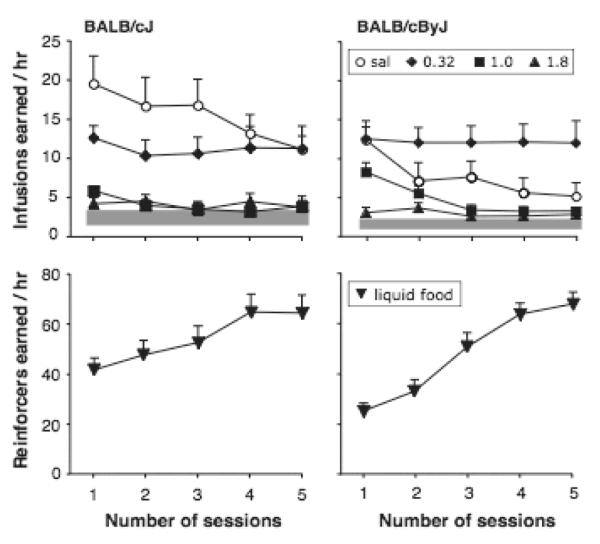
Figure 5 (top panels) shows self-administration of saline or three doses of cocaine over 5 consecutive sessions following food-maintained behavior, in BALB/cJ and BALB/cByJ mice. ANOVA on cocaine self-administration behavior showed a significant effect of strain [F(1,48)=4.4, p<0.05], cocaine dose [F(3,48)=16.6, p<0.0001], and session [F(4,192)=8.5, p<0.0001], and a significant dose by session interaction [F(12,192)=2.3, p<0.01]. There was no significant effect of sex and no other interactions. In the BALB/cJ strain, responding persisted with only slow extinction, but no dose of cocaine maintained responding above saline levels. Rather, cocaine dose-dependently decreased responding, consistent with direct rate-decreasing effects or aversive effects. ANOVA confirmed an effect of session [F(4,124)=4.1, p<0.0001] and cocaine dose [F(3,31)=4.1, p<0.0001], but no interaction. In the BALB/cByJ strain, response rates appeared lower than in the BALB/cJ mice when saline was present. In contrast to the BALB/cJ strain, in the BALB/cByJ mice there was a significant session by cocaine dose interaction [F(12,100)=64.6, p<0.05] in addition to the main effects of session [F(4,100)=6.4, p<0.001] and dose [F(3,25)=9.5, p<0.001]. A moderate dose of cocaine, 0.32 mg/kg/infusion, appeared to maintain nose-poking above saline levels in the BALB/cByJ mice, although this did not reach significance post-hoc.
Figure 5.
Intravenous self-administration of vehicle (“Veh”) or three doses of cocaine (top panels) and operant behavior maintained by liquid food (bottom panels) in BALB/cJ and BALB/cByJ mice. Shaded area represents mean inactive pose pokes ± one SEM. Abscissa: session number; ordinate: drug or food reinforcers earned / hr. Data are group means, bars represent one SEM. Saline: N=6-9, cocaine 0.32 mg/kg/infusion N=6-7, cocaine 1.0 mg/kg/infusion N=13-15, cocaine 1.8 mg/kg/infusion N=4-5. Food: N=20.
Figure 5 lower panels show acquisition of food-maintained behavior in the same mice. Both strains readily acquired food-maintained nose-poking, median number of sessions to acquisition criteria was 3.5 in both strains, number of reinforcers and % active responses were also comparable between strains (peak mean reinforcers 64.6 vs. 67.7, peak mean % active 79.4 vs. 81.3). ANOVA on reinforcers earned over the 5 sessions showed no main effect of strain or sex, but an effect of session F(4,152)=55.2, p<0.001] a strain by session interaction F(4,152)=5.2, p=0.001]. The interaction probably reflected a significantly higher number of reinforcers earned by the BALB/cJ mice in the first session (p<0.01).
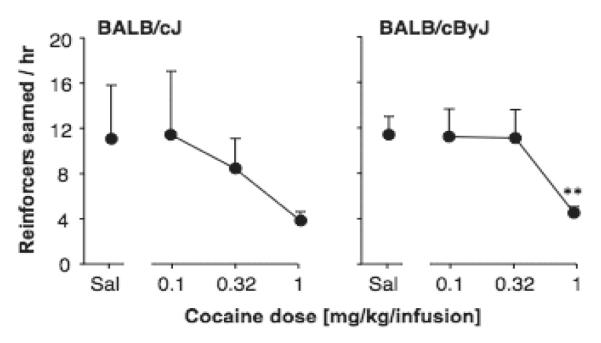
After exposure to 5 consecutive days of various cocaine doses or saline, a dose-effect determination was made by presenting saline, 0.1, 0.32 and 1.0 mg/kg/infusion cocaine once in daily sessions. As shown in Figure 6, under those conditions, no dose of cocaine maintained higher responding than saline in either strain. ANOVA showed a significant effect of cocaine dose F(3,30)= 5.0, p<0.01], but no effect of strain or sex and no significant interactions. The 1.0 mg/kg/infusion dose decreased responding significantly relative to saline only in the BALB/cByJ mice (p=0.001), but a similar trend was observed in the BALB/cJ mice.
Figure 6.
Dose-effect curves of intravenous cocaine self-administration in BALB/cJ and BALB/cByJ mice. Abscissa: cocaine dose [mg/kg/infusion]; ordinate: reinforcers earned / hr. Data are group means, bars represent one SEM. N=6-8. “Veh” indicates vehicle. **p<0.01 paired sample t-test vs. vehicle.
