Abstract
Object
Brain tumors are rare in infants under 6-months of age. These tumors can be challenging to treat surgically. We analyzed a modern series of patients treated by a multidisciplinary team at a tertiary care center and performed a literature review of this unique population.
Methods
Retrospective clinical data was collected for patients surgically treated for intracranial mass lesions at The Children’s Hospital of Philadelphia from 1998 to 2007. Dermoid cysts and other skull-based lesions were excluded from the analysis.
Results
Sixteen patients under 6-months of age underwent surgery for primary intracranial mass lesions. The median age of the patients at surgery was 5.2 months (range 1.4 to 6 months of age). Children most often presented with a bulging fontanelle, hydrocephalus, or macrocephaly (7 patients). Vomiting was seen in 5 patients, cranial nerve palsies in 1 patient, and seizures in 3 patients.
All patients had tumor resections and post-operatively were monitored in the intensive care unit. The final pathology consisted of atypical teratoid/rhabdoid tumor (3 cases), primitive neuroectodermal tumor/medulloblastoma (3 cases), choroid plexus papilloma (2 cases), astrocytoma (2 cases), ganglioglioma (2 cases), desmoplastic infantile ganglioglioma (2), glioblastoma multiforme (1), and choroid plexus carcinoma (1).
Two intra-operative deaths occurred. Of the surviving 14, a gross total resection was achieved in 4. Adjuvant therapy was determined by a multidisciplinary team composed of neuro-oncology, neurosurgery, and radiation oncology. Seven patients were treated with chemotherapy, 1 patient had proton beam therapy. Five-year overall survival was 45%. The eight surviving patients had neurological sequelae, and developmental outcome was variable.
Conclusions
Brain tumors are uncommon in children under 6-months of age. Patients present with a variety of tumor pathologies. Children who survive have neurological sequelae. More studies are necessary to understand the impact that different treatment options, tumor pathology, and tumor location have on neurological outcome.
Keywords: brain tumor, surgery, neonates, congenital
Introduction
Brain tumors are rare in infants less than 6 months of age. Although brain tumors in children have been well characterized, there are relatively few studies that have looked specifically at children less than 6 months of age.
Treatment of very young children with malignant brain tumors requires an interdisciplinary approach36. Surgery remains a vital component of most treatment algorithms28,40,48,50. In order to minimize the harmful effects of radiation, chemotherapy has become the critical component of adjuvant therapy in the neonatal age group14,16,20,22,28. Chemotherapy is also used as a time “bridge” in order to delay radiation therapy, especially in younger children.
The treatment algorithms for childhood brain tumors are well defined for older children but are not as well defined for infants less than 6 months of age40. Therefore, a more thorough examination of the treatment modalities used, and the associated outcomes, will help establish the best methods for managing these patients. Additionally, characterizing the pathology and frequency of the various tumor types in this patient population is paramount to defining and developing the best treatment options47.
Characterizing the types of tumors, and the treatment options that provide the best results may improve the chances of meaningful neurological and developmental recovery. This study illustrates the experience of a large tertiary care children’s hospital in treating infants less than 6 months of age with brain tumors. Additionally, since brain tumors in this age group are rare, we performed a literature review of published cases.
Clinical Materials and Methods
Study design and subjects
A neurosurgical database of brain tumor patients who were treated surgically was queried to identify all patients less than 6 months of age who underwent resection of primary intracranial mass lesions between 1998 and 2007 at The Children’s Hospital of Philadelphia (CHOP). With institutional review board approval, retrospective analysis of these patients was performed.
Clinical data
Data including patient demographics, clinical presentation, and treatment were abstracted from inpatient hospital records, neurosurgical outpatient clinic charts, and neuro-oncology outpatient clinic charts. Extent of resection was categorized based on the immediate (within one day) post-operative MRI. Categories were gross total resection (no residual disease), subtotal resection (50–99% reduction in tumor size), and biopsy (less than 50% reduction in tumor size). The Kaplan-Meier estimate of survival was calculated using STATA 11.1 (STATA Corporation, College Station, TX). Survival time was calculated from the date of the first neurosurgical intervention. Neurological outcomes were retrieved from neuro-oncology and/or neurosurgery clinic charts.
Literature Review
PubMed was searched for key words including infant, neonatal, and brain tumor (as well as specific brain tumor types). References of included papers were examined to find additional papers. A large literature review was used to identify potential cases that were missed in the original PubMed Search (Isaacs). The majority of the relevant publications reviewed patients up to 3 years of age. Only studies in which subjects less than 6 month of age could be identified clearly were included in our review.
Results
Patient characteristics and presentation
From 1998 to 2007, 16 patients 6 months old and younger with pathologically confirmed malignant and benign intracranial brain tumors were identified. Five (31%) were male and 11 (69%) were female. The median age at time of surgical treatment was 5.2 months (range 1.4 to 6.0 months), and only 2 patients were less than 3 months of age at the time of surgery. The most common presenting clinical signs were bulging fontanelle, hydrocephalus, or macrocephaly, occurring in 7 patients. Vomiting was seen in 5 patients, irritability or lethargy in 5 patients, seizures in 3 patients, and cranial nerve palsies in 1 patient. One patient (#5) was noted to have hydrocephalus on prenatal ultrasound (Table 1).