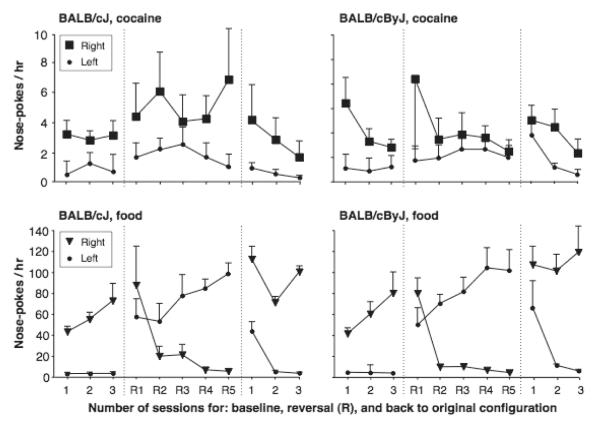
Finally, a reversal procedure was used to further determine whether cocaine served as a positive reinforcer in either of the BALB strains. Figure 7 shows nose-pokes in the right hole (reinforced under standard conditions) and left hole (inactive under standard conditions) during baseline, five days of reversal (left hole reinforced, right hole inactive), and reversal back to standard configuration. When reinforced with cocaine (top panels), both strain generally failed to reallocate their behavior to the newly reinforced hole; only one BALB/cByJ mouse showed a reversal, and none of the BALB/cJ mice. In contrast when the procedure was repeated with food as the reinforcer, all mice successfully reallocated their behavior to maintain their intake of liquid food (Figure 7 lower panels).
Figure 7.
Nose-poking behavior maintained by cocaine (1.0 mg/kg/infusion) or food in a reversal procedure, in BALB/cJ and BALB/cByJ mice. Abscissa: session number for baseline (three sessions), reversal of the reinforced and inactive nose-poke holes (five sessions, indicated by R), then reversal back to the original configuration of the holes (three sessions); ordinate: nose-pokes / hr. Data are group means, bars represent one SEM. N=5.
DISCUSSION
The present data extend a fairly large body of literature showing differences in the psychomotor stimulant effects of cocaine between mouse strains. Unlike most previous studies, which compared smaller numbers of strains, this investigation provided data on 13 inbred mouse strains and the outbred Swiss-Webster and CD-1 stocks in a direct comparison, using uniform methods, in both male and female rodents. Novelty-induced activity and habituation were also evaluated. All but BALB/cJ mice showed locomotor habituation. Intriguingly, we also found all but BALB/cJ mice showed significant cocaine-induced hyperactivity. This atypical phenotype of the BALB/cJ mice prompted us to test whether the BALB/cJ strain, and the closely related BALB/cJ strain, also lacked reinforcing effects of cocaine. The self-administration paralleled the locomotor activity data: the BALB/cJ mice also failed to self-administer cocaine, while the BALB/cByJ mice showed modest locomotor habituation, cocaine-induced locomotion, and cocaine self-administration. Thus there may be some correlation between all-or-none locomotion in a novel environment, and stimulant and reinforcing effects of cocaine. However, neither novelty- nor cocaine-induced activity offered a clear prediction of relative reinforcing effects among strains, as assessed from these and previous data.
Spontaneous locomotor activity, and efficacy and potency of cocaine in locomotor stimulation
While a comprehensive review of previous accounts of spontaneous and cocaine-stimulated locomotor activity across strains of mice is beyond the scope of this article, a non-exhaustive list of references can be found in Table IV, for accounts that included strains tested in the present article (plus outbred CD-1). For drug-free activity levels, the ranking we observed generally matched well with previous studies, although few studies tested more than 3 or 4 of these strains/stocks. For example, C57BL/6J mice, substrains of BALB, and outbred CD-1 mice consistently had among the highest activity levels, while A/J mice and substrains of 129 consistently had the lowest activity levels (Anisman & Cygan, 1975; Baron & Meltzer, 2001; Bolivar, Caldarone, Reilly & Flaherty, 2000; Bothe, Bolivar, Vedder & Geistfeld, 2005; Downing, Rodd-Henricks, Marley & Dudek, 2003; Fowler, Zarcone, Vorontsova & Chen, 2002; McKerchar, Zarcone & Fowler, 2005; see also Table IV). The DBA/2J showed very variable ranking relative to other commonly tested strains across studies (see Table IV for references). Although few studies have used extended sessions of spontaneous behavior (e.g., longer than 30 min), our findings on habituation also agree with previous studies in that most strains/stocks showed within-session habituation when tested in a novel environment, with the notable exception of the BALB/cJ strain (Cabib, Algeri , Perego & Puglisi-Allegra, 1990; Fowler, Zarcone, Vorontsova & Chen, 2002; Helmeste & Seeman, 1982; Miner, 1997; Tang, Orchard & Sanford, 2002, Wahlsten, Rustay, Metten & Crabbe, 2003).
Table IV.
Locomotor activity studies in various strains
Note: Only studies in which mice were tested in a novel environment and without injections of vehicle are reported in the first columns.
Cocaine produced dose-dependent locomotor stimulation in all strains/stocks but the BALB/cJ. For cocaine-induced hyperactivity also, our results are generally in agreement with previous studies in terms of peak effects. For example our peak difference scores rankings of C57BL/6J>DBA/2J>C3H/HeJ>A/J is consistent with previous investigations (Downing, Rodd-Henricks, Marley & Dudek, 2003; Gill & Boyle, 2008; Mead, Katz & Rocah, 2002). It is intriguing that the same strain that failed to show habituation in the drug-free “novelty” test, the BALB/cJ, also was the only strain that failed to show a locomotor stimulant response to cocaine in the present study. Locomotor hyperactivity has been shown in BALB/c mice after d-amphetamine administration, but not methamphetamine (Davis, Babbini, Pong, King & White, 1974; Helmeste & Seeman, 1982; Ito, Mori, Namiki, Suzuki & Sawaguchi, 2007; McKerchar, Zarcone & Fowler, 2006). BALB/cJ mice were also the only strain out of six tested in which amphetamine induced jumping, and were found hypersensitive to amphetamine lethality under some conditions (Davis, Babbini, Pong, King & White, 1974; McKerchar, Zarcone & Fowler, 2006; see also Halladay et al., 2003). While those data suggest differential sensitivity of BALB/cJ mice between psychostimulant drugs, they also suggest an unusual response profile in general. We observed significant increases in activity in the closely related BALB/cByJ strain. Some previous studies have reported significant locomotor stimulant effects of cocaine in BALB/cByJ mice, while others have not (Deroche et al., 1997; Elmer, Gorelick, Goldberg & Rothman 1996; Koff, Shuster & Miller, 1994; Reith & Selmeci, 1991; Ruth, Ullman &, Collins, 1988; Seale & Carney, 1991; Wiener & Reith, 1990). Thus the difference in sensitivity between these BALB substrains may be relatively subtle and dependent upon test conditions. However, confirmation of this difference in cocaine sensitivity between two such closely related substrains would provide a useful genetic tool in identifying potential candidate genes for insensitivity to cocaine (Velez, Sokoloff, Miczek, Palmer & Dulawa, 2010). Direct comparisons between BALB/cJ and BALB/cByJ mice in abuse-related effects of cocaine may therefore warrant further attention (see also below about reinforcing effects of cocaine in these mice).