Table 1.
Clinical Information on Subjects
| Patient | Age at Surgery (months) |
Presenting Symptoms and Signs |
Tumor Location | Diagnosis | Extent of Resection | Adjuvant therapy |
Age at Death or Last Follow- up (years) |
Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.6 | Irritability, frontal bossing | 3rd ventricle with extension into lateral ventricles | Choroid plexus papilloma | GTR | None | 0.9 | Difficulty cruising and standing |
| 2 | 3.8 | Not available | Superior cerebellar and pineal region | Atypical teratoid/rhabdoid tumor | GTR | None | 0.5 | Deceased |
| 3 | 5.6 | Seizures, bulging fontanelle | R temporal lobe | Desmoplastic infantile ganglioglioma | STR (>90%) | VP shunt | 7.5 | Partial R cranial nerve III palsy, L hemiparesis, aggressive behavior and learning difficulties |
| 4 | 5.7 | Vomiting, irritability | L lateral ventricle | Choroid plexus carcinoma | STR (>90%) | Chemotherapy | 1.9 | Deceased |
| 5 | 2.1 | Hydrocephalus on fetal ultrasound | Hypothalamic/suprasellar region | Ganglioglioma | STR (>70%) | B VP shunt Chemotherapy | 4.8 | Precocious puberty, seizures |
| 6 | 5.2 | Hydrocephalus, limited gaze, increased tone | L parietal/occipital lobe | Anaplastic astrocytoma | STR (>70%) | VP shunt Chemotherapy | 8.4 | Seizures, spastic gait, B adduction palsies, severe cognitive and developmental delay |
| 7 | 5.9 | Vomiting, irritability, hydrocephalus | L lateral ventricle | Choroid plexus papilloma | Intraoperative death | None | 0.5 | Deceased (intraoperative death) |
| 8 | 6 | Vomiting, large fontanelle | Pineal region | Atypical teratoid/rhabdoid tumor | STR (>90%) | VP shunt Chemotherapy | 1.5 | Deceased |
| 9 | 3.5 | Not available | Pineal region | PNET | STR (>70%) | None | 1.1 | Deceased |
| 10 | 4.0 | Not available | B cerebellum | Anaplastic ganglioglioma | STR (>90%) | None | 8.9 | Dysarthric speech, nystagmus, significant learning problems (requires classroom aide) |
| 11 | 5.6 | Vomiting, multiple cranial nerve deficits | R cerebellar-pontine angle | Atypical teratoid/rhabdoid tumor | Biopsy | None | 0.5 | Deceased |
| 12 | 3.9 | Lethargy, strabismus, herniation | R cerebral hemisphere | Glioblastoma multiforme | Intraoperative death | None | 0.3 | Deceased, (intra operative death, herniation prior to resection) |
| 13 | 5.7 | Not available | R hippocampal region extending to lateral basal ganglia and thalamus | Pilocytic astrocytoma | STR (>70%) | Chemotherapy | 6.9 | L spastic hemiparesis |
| 14 | 5.2 | Not available | Cerebellar vermis | Medulloblastoma | STR (>90%) | VP shunt Chemotherapy Radiation (proton) | 1.2 | Does not crawl, cruise or stand, few spoken words, little developmental progress |
| 15 | 4.4 | Partial seizures with staring, apnea, cyanosis | R temporal, occipital, and posterior parietal lobes | Fibrillary astrocytoma | GTR* | None | 2.1 | Seizure free, mild L hemiparesis, no behavioral problems |
| 16 | 1.4 | Irritability, bulging fontanelle | B cerebellum | PNET (medullo-myoblastoma) | GTR | VP shunt | 0.5 | Deceased |
After initial surgery, post-operative day 1 MRI revealed residual enhancement. The patient underwent additional surgery within weeks and post-operative MRI demonstrated gross total resection.
GTR, gross total resection; STR, subtotal resection; VP, ventriculoperitoneal; R, right; L, left; B, bilateral
Pre-treatment imaging
Eleven tumors were supratentorial, and 5 were infratentorial. Specific tumor locations are presented in Table 1. Figure 1 and Figure 2 show examples of pre-treatment imaging.
Figure 1.
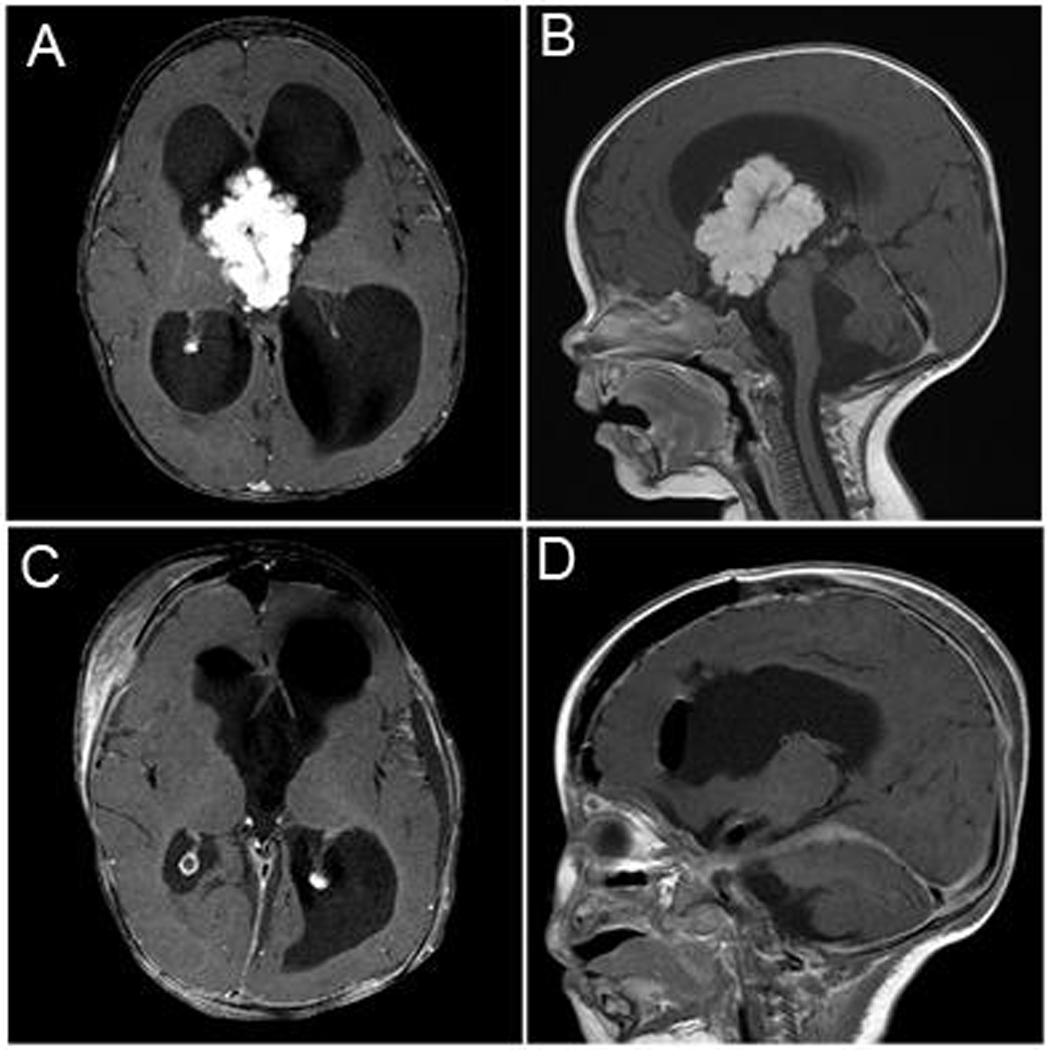
Patient 1 was a 5 month-old girl that presented with increased head size and neck pain and stiffness. Axial (A) and sagittal (B) post-gadolinium T1 MR images demonstrated a large enhancing intraventricular mass with significant hydrocephalus. The patient underwent a craniotomy and resection. Post-operative axial (C) and coronal (D) post-gadolinium T1 imaging demonstrated gross total resection of the mass. On pathology the patient was found to have a choroid plexus papilloma.
Figure 2.
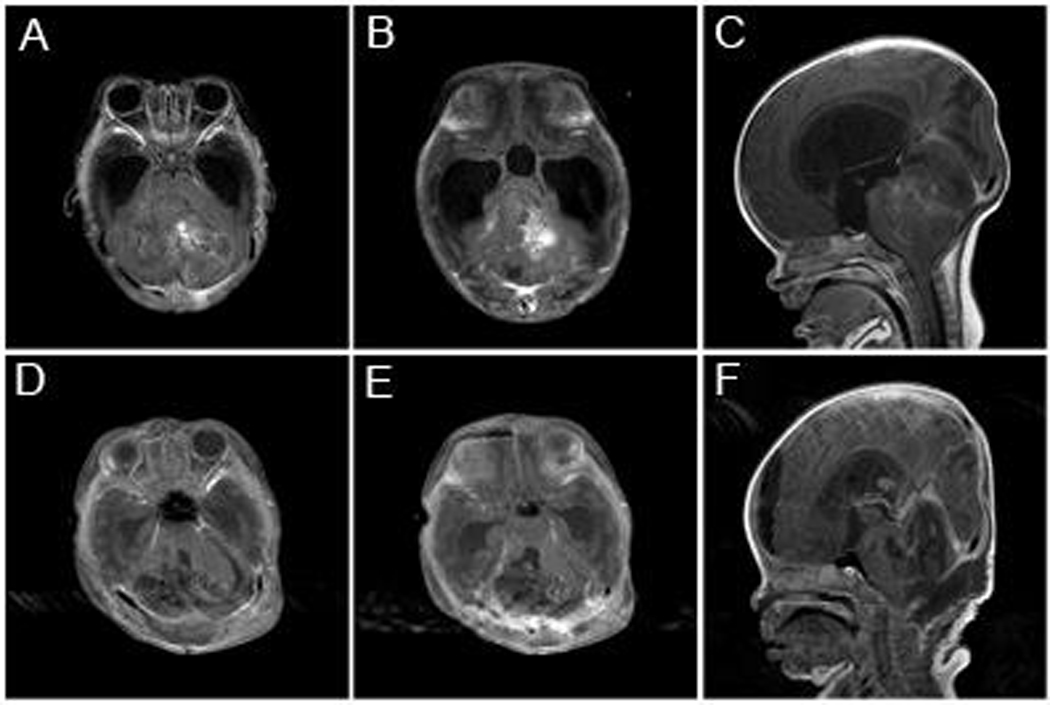
Patient 16 was a 1 month-old girl that presented with increased head size and fussiness. Axial (A and B) and sagittal (C) post-gadolinium T1 MR images a demonstrated a large enhancing posterior fossa mass with significant hydrocephalus. The patient underwent a suboccipital craniotomy and resection. Post-operative axial (D and E) and sagittal (F) post-gadolinium T1 images demonstrated resection of the mass. On pathology the patient was found have a medulloblastoma with myoblastic differentiation.