The calculated A50 values in the present investigation indicated that the mouse strains and outbred stocks could be divided into two groups of lower and higher potency, with a roughly 2-fold difference in potency. Few previous studies reported A50 values, and findings varied between studies. For example, Tolliver & Carney (1995) reported approximately 2-fold lower potency in DBA/2J relative to C57BL/6J, comparable to our present findings. In contrast, Elmer and co-workers (1996) found cocaine to be significantly more potent, but less efficacious (as % of saline activity levels), in C57BL/6J mice relative to C3H/HeJ mice, which were comparable in potency and efficacy (as % saline) in the present study. As noted for baseline levels, apparent potency and efficacy of cocaine between the C57BL/6J strain and the DBA/2J strain have been variable across investigations (e.g., Elmer, Gorelick, Goldberg & Rothman, 1996; Tolliver & Carney, 1995; Womer, Jones & Erwin, 1994). More generally, ranking of mouse strains/stocks in cocaine potency or efficacy between studies appear highly variable, not only across different behavioral endpoints (as already remarked by Seale & Carney, 1991), but also within the same endpoint (e.g., locomotor stimulation, seizure induction, suppression of operant behavior), indicating that such strain comparisons should be interpreted with caution (Golden et al., 2001; Heyser, McDonald, Beauchamp, Koob & Gold 1997; Marley, Witkin & Goldberg, 1991).
We did a second dose-effect determination in each strain and stock, to increase our confidence in the data and assess within-subjects replicability (the experiment was not designed to produce or measure sensitization). Both determinations generally yielded very comparable results (notably, saline activity levels only differed between determinations in one strain, the A/J). In the strains that showed a significant effect of determinations, this was always due to increases in activity for the second determination (i.e., never decreases), consistent with the development of sensitization. The degree of increase varied among strains, was absent in more than half the strains, and was clearest in the FVB/NJ and DBA/2J strains. Demonstration of sensitization to cocaine’s locomotor stimulant effects can be strongly affected by the experimental parameters, thus it is perhaps not surprising that strain comparisons in this regard vary between studies. For example, previous investigations have reported sensitization comparably in DBA/2J and C57BL/6J mice (Phillips et al., 1998), more in C57BL/6J mice than in A/J mice (Mead, Katz & Rocha, 2002), or in C57BL/6J, C3H/HeJ, and BALB/cByJ mice but not in DBA/2J mice (Elmer, Gorelick, Goldberg & Rothman, 1996). We also tested separate cohorts of C57BL/6J, 129X1/SvJ and DBA/2J mice to further assess within-laboratory replicability, and found good consistency between cohorts as well.
Qualitative effects of cocaine on locomotor behavior: distribution of beam breaks between consecutive, repeated, and elevated
Data from the first cocaine dose-effect determination were also analyzed as the distribution of beam breaks between breaks of consecutive beams (suggesting ambulation), repeated breaks of the same beam (suggesting finer movements that may include stereotyped behaviors), and breaks of the elevated beams (suggesting rearing or jumping). Strains/stocks varied significantly in their baseline distribution of behavior, with the most active strains and stocks typically showing more consecutive beam breaks, and the least active strains showing higher percentages of repeated beam breaks. Despite these differences, high-activity strains/stocks tended to show high counts in all three categories, and low-activity strains showed low counts in all three categories. Strain/stock ranking in terms of activity by break type, including vertical activity, was in agreement with most previous reports (Bothe, Bolivar, Vedder & Geistfeld, 2004, 2005; Rodgers, Boullier, Chatzimichalaki, Cooper &, Shorten, 2002; Tang, Orchard & Sanford, 2002; Wahlsten et al., 2003), but not all (Velez, Sokoloff, Miczek , Palmer & Dulawa, 2010: BALB/cJ vs. BALB/cByJ).
Cocaine tended to increase the proportion of consecutive beam breaks, suggesting increased ambulation, with the exception of the low-baseline strains, such as substrains of 129. In those latter strains, cocaine tended to increase the proportion of repeated beam breaks (or to not affect beam type distribution), consistent with induction of stereotyped behaviors. Cocaine also decreased the proportion of elevated beam breaks in most strains/stocks, consistent with previous observations in various strains (Wahlsten et al., 2003). Cocaine also affected the beam break type distribution in the BALB/cJ strain, despite the lack of effect on total activity in this strain: as in most other strains, cocaine increased the percent consecutive breaks, and decreased percent elevated beam breaks. However, unlike other strains, in which cocaine both increased the absolute count of consecutive beam breaks (indicating increased ambulation) and (typically) decreased counts of elevated breaks, cocaine only decreased counts of elevated beam breaks in the BALB/cJ mice. Cocaine produced no significant increases in any break type in the BALB/cJ mice. The only other strain in which absolute increases in consecutive beam breaks were not observed was the 129/SvEvTac, but in this strain cocaine did produce significant increases in repeated breaks (data not shown). Thus even taking into account the change in distribution of beam breaks, the BALB/cJ remains the only strain tested in which cocaine failed to increase any aspect of locomotor behavior.