Treatment
The patients were evaluated and treated by an interdisciplinary team as clinically indicated including pediatric neurosurgeons, neuroradiologists, neuropathologists, neurologists, neuro-oncologists, and radiation oncologists. All patients underwent surgical resection of their tumors with subsequent histopathological evaluation. Post-operatively all patients were monitored in the intensive care unit. Four patients (25%) had gross total surgical resection, nine patients (56%) had a subtotal surgical resection, and one patient (6%) had a biopsy. Two patients (13%) did not have a post-operative MRI because of intraoperative death. Seven patients (44%) received post-operative chemotherapy. One patient (6%) underwent proton beam therapy at another facility.
Pathology
Table 1 shows the pathological features of the sixteen patients’ tumors.
Survival and neurological outcomes
Follow-up data was available in all patients. The median follow-up time was 0.9 years (interquartile range 0.3 years to 5.5 years). The median survival among the subjects was 1.4 years. Using the Kaplan-Meier method, the 1-year, 2-year, and 5-year survival were 53%, 45%, 45%, respectively (Figure 3). There were 8 deaths that occurred at a median of 6.4 months of age (interquartile range 5.9 to 15.5 months) and a median of 3.9 months from surgical resection (interquartile range 0.2 to 10.7 months). Two infants died intraoperatively, one of whom presented with herniation and was operated upon emergently. The other 6 deaths were a result of tumor progression. Of the 8 surviving patients, all are in continuous remission (as demonstrated by yearly MRIs) at a median age of 5.9 years (interquartile range 1.6–8 years). Of the four patients in whom a gross total resection was achieved, two continue to be tumor-free; the other two patients (both with atypical rhabdoid teratoid tumors) died of progression of recurrent tumor (Table 1). All 8 surviving patients had neurological sequelae. Deficits and their severity varied, ranging from mild hemiparesis to severe motor deficits with cognitive and behavioral problems (Table 1).
Figure 3.
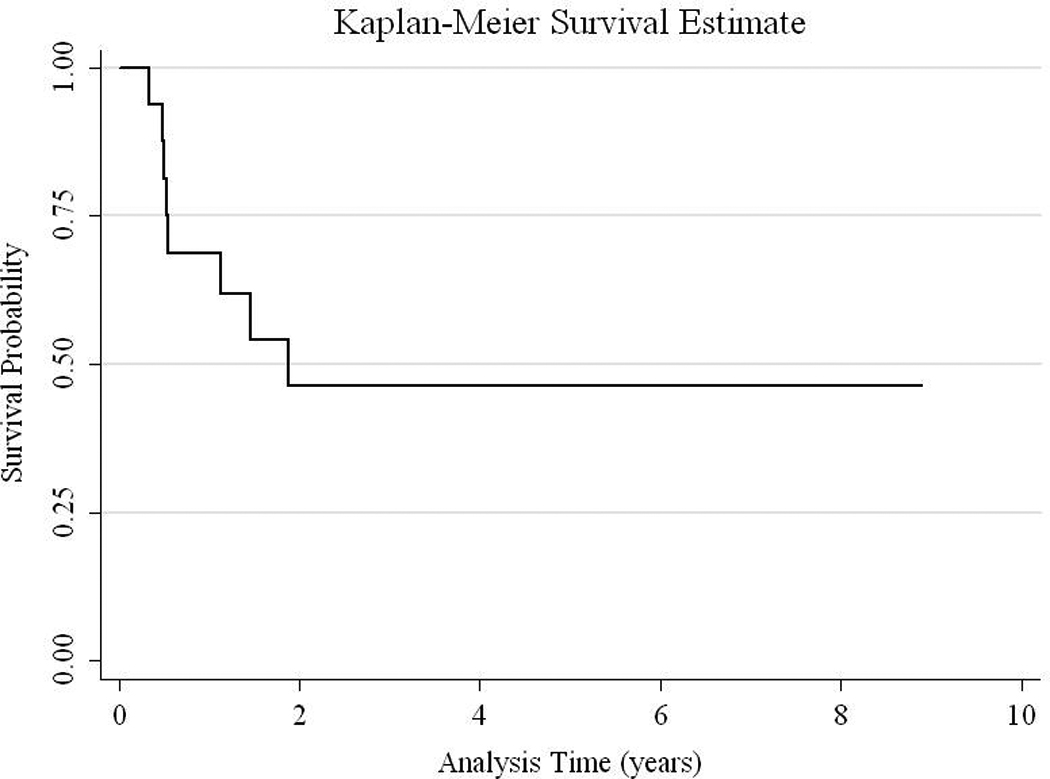
Kaplan-Meier Survival Curve
Literature review
One hundred twenty-three papers were reviewed and 44 papers that were suitable for the current study were identified. These included 29 case reports or series and 15 retrospective studies. The number of patients included in each reviewed paper ranged from a single patient in a case report to 250 patients in a literature review24,25. These 44 papers yielded 468 cases of intracranial tumors in children 6 months of age and less1,3–5,8–10,12,13,15,17–19,21,26,27,29–31,33–35,37,38,40–46,51–55,57–59,61,62,65–67. There was significant variability in brain tumor histologies (Table 2). Teratoma was the most common tumor reported in the existing literature and was mainly found in neonates within a few weeks of life; up to 83.7% of these patients died. The next most common tumor types were choroid plexus tumors and astrocytomas. The estimated survival of the 445 subjects who had follow-up data available was 30.8% (Table 2).
Table 2.
Literature Review Tumor Types and Survival
| Tumor Type | Number (%) * | Living | Dead | Percent Survival § |
|---|---|---|---|---|
| Astroblastoma | 2 (0.4%) | 1 | 1 | 50 |
| Astrocytoma (most grades not specified) | 85 (18.2%) | 34 | 48 3 additional lost to follow-up |
40 |
| Atypical teratoid rhabdoid tumor | 11 (2.4%) | 0 | 11 | 0 |
| Craniopharyngioma | 20 (4.3%) | 4 | 16 | 20 |
| Choroid plexus papilloma | 38 (8.1%) | 33 | 4 1 additional lost to follow-up |
87 |
| Choroid plexus carcinoma | 24 (5.1%) | 10 | 14 | 42 |
| Desmoplastic infantile ganglioglioma | 2 (0.4%) | 2 | 0 | 100 |
| Ependymoma | 16 (3.4%) | 2 | 13 1 additional lost to follow-up |
12.5 |
| Ependymoblastoma | 4 (0.9%) | 0 | 4 | 0 |
| Ganglioglioma | 8 (1.7%) | 8 | 0 | 100 |
| Gangliocytoma | 3 (0.6%) | 3 | 0 | 100 |
| Glioma (most grades not specified) | 6 (1.3%) | 3 | 3 | 50 |
| Glioblastoma multiforme | 23 (4.9%) | 3 | 20 | 13 |
| Gliosarcoma | 3 (0.6%) | 1 | 1 1 additional lost to follow-up |
33 |
| Hemangioblastoma | 2 (0.4%) | 1 | 1 | 50 |
| Medulloblastoma | 22 (4.7%) | 2 | 17 3 additional lost to follow-up |
9 |
| Medulloepithelioma | 4 (0.9%) | 0 | 4 | 0 |
| Meningioma | 8 (1.7%) | 5 | 3 | 62.5 |
| Meningeal sarcoma | 8 (1.7%) | 2 | 6 | 25 |
| Oligodendroglioma | 3 (0.6%) | 1 | 2 | 33 |
| Oligoastrocytoma | 1 (0.2%) | 1 | 0 | 100 |
| Pineoblastoma | 3 (0.6%) | 0 | 2 1 additional lost to follow-up |
0 |
| Primitive neuroectodermal tumor | 44 (9.4%) | 6 | 36 2 additional lost to follow-up |
13.6 |
| Sarcoma | 3 (0.6%) | 0 | 2 1 additional lost to follow-up |
0 |
| Subependymal giant cell astrocytoma | 2 (0.4%) | 1 | 1 | 50 |
| Spongioblastoma | 4 (0.9%) | 0 | 4 | 0 |
| Teratoma | 98 (20.9%) | 16 | 78 4 additional lost to follow-up |
16.3 |
| Miscellaneous ** | 21 (4.5%) | 5 | 10 6 additional lost to follow-up |
23.8 |
| TOTAL | 468 (100%) | 144 alive | 301 dead 23 lost to follow-up |
30.8 |
Percent of total cases
9 choroid plexus tumors not otherwise specified, 1 atypical choroid plexus papilloma, 1 middle fossa neuroblastoma,1 gliofibroma, 1 malignant embryonic tumor, 1 plexus tumor, 1 oligodendrocytoma, 1 midbrain cystic blastoma, 1 melanotic prognoma, 1 hemangioendothelioma, 3 not otherwise specified
Patients lost to follow-up were not included in the calculation of percentage survival.
Discussion
Location of tumors in infants less than 6 months does not appear to follow the trends seen in older pediatric populations. In our series, nearly 70% of tumors were located supratentorially, a finding consistent with prior reports55. This contrasts with older pediatric patients in whom infratentorial tumors are more common7,11,22–25,32,39,40,56. As with older children, the histopathological diagnoses varied widely; however, there was a higher incidence of atypical teratoid rhabdoid tumors and choroid plexus tumors.