Cocaine self-administration in BALB mice
Of 15 inbred mouse strains /outbred stocks tested, only the BALB/cJ failed to show locomotor activating effects of cocaine in the present investigation. Furthermore, the closely related BALB/cByJ substrain has previously been found not to self-administer cocaine without previous operant training (Deroche et al, 1997; Roberts, Polis & Gold, 1997). These two findings, taken together, prompted the question whether BALB/cJ mice could also lack reinforcing effects of cocaine. To our knowledge, the BALB/cJ substrain has not previously been tested for cocaine self-administration. We therefore evaluated both BALB substrains in chronic intravenous cocaine self-administration to test the hypothesis that under some conditions, cocaine would be self-administered in the BALB/cByJ, but not the BALB/cJ mice. We trained the mice to respond for a food reinforcer prior to exposure to cocaine to allow conditions that would facilitate acquisition of cocaine self-administration.
Over a range of doses, the BALB/cJ mice showed no indication of self-administering cocaine, as cocaine maintained lower or similar responding compared to saline. The BALB/cByJ strain showed some evidence of reinforcing effects at a moderate cocaine dose when each dose was available for five successive sessions, but failed to maintain responding above saline levels when doses were switched daily, and mostly failed to reallocate their behavior in a reversal procedure. These mixed results are consistent with an earlier report which indicated that cocaine has reinforcing properties in BALB/cByJ mice, but also anxiogenic/aversive effects that limit self-administration (David, Gold, Koob & Cazala, 2001). The use of food-training combined with the slow rates of extinction of nose-poking behavior when saline was available make a definite demonstration of reinforcing effects (or lack thereof) difficult, as high response rates were observed when saline was available, especially when doses were switched daily. Perseveration of a previously reinforced behavior may also have impeded performance in the reversal procedure, although all mice performed the reversal when tested with food. Also, the sample size was modest in the reversal test (five per strain). Despite these limitations of the current study, our data suggest that cocaine may serve as a relatively weak reinforcer at some doses in the BALB/cByJ strain, under more permissive conditions than previously used, while the BALB/cJ mice did not show any convincing indication of self-administering cocaine at any dose.
For both BALB substrains, it is unlikely that lack of cocaine self-administration was attributable to general performance or learning deficits, or to a general unresponsiveness to reinforcers, as both strains performed at comparable levels in food-maintained operant behavior, including a reversal procedure. Furthermore, BALB/cByJ mice were previously shown to self-administer intravenous heroin or cocaine-heroin mixtures, without food pre-training (Deroche et al, 1997; Roberts Polis & Gold, 1997). Similarly, BALB/cJ mice maintained lever-pressing behavior reinforced orally with the opioid agonist etonitazene (Elmer, Pieper, Goldberg & George, 1995). Finally, both substrains have performed readily under various schedules of food reinforcement (Baron & Meltzer, 2001; Deroche et al., 1997; Heyser, McDonald, Beauchamp, Koob & Gold, 1997; Isles, Humby , Walters & Wilkinson, 2004; McKerchar, Zarcone & Fowler, 2005; Wenger, McMillan & Chang, 1984; Zarcone, Chen & Fowler, 2004) or shock termination (Brennan, 2004), and BALB/c mice showed high rates of bar pressing in intracranial self-stimulation procedures (Garrigues & Cazala, 1983; Cazala, Galey & Durkin, 1988). It should be noted also that cocaine was not devoid of effects in either BALB substrain. In the locomotor assay, cocaine did dose-dependently decrease elevated beam breaks (rearing) in the BALB/cJ mice, as discussed above. In the self-administration assay, there was a significant effect of cocaine dose in both substrains reflecting lower responding when higher cocaine doses were presented, suggesting the use of higher doses would not likely have produced measurable self-administration. Rate-decreasing effects of moderate to high doses of cocaine in operant behavior are well-known and have been demonstrated in the BALB/cByJ strain when cocaine was self-administered, or when cocaine was administered as a pretreatment to food-maintained behavior (Deroche et al., 1997; Heyser, McDonald, Beauchamp, Koob & Gold, 1997).
Parallels in locomotor activity in a novel environment, and stimulant and reinforcing effects of cocaine
Some studies suggested positive correlations between locomotor response to novelty, locomotor response to amphetamine/cocaine, and rewarding or reinforcing effects of amphetamine/cocaine (Hooks, Jones, Smith, Neill & Justice, 1991; Klebaur, Bevins, Segar & Bardo, 2001; Orsini, Buchini, Piazza, Puglisi-Allegra & Cabib, 2004; Piazza, Deminiere, Le Moal & Simon 1989; Pierre & Vezina, 1997). Other studies showed no correlation between novelty- or cocaine-induced locomotor activity and cocaine-conditioned CPP or amount of cocaine self-administered (Brabant, Quertemont & Tirelli, 2005; Gong, Neill & Justice, 1996; Mitchell, Cunningham & Mark, 2005). Here we looked for correlations between activity in a novel environment and the potency or efficacy of cocaine to increase locomotor activity across strains. We found that peak activity levels in a novel environment (as well as habituated baseline activity), correlated positively with peak activity levels after cocaine administration. However, no measures of spontaneous activity correlated with cocaine’s potency, or with the magnitude of cocaine-induced hyperactivity relative to baseline. Recombinant inbred strain studies similarly showed correlations between baseline activity and peak cocaine-stimulated activity, but not between baseline and the degree of stimulation by cocaine, ethanol or morphine (Gill & Boyle, 2008; Miner & Marley, 1995). Among the strains/stocks that did show locomotor stimulating effects of cocaine, we also found no clear indication that the strain differences in potency or efficacy of cocaine in the present study correlated with strain differences in cocaine-conditioned CPP or self-administration. For instance, we reported lower rates of cocaine self-administration in 129X1/SvJ mice, but not 129S6/SvEvTac mice, relative to C57BL/6J mice (Thomsen & Caine, 2006). In the present study, the 129X1/SvJ and C57BL/6J mice showed higher levels of cocaine-induced hyperlocomotion than did the 129S6/SvEvTac mice, while the calculated potency of cocaine was lower in the 129X1/SvJ mice relative to both 129S6/SvEvTac and C57BL/6J mice. Previous studies suggested that, while cocaine is self-administered by both DBA/2J mice and C57BL/6J mice, dose-effect functions are shifted down and/or to the left in the DBA/2J mice relative to the C57BL/6J, and that the DBA/2J may be more sensitive to rate-decreasing effects of cocaine (Grahame & Cunningham, 1995; Kuzmin & Johansson, 2000; Rocha et al., 1998; van der Veen, Piazza & Deroche-Gamonet, 2007; see also Thomsen & Caine, 2007 for review). In the present study, the DBA/2J mice were less, not more, sensitive to cocaine’s locomotor stimulating effects, based on the calculated potency. The only apparent correlation for those two strains may be that DBA/2J mice showed lower baseline activity in the present study as well as low spontaneous nose-poking behavior in operant studies (Kuzmin & Johansson, 2000).