Our patients had a 5-year survival of 45%, compared with an estimated survival of 30.8% in the literature review. However, follow-up time was not available for all the subjects in the literature review, limiting our ability to calculate a 5-year survival for the patients represented in the literature and thus to compare the survival in the literature to that of our patients. Additionally, in contrast to prior studies55 our study had no subjects with teratoma, a tumor type with high mortality. The absence of teratoma patients may explain the higher survival of our patient population compared with the literature. However, since our selection of patients utilized a surgical database, it is possible that infants with teratoma may have been missed if no surgical treatment was indicated or elected due to poor prognosis, thereby inflating the survival estimate. Additionally, our series had 5 patients with low-grade tumors which have the highest survival of the infantile brain tumors. Strengths of this study include its relatively long follow-up compared with historical data. In addition, given that all patients presented since 1998, it represents a relatively modern surgical series.
Developmental and neurological outcomes in very young children with tumors are variable. All surviving children in our series had residual neurological or developmental deficits at follow-up. However, the degree of disability varied widely between patients. Post-operative and long-term quality of life is a topic seldom discussed in the neurosurgical literature. However, neurological sequelae are important. Compared to other pediatric tumor patients and even to adult brain tumor patients, the pediatric brain tumor population faces significantly more devastating neurological, psychosocial, and economic effects63. Further characterization of the neuro-cognitive deficits of this population as the children enter school, utilizing formal neuro-psychological testing, will be crucial to characterizing the scope of issues facing survivors of infant brain tumors.
The primary mode of treatment for infantile brain tumors remains surgery, although the prognosis for many of these tumors remains poor. Importantly, in our experience it can be difficult to predict the tumor type based on the preoperative imaging for some of these young patients, necessitating surgical treatment to establish the diagnosis and in some cases to provide definitive treatment. The use of adjuvant chemotherapy and its effects on outcome need to be explored further, especially since radiation therapy is not usually an option in infants16. Re-operating on stable residual masses has also been questioned by some authors, citing that a “wait and see” method may be better than subjecting the child to a second operation6. It is our practice to determine the role for reoperation based on the tumor histology and the neurological status of the child. As more data for this patient population becomes available, the characterization and best treatment strategies will become further refined. Given the many advancements and improvements in surgical technique, radiation, and chemotherapy options, the population of pediatric brain tumor survivors continues to grow. The advent and increasing availability of proton beam radiation, with the goal of increasing survival while minimizing neurological repercussions, may allow for the use of radiotherapy in patients younger than was previously believed to be safe. A thorough understanding of the residual neurological deficits and quality of life in survivors is required in order to improve the care of these patients. More research is being dedicated to treatment-related effects, such as the long-term impact of focal proton beam radiation on the growing brain and body2,16,20,49,60,64. Larger series will be necessary to correlate outcome with tumor type, extent of resection, and/or response to adjuvant therapies.
Abbreviations
- R
right
- L
left
- B
bilateral
- PNET
primitive neuroectodermal tumor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has any disclosures or conflicts of interest.
References
- 1.Alexiou GA, Stefanaki K, Sfakianos G, Prodromou N. Desmoplastic infantile ganglioglioma: a report of 2 cases and a review of the literature. Pediatr Neurosurg. 2008;44:422–425. doi: 10.1159/000149913. [DOI] [PubMed] [Google Scholar]
- 2.Allen JC. What infants can teach us about brain tumors. N Engl J Med. 1993;328:1780–1781. doi: 10.1056/NEJM199306173282410. [DOI] [PubMed] [Google Scholar]
- 3.Araki K, Aori T, Takahashi JA, Nozaki K, Nagata I, Kikuchi H, Yokoyama M, Hattori H, Akiyama Y, Kubota Y, Yokomizo H. [A case report of choroid plexus carcinoma] No Shinkei Geka. 1997;25:853–857. [PubMed] [Google Scholar]