Those findings taken together suggest that any correlations between spontaneous or cocaine-stimulated locomotor activity and self-administration behavior is more easily explained by general underlying activity levels, which can be observed as locomotion or as operant behavior, rather than by prediction of cocaine’s reinforcing potency or efficacy. In other words, high spontaneous activity levels tend to predict high drug-stimulated activity levels (as we observed in the present study). And high locomotor activity levels may also tend to predict high lever-pressing or nose-poking activity, as indicated by previous investigations using both comparisons across mouse strains and individual differences in rats (Baron & Meltzer, 2001; McKerchar, Zarcone & Fowler, 2005; Mitchell, Cunningham & Mark, 2005). Therefore, while high spontaneous activity levels likely facilitate acquisition of an operant response, it remains to be shown that this initial facility predicts increased reinforcing effects of cocaine (or any other drug).
Intriguingly, in the present study we found that the BALB/cJ strain failed to show habituation in a novel environment in a locomotor activity assay, and lacked both locomotor stimulant and reinforcing effects of cocaine. The closely related BALB/cByJ substrain showed modest but significant habituation and locomotor activating effects of cocaine, and showed evidence of modest reinforcing effects of cocaine. In previous studies, BALB/c mice also failed to show CPP to cocaine or amphetamine, under conditions that produced CPP in C57BL/6 mice, and morphine-induced CPP in both strains (Belzung & Barreau, 2000). In contrast, cocaine, amphetamine, and the dopamine reuptake inhibitor GBR 12909 all produced CPP in BALB/cByJ mice (Seale & Carney, 1991). Thus, results from locomotor activity assays (novelty or cocaine), self-administration, or CPP, all correlate well in showing detectable (if often modest) effects in the BALB/cByJ substrain but no detectable effects in the BALB/cJ substrain across the behavioral endpoints.
Thus while we found no evidence of quantitative correlation between locomotor response to novelty or cocaine and rewarding/reinforcing effects of cocaine, it is possible that an absolute lack of psychomotor stimulant effects of cocaine may “predict” a lack of reinforcing effects of cocaine. It is further possible that a complete lack of locomotor response/habituation to a novel environment may predict lack of cocaine effects. Because we only found only one strain showing this “unresponsive” profile under the present test conditions, we cannot determine at this point whether it is a coincidence or reflects some more general correlation (“predictive value”) between endpoints. Cocaine-conditioned CPP has been demonstrated in C57BL/6, DBA/2, A/J, and C3H strains, as well as in outbred CD-1 and Swiss-Webster mice, while 129X1/SvJ mice failed to show CPP over a range of doses (Dell’Omo, Laviola, Chiarotti, Alleva & Bignami, 1993; Itzhak, Martin, Black & Huang, 1998; Kurtuncu, Arslan, Akhisaroglu, Manev & Uz, 2004; Laviola, Dell’Omo, Alleva & Bignami 1992; Miner, 1997; Orsini, Bonito-Oliva, Conversi & Cabib, 2005; Orsini, Bonito-Oliva, Conversi & Cabib, 2008; Seale & Carney, 1991; Zhang, Mantsch, Schlussman, Ho & Kreek 2002). Cocaine self-administration has also been established in several strains, including the C57BL/6J, DBA/2J, 129S6/SvEvTac, 129X1/SvJ, C57BL/6xSJL hybrid, and outbred Swiss Webster (Caine, Negus & Mello, 1999; Carney, Landrum, Cheng & Seale 1991; Deroche et al., 1997; Grahame, Phillips, Burkhart-Kasch & Cunningham 1995; Highfield, Mead, Grimm, Rocha &, Shaham 2002; Kuzmin & Johansson, 2000; Rocha et al., 1998; Thomsen & Caine, 2006; Thomsen et al., 2010; Tsibulsky & Norman, 2001; van der Veen, Piazza & Deroche-Gamonet, 2007; van der Veen et al., 2008;). All those strains/stock showed significant effects of cocaine in the locomotor assay in the present study. Conversely, mutant strains of mice that failed to self-administer cocaine, such as mice lacking functional dopamine D1 receptors (D1−/−) or dopamine transporters (DAT−/−), or carrying a mutant cocaine-insensitive dopamine transporter, also lacked locomotor hyperactivity responses to cocaine (Caine et al., 2007; Centonze et al., 2003; Chen et al., 2006; Chiamulera et al., 2001; Karasinska, George, Cheng & O’Dowd, 2005; Karlsson, Hefner, Sibley & Holmes, 2008; Mead, Rocha, Donovan & Katz, 2002; Morice, Denis, Giros & Nosten-Bertrand 2004; Thomsen, Hall, Uhl & Caine, 2009; Thomsen, Han, Gu & Caine, 2009 ). Note that the D1 and both DAT mutant strains were either maintained on a mixed 129 substrain X C57BL/6J background or backcrossed to the C57BL/6J strain, and that both 129X1/SvJ, 129S6/SvSvTac, and C57BL/6J mice self-administered cocaine under experimental conditions similar to those reported for the mutant mice (Caine et al., 2007; Chen et al., 2006; Sora et al., 1998; Thomsen & Caine, 2006). Intriguingly, both D1−/− and DAT−/− mice also showed delayed/disrupted habituation to novelty-induced hyperlocomotion, most markedly so in the DAT−/− mice (Centonze et al., 2003; Gainetdinov et al., 1999; Karlsson, Hefner, Sibley & Holmes, 2008; Mead, Rocha, Donovan & Katz, 2002; Wong et al., 2003). In summary, a lack of psychomotor stimulant response to cocaine appears to be a fairly good predictor of lack of reinforcing effects of cocaine, across several mouse strains and outbred stocks. Furthermore, present and previous findings are compatible with the notion that delayed or disrupted habituation in the locomotor response to a novel environment may predict a lack of psychomotor stimulant or reinforcing effects of cocaine, although this hypothesis remains to be substantiated.
ACKNOWLEDGMENTS
We thank Dr. Rebecca J. Ralph for contributing to portions of this work. We thank Cynde Harmon, Jill Berkowitz, Gloria Yu, Justin Hamilton, and Jennifer Dohrmann for technical assistance. This work was supported predominantly by the Zaffaroni Foundation, and in part by the National Institute on Drug Abuse (DA07252, DA12142, DA14528).
REFERENCES
- Anisman H, Cygan D. Central effects of scopolamine and (+)-amphetamine on locomotor activity: interaction with strain and stress variables. Neuropharmacology. 1975;14:835–40. doi: 10.1016/0028-3908(75)90111-2. [DOI] [PubMed] [Google Scholar]
- Anisman H, Wahlsten D, Kokkinidis L. Effects of d-amphetamine and scopolamine on activity before and after shock in three mouse strains. Pharmacol Biochem Behav. 1975;3:819–24. doi: 10.1016/0091-3057(75)90112-4. [DOI] [PubMed] [Google Scholar]
- Baron SP, Meltzer LT. Mouse strains differ under a simple schedule of operant learning. Behav Brain Res. 2001;118:143–52. doi: 10.1016/s0166-4328(00)00322-3. [DOI] [PubMed] [Google Scholar]
- Belzung C, Barreau S. Differences in drug-induced place conditioning between BALB/c and C57Bl/6 mice. Pharmacol Biochem Behav. 2000;65:419–23. doi: 10.1016/s0091-3057(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behav Genet. 2000;30:285–93. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3:149–57. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005;55:326–34. [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Tirelli E. Evidence that the relations between novelty-induced activity, locomotor stimulation and place preference induced by cocaine qualitatively depend upon the dose: a multiple regression analysis in inbred C57BL/6J mice. Behav Brain Res. 2005;158:201–10. doi: 10.1016/j.bbr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Brennan FX. Genetic differences in leverpress escape/avoidance conditioning in seven mouse strains. Genes Brain Behav. 2004;3:110–4. doi: 10.1111/j.1601-183x.2003.0057.x. [DOI] [PubMed] [Google Scholar]
- Cabib S, Algeri S, Perego C, Puglisi-Allegra S. Behavioral and biochemical changes monitored in two inbred strains of mice during exploration of an unfamiliar environment. Physiol Behav. 1990;47:749–53. doi: 10.1016/0031-9384(90)90089-m. [DOI] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–5. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 1999;147:22–4. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–50. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991;2:477–80. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- Cazala P, Galey D, Durkin T. Electrical self-stimulation in the medial and lateral septum as compared to the lateral hypothalamus: differential intervention of reward and learning processes? Physiol Behav. 1988;44:53–9. doi: 10.1016/0031-9384(88)90345-9. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–12. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–8. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–11. [PubMed] [Google Scholar]
- David V, Gold LH, Koob GF, Cazala P. Anxiogenic-like effects limit rewarding effects of cocaine in balb/cbyj mice. Neuropsychopharmacology. 2001;24:300–18. doi: 10.1016/S0893-133X(00)00205-0. [DOI] [PubMed] [Google Scholar]
- Davis WM, Babbini M, Pong SF, King WT, White CL. Motility of mice after amphetamine: effects of strain, aggregation and illumination. Pharmacol Biochem Behav. 1974;2:803–9. doi: 10.1016/0091-3057(74)90113-0. [DOI] [PubMed] [Google Scholar]
- Dell’omo G, Laviola G, Chiarotti F, Alleva E, Bignami G. Prenatal oxazepam effects on cocaine conditioned place preference in developing mice. Neurotoxicol Teratol. 1993;15:207–10. doi: 10.1016/0892-0362(93)90017-i. [DOI] [PubMed] [Google Scholar]
- Deroche V, Caine SB, Heyser CJ, Polis I, Koob GF, Gold LH. Differences in the liability to self-administer intravenous cocaine between C57BL/6 x SJL and BALB/cByJ mice. Pharmacol Biochem Behav. 1997;57:429–40. doi: 10.1016/s0091-3057(96)00439-x. [DOI] [PubMed] [Google Scholar]
- Downing C, Rodd-Henricks K, Marley RJ, Dudek BC. Genetic variation in the psychomotor stimulant properties of cocaine in Mus musculus. Psychopharmacology (Berl) 2003;167:159–66. doi: 10.1007/s00213-003-1387-0. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Gorelick DA, Goldberg SR, Rothman RB. Acute sensitivity vs. context-specific sensitization to cocaine as a function of genotype. Pharmacol Biochem Behav. 1996;53:623–8. doi: 10.1016/0091-3057(95)02062-4. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Goldberg SR, George FR. Opioid operant self-administration, analgesia, stimulation and respiratory depression in mu-deficient mice. Psychopharmacology (Berl) 1995;117:23–31. doi: 10.1007/BF02245094. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Zarcone TJ, Vorontsova E, Chen R. Motor and associative deficits in D2 dopamine receptor knockout mice. Int J Dev Neurosci. 2002;20:309–21. doi: 10.1016/s0736-5748(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Garrigues AM, Cazala P. Central catecholamine metabolism and hypothalamic self-stimulation behaviour in two inbred strains of mice. Brain Res. 1983;265:265–71. doi: 10.1016/0006-8993(83)90341-4. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Gill KJ, Boyle AE. Genetic influences on drug-induced psychomotor activation in mice. Genes Brain Behav. 2008;7:859–68. doi: 10.1111/j.1601-183X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- Golden GT, Ferraro TN, Smith GG, Snyder RL, Jones NL, Berrettini WH. Acute cocaine-induced seizures: differential sensitivity of six inbred mouse strains. Neuropsychopharmacology. 2001;24:291–9. doi: 10.1016/S0893-133X(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr. Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav. 1996;53:191–6. [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Genetic differences in intravenous cocaine self-administration between C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 1995;122:281–91. doi: 10.1007/BF02246549. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Phillips TJ, Burkhart-Kasch S, Cunningham CL. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol Biochem Behav. 1995;51:827–34. doi: 10.1016/0091-3057(95)00047-z. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Kusnecov A, Michna L, Kita T, Hara C, Wagner GC. Relationship between methamphetamine-induced dopamine release, hyperthermia, self-injurious behaviour and long term dopamine depletion in BALB/c and C57BL/6 mice. Pharmacol Toxicol. 2003;93:33–41. doi: 10.1034/j.1600-0773.2003.930105.x. [DOI] [PubMed] [Google Scholar]