- 4.Arico M, Raiteri E, Bossi G, Giordana MT, Corbella F, Locatelli D, Pezzotta S. Choroid plexus carcinoma: report of one case with favourable response to treatment. Med Pediatr Oncol. 1994;22:274–278. doi: 10.1002/mpo.2950220412. [DOI] [PubMed] [Google Scholar]
- 5.Arnstein LH, Boldrey E, Naffziger HC. A case report and survey of brain tumors during the neonatal period. J Neurosurg. 1951;8:315–319. doi: 10.3171/jns.1951.8.3.0315. [DOI] [PubMed] [Google Scholar]
- 6.Benesch M, Eder HG, Sovinz P, Raith J, Lackner H, Moser A, Urban C. Residual or recurrent cerebellar low-grade glioma in children after tumor resection: is re-treatment needed? A single center experience from 1983 to 2003. Pediatr Neurosurg. 2006;42:159–164. doi: 10.1159/000091859. [DOI] [PubMed] [Google Scholar]
- 7.Blaney SMH-KD, Poussaint TY, Santi M, Gibertson R, Parsons D, Pollack I, editors. Principles and Practice of Pediatric Oncology, ed 6th. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 8.Borch K, Jacobsen T, Olsen JH, Hirsch F, Hertz H. Neonatal cancer in Denmark 1943–1985. Pediatr Hematol Oncol. 1992;9:209–216. doi: 10.3109/08880019209016588. [DOI] [PubMed] [Google Scholar]
- 9.Boyd MC, Steinbok P. Choroid plexus tumors: problems in diagnosis and management. J Neurosurg. 1987;66:800–805. doi: 10.3171/jns.1987.66.6.0800. [DOI] [PubMed] [Google Scholar]
- 10.Carstensen H, Juhler M, Bogeskov L, Laursen H. A report of nine newborns with congenital brain tumours. Childs Nerv Syst. 2006;22:1427–1431. doi: 10.1007/s00381-006-0115-6. [DOI] [PubMed] [Google Scholar]
- 11.CBTRUS: Central Brain Tumor Registry of the United States. Primary brain tumors in the United States statistical report 2000–2004, in, 2007–2008 [Google Scholar]
- 12.Chadduck WM, Gollin SM, Gray BA, Norris JS, Araoz CA, Tryka AF. Gliosarcoma with chromosome abnormalities in a neonate exposed to heptachlor. Neurosurgery. 1987;21:557–559. doi: 10.1227/00006123-198710000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Chung SK, Wang KC, Nam DH, Cho BK. Brain tumor in the first year of life: a single institute study. Journal of Korean Medical Science. 1998;3:65–70. doi: 10.3346/jkms.1998.13.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen BH, Packer RJ, Siegel KR, Rorke LB, D'Angio G, Sutton LN, Bruce DA, Schut L. Brain tumors in children under 2 years: treatment, survival and long-term prognosis. Pediatric Neurosurgery. 1993;19:171–179. doi: 10.1159/000120727. [DOI] [PubMed] [Google Scholar]
- 15.de Leon-Bojorge B, Rueda-Franco F, Anaya-Jara M. Central nervous system atypical teratoid rhabdoid tumor: experience at the National Institute of Pediatrics, Mexico City. Childs Nerv Syst. 2008;24:307–312. doi: 10.1007/s00381-007-0464-9. [DOI] [PubMed] [Google Scholar]
- 16.Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, Sanford RA, Mulhern RK, James HE, Freeman CR, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 17.Fenton LZ, Foreman NK. Atypical teratoid/rhabdoid tumor of the central nervous system in children: an atypical series and review. Pediatr Radiol. 2003;33:554–558. doi: 10.1007/s00247-003-0934-5. [DOI] [PubMed] [Google Scholar]
- 18.Galassi E, Godano U, Cavallo M, Donati R, Nasi MT. Intracranial tumors during the 1st year of life. Childs Nerv Syst. 1989;5:288–298. doi: 10.1007/BF00274516. [DOI] [PubMed] [Google Scholar]
- 19.Gianella-Borradori A, Zeltzer PM, Bodey B, Nelson M, Britton H, Marlin A. Choroid plexus tumors in childhood. Response to chemotherapy, and immunophenotypic profile using a panel of monoclonal antibodies. Cancer. 1992;69:809–816. doi: 10.1002/1097-0142(19920201)69:3<809::aid-cncr2820690333>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Gottardo NG, Gajjar A. Chemotherapy for malignant brain tumors of childhood. J Child Neurol. 2008;23:1149–1159. doi: 10.1177/0883073808321765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn JS, Bejar R, Gladson CL. Neonatal subependymal giant cell astrocytoma associated with tuberous sclerosis: MRI, CT, and ultrasound correlation. Neurology. 1991;41:124–128. doi: 10.1212/wnl.41.1.124. [DOI] [PubMed] [Google Scholar]
- 22.Hargrave DR, Zacharoulis S. S: Pediatric CNS tumors: current treatment and future directions. Expert Rev Neurother. 2007;7:1029–1042. doi: 10.1586/14737175.7.8.1029. [DOI] [PubMed] [Google Scholar]
- 23.Heuer GG, Jackson EM, Magge SN, Storm PB. Surgical management of pediatric brain tumors. Expert Rev Anticancer Ther. 2007;7:S61–S68. doi: 10.1586/14737140.7.12s.S61. [DOI] [PubMed] [Google Scholar]
- 24.Isaacs H., Jr I. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol. 2002;27:249–261. doi: 10.1016/s0887-8994(02)00472-1. [DOI] [PubMed] [Google Scholar]
- 25.Isaacs H., Jr II. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol. 2002;27:333–342. doi: 10.1016/s0887-8994(02)00459-9. [DOI] [PubMed] [Google Scholar]