- Hano J, Vetulani J, Sansone M, Oliverio A. Effect of clonidine, amphetamine, and their combinations on the locomotor activity of CD-1 and C57BL/6 mice. Pharmacol Biochem Behav. 1978;9:741–6. doi: 10.1016/0091-3057(78)90350-7. [DOI] [PubMed] [Google Scholar]
- Helmeste DM, Seeman P. Amphetamine-induced hypolocomotion in mice with more brain D2 dopamine receptors. Psychiatry Res. 1982;7:351–9. doi: 10.1016/0165-1781(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, McDonald JS, Beauchamp V, Koob GF, Gold LH. The effects of cocaine on operant responding for food in several strains of mice. Psychopharmacology (Berl) 1997;132:202–8. doi: 10.1007/s002130050337. [DOI] [PubMed] [Google Scholar]
- Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology (Berl) 2002;161:417–24. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol Biochem Behav. 1999;63:21–6. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–8. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. J Neurosci. 2004;24:6733–40. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Mori T, Namiki M, Suzuki T, Sawaguchi T. Complicated interaction between psychostimulants and morphine in expression of phenotype of behavior in the dopaminergic system of BALB/c mice. J Pharmacol Sci. 2007;105:326–33. doi: 10.1254/jphs.fp0070653. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Huang PL. The role of neuronal nitric oxide synthase in cocaine-induced conditioned place preference. Neuroreport. 1998;9:2485–8. doi: 10.1097/00001756-199808030-00011. [DOI] [PubMed] [Google Scholar]
- Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464–77. doi: 10.1037/0735-7044.117.3.464. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–50. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200:117–27. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–9. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–75. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Koff JM, Shuster L, Miller LG. Chronic cocaine administration is associated with behavioral sensitization and time-dependent changes in striatal dopamine transporter binding. J Pharmacol Exp Ther. 1994;268:277–82. [PubMed] [Google Scholar]
- Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–5. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Fredholm BB, Ogren SO. Genetic evidence that cocaine and caffeine stimulate locomotion in mice via different mechanisms. Life Sci. 2000;66:PL113–8. doi: 10.1016/s0024-3205(99)00647-5. [DOI] [PubMed] [Google Scholar]
- Lathe R. Mice, gene targeting and behaviour: more than just genetic background. Trends Neurosci. 1996;19:183–6. doi: 10.1016/s0166-2236(96)20022-0. discussion 188-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Dell’Omo G, Alleva E, Bignami G. Ontogeny of cocaine hyperactivity and conditioned place preference in mice. Psychopharmacology (Berl) 1992;107:221–8. doi: 10.1007/BF02245141. [DOI] [PubMed] [Google Scholar]
- Marley RJ, Witkin JM, Goldberg SR. Genetic factors influence changes in sensitivity to the convulsant properties of cocaine following chronic treatment. Brain Res. 1991;542:1–7. doi: 10.1016/0006-8993(91)90989-9. [DOI] [PubMed] [Google Scholar]
- McKerchar TL, Zarcone TJ, Fowler SC. Differential acquisition of lever pressing in inbred and outbred mice: comparison of one-lever and two-lever procedures and correlation with differences in locomotor activity. J Exp Anal Behav. 2005;84:339–56. doi: 10.1901/jeab.2005.95-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerchar TL, Zarcone TJ, Fowler SC. Use of a force-plate actometer for detecting and quantifying vertical leaping induced by amphetamine in BALB/cJ mice, but not in C57BL/6J, DBA/2J, 129X1/SvJ, C3H/HeJ, and CD-1 mice. J Neurosci Methods. 2006;153:48–54. doi: 10.1016/j.jneumeth.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Mead AN, Katz JL, Rocha BA. Intravenous cocaine-induced activity in A/J and C57BL/6J mice: behavioral sensitization and conditioned activity. Neuropharmacology. 2002;42:976–86. doi: 10.1016/s0028-3908(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Mead AN, Rocha BA, Donovan DM, Katz JL. Intravenous cocaine induced-activity and behavioural sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur J Neurosci. 2002;16:514–20. doi: 10.1046/j.1460-9568.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- Meier GW, Hatfield JL, Foshee DP. Genetic and Behavioral Aspects of Pharmacologically Induced Arousal. Psychopharmacologia. 1963;4:81–90. doi: 10.1007/BF00413326. [DOI] [PubMed] [Google Scholar]
- Mhyre TR, Chesler EJ, Thiruchelvam M, Lungu C, Cory-Slechta DA, Fry JD, Richfield EK. Heritability, correlations and in silico mapping of locomotor behavior and neurochemistry in inbred strains of mice. Genes Brain Behav. 2005;4:209–28. doi: 10.1111/j.1601-183X.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol Biochem Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Miner LL, Marley RJ. Chromosomal mapping of the psychomotor stimulant effects of cocaine in BXD recombinant inbred mice. Psychopharmacology (Berl) 1995;122:209–14. doi: 10.1007/BF02246541. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–72. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice E, Denis C, Giros B, Nosten-Bertrand M. Phenotypic expression of the targeted null-mutation in the dopamine transporter gene varies as a function of the genetic background. Eur J Neurosci. 2004;20:120–6. doi: 10.1111/j.1460-9568.2004.03465.x. [DOI] [PubMed] [Google Scholar]
- Morse AC, Erwin VG, Jones BC. Strain and housing affect cocaine self-selection and open-field locomotor activity in mice. Pharmacol Biochem Behav. 1993;45:905–12. doi: 10.1016/0091-3057(93)90138-j. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression : a practical guide to curve fitting. Oxford University Press; Oxford ; New York, NY: 2004. [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 2005;181:327–36. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Genetic liability increases propensity to prime-induced reinstatement of conditioned place preference in mice exposed to low cocaine. Psychopharmacology (Berl) 2008;198:287–96. doi: 10.1007/s00213-008-1137-4. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172:264–70. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Mark AG. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Hen R, Crabbe JC. Complications associated with genetic background effects in research using knockout mice. Psychopharmacology (Berl) 1999;147:5–7. doi: 10.1007/s002130051128. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci. 1998;18:3023–34. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–84. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Geyer MA. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J Pharmacol Exp Ther. 2001;298:148–55. [PubMed] [Google Scholar]
- Reith ME, Selmeci G. Cocaine binding sites in mouse striatum, dopamine autoreceptors, and cocaine-induced locomotion. Pharmacol Biochem Behav. 1992;41:227–30. doi: 10.1016/0091-3057(92)90087-v. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Polis IY, Gold LH. Intravenous self-administration of heroin, cocaine, and the combination in Balb/c mice. Eur J Pharmacol. 1997;326:119–25. doi: 10.1016/s0014-2999(97)85405-2. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ. Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 1998;138:82–8. doi: 10.1007/s002130050648. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol Behav. 2002;77:301–10. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- Ruth JA, Ullman EA, Collins AC. An analysis of cocaine effects on locomotor activities and heart rate in four inbred mouse strains. Pharmacol Biochem Behav. 1988;29:157–62. doi: 10.1016/0091-3057(88)90289-4. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60:593–9. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–31. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Seale TW, Carney JM. Genetic determinants of susceptibility to the rewarding and other behavioral actions of cocaine. J Addict Dis. 1991;10:141–62. doi: 10.1300/J069v10n01_10. [DOI] [PubMed] [Google Scholar]
- Skrinskaya JA, Nikulina EM, Popova NK. Role of genotype in brain dopamine metabolism and dopamine-dependent behavior of mice. Pharmacol Biochem Behav. 1992;42:261–7. doi: 10.1016/0091-3057(92)90525-k. [DOI] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–69. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 2006;184:145–54. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behav Genet. 2007;37:101–18. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, Fink-Jensen A, Caine SB. Attenuation of cocaine’s reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332:959–69. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–92. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331:204–11. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, Carney J/M/ Comparison of cocaine and GBR 12935: effects on locomotor activity and stereotypy in two inbred mouse strains. Pharmacol Biochem Behav. 1994;48:733–9. doi: 10.1016/0091-3057(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, Carney JM. Locomotor stimulant effects of cocaine and novel cocaine analogs in DBA/2J and C57BL/6J inbred mice. Pharmacol Biochem Behav. 1995;50:163–9. doi: 10.1016/0091-3057(94)00277-p. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norma AB. Satiety threshold during maintained cocaine self-administration in outbred mice. Neuroreport. 2001;12:325–8. doi: 10.1097/00001756-200102120-00029. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V. Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS One. 2008;3:e2245. doi: 10.1371/journal.pone.0002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl) 2007;193:179–86. doi: 10.1007/s00213-007-0777-0. [DOI] [PubMed] [Google Scholar]
- Velez L, Sokoloff G, Miczek KA, Palmer AA, Dulawa SC. Differences in aggressive behavior and DNA copy number variants between BALB/cJ and BALB/cByJ substrains. Behav Genet. 2010;40:201–10. doi: 10.1007/s10519-009-9325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Rustay NR, Metten P, Crabbe JC. In search of a better mouse test. Trends Neurosci. 2003;26:132–6. doi: 10.1016/S0166-2236(03)00033-X. [DOI] [PubMed] [Google Scholar]
- Wenger GR. The role of control activity levels in the reported strain differences to the behavioral effects of drugs in mice. Pharmacol Biochem Behav. 1989;32:241–7. doi: 10.1016/0091-3057(89)90240-2. [DOI] [PubMed] [Google Scholar]
- Wenger GR, McMillan DE, Chang LW. Behavioral effects of trimethyltin in two strains of mice. II. Multiple fixed ratio, fixed interval. Toxicol Appl Pharmacol. 1984;73:89–96. doi: 10.1016/0041-008x(84)90056-5. [DOI] [PubMed] [Google Scholar]
- Wiener HL, Reith ME. Correlation between cocaine-induced locomotion and cocaine disposition in the brain among four inbred strains of mice. Pharmacol Biochem Behav. 1990;36:699–701. doi: 10.1016/0091-3057(90)90277-o. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Womer DE, Jones BC, Erwin VG. Characterization of dopamine transporter and locomotor effects of cocaine, GBR 12909, epidepride, and SCH 23390 in C57BL and DBA mice. Pharmacol Biochem Behav. 1994;48:327–35. doi: 10.1016/0091-3057(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Wong JY, Clifford JJ, Massalas JS, Kinsella A, Waddington JL, Drago J. Essential conservation of D1 mutant phenotype at the level of individual topographies of behaviour in mice lacking both D1 and D3 dopamine receptors. Psychopharmacology (Berl) 2003;167:167–73. doi: 10.1007/s00213-003-1415-0. [DOI] [PubMed] [Google Scholar]
- Zarcone TJ, Chen R, Fowler SC. Differential acquisition of food-reinforced disk pressing by CD-1, BALB/cJ and C57BL/6J mice. Behav Brain Res. 2004;152:1–9. doi: 10.1016/j.bbr.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–62. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Orsini C, Cabib S, Puglisi-Allegra S. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82:521–8. doi: 10.1016/s0306-4522(97)00276-5. [DOI] [PubMed] [Google Scholar]