- 26.Itoh Y, Kowada M, Mineura K, Kojima H. Congenital glioblastoma of the cerebellum with cytofluorometric deoxyribonucleic acid analysis. Surg Neurol. 1987;27:163–167. doi: 10.1016/0090-3019(87)90289-8. [DOI] [PubMed] [Google Scholar]
- 27.Jung WH, Choi S, Oh KK, Chi JG. Congenital glioblastoma multiforme--report of an autopsy case. J Korean Med Sci. 1990;5:225–231. doi: 10.3346/jkms.1990.5.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knab B, Connell PP. Radiotherapy for pediatric brain tumors: when and how. Expert Rev Anticancer Ther. 2007;7:S69–S77. doi: 10.1586/14737140.7.12s.S69. [DOI] [PubMed] [Google Scholar]
- 29.Kumabe T, Tominaga T, Kondo T, Yoshimoto T, Kayama T. Intraoperative radiation therapy and chemotherapy for huge choroid plexus carcinoma in an infant--case report. Neurol Med Chir (Tokyo) 1996;36:179–184. doi: 10.2176/nmc.36.179. [DOI] [PubMed] [Google Scholar]
- 30.Kwinta P, Kwiatkowski S, Tomasik T, Grudzien A, Korab-Chrzanowska E, Adamek D, et al. [Congenital brain tumor in neonate--case report and review of literature] Przeglad Lekarski. 2005;62:1302–1307. [PubMed] [Google Scholar]
- 31.Lalitha VS, Rubinstein LJ. Reactive glioma in intracranial sarcoma: a form of mixed sarcoma and glioma ("sarcoglioma"): report of eight cases. Cancer. 1979;43:246–257. doi: 10.1002/1097-0142(197901)43:1<246::aid-cncr2820430136>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Larouche V, Huang A, Bartels U, Bouffet E. Tumors of the central nervous system in the first year of life. Pediatr Blood Cancer. 2007;49:1074–1082. doi: 10.1002/pbc.21351. [DOI] [PubMed] [Google Scholar]
- 33.Lasky JL, Choi EJ, Johnston S, Yong WH, Lazareff J, Moore T. Congenital brain tumors: case series and review of the literature. J Pediatr Hematol Oncol. 2008;30:326–331. doi: 10.1097/MPH.0b013e3181647bf0. [DOI] [PubMed] [Google Scholar]
- 34.Mapstone TB, Warf BC. Intracranial tumor in infants: characteristics, management, and outcome of a contemporary series. Neurosurgery. 1991;28:343–348. [PubMed] [Google Scholar]
- 35.Marsh JS. Supratentorial glioblastoma multiforme in infancy; report of three cases occurring before the age of one. Bull Los Angel Neuro Soc. 1956;21:30–36. [PubMed] [Google Scholar]
- 36.Mazewski CM, Hudgins RJ. Neonatal brain tumors: a review. Semin Perinatol. 1999;23:286–298. doi: 10.1016/s0146-0005(99)80037-8. [DOI] [PubMed] [Google Scholar]
- 37.Medhkour A, Traul D, Husain M. Neonatal subependymal giant cell astrocytoma. Pediatr Neurosurg. 2002;36:271–274. doi: 10.1159/000058432. [DOI] [PubMed] [Google Scholar]
- 38.Mehrotra N, Shamji MF, Vassilyadi M, Ventureyra EC. Intracranial tumors in first year of life: the CHEO experience. Childs Nerv Syst. 2009;25:1563–1569. doi: 10.1007/s00381-009-0936-1. [DOI] [PubMed] [Google Scholar]
- 39.Merchant TE, Pollack IF, Loeffler JS. Brain tumors across the age spectrum: biology, therapy, and late effects. Semin Radiat Oncol. 2010;20:58–66. doi: 10.1016/j.semradonc.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nejat F, El Khashab M, Rutka JT. Initial management of childhood brain tumors: neurosurgical considerations. J Child Neurol. 2008;23:1136–1148. doi: 10.1177/0883073808321768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newbould MJ, Kelsey AM, Arango JC, Ironside JW, Birch J. The choroid plexus carcinomas of childhood: histopathology, immunocytochemistry and clinicopathological correlations. Histopathology. 1995;26:137–143. doi: 10.1111/j.1365-2559.1995.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 42.Packer RJ, Perilongo G, Johnson D, Sutton LN, Vezina G, Zimmerman RA, Ryan J, Reaman G, Schut L. Choroid plexus carcinoma of childhood. Cancer. 1992;69:580–585. doi: 10.1002/1097-0142(19920115)69:2<580::aid-cncr2820690250>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Papadakis N, Millan J, Grady DF, Segerberg LH. Medulloblastoma of the neonatal period and early infancy. Report of two cases. J Neurosurg. 1971;34:88–91. doi: 10.3171/jns.1971.34.1.0088. [DOI] [PubMed] [Google Scholar]
- 44.Parkes SE, Muir KR, Southern L, Cameron AH, Darbyshire PJ, Stevens MCG. Neonatal Tumors. A Thirty-Year Population-Based Study. Medical and Pediatric Oncology. 1994;22:309–317. doi: 10.1002/mpo.2950220503. [DOI] [PubMed] [Google Scholar]
- 45.Pascual-Castroviejo I, Villarejo F, Perez-Higueras A, Morales C, Pascual-Pascual SI. Childhood choroid plexus neoplasms. A study of 14 cases less than 2 years old. Eur J Pediatr. 1983;140:51–56. doi: 10.1007/BF00661906. [DOI] [PubMed] [Google Scholar]
- 46.Raimondi AJ, Tomita T. Brain tumors during the first year of life. Childs Brain. 1983;10:193–207. doi: 10.1159/000120114. [DOI] [PubMed] [Google Scholar]
- 47.Reddy AT. Advances in biology and treatment of childhood brain tumors. Curr Neurol Neurosci Rep. 2001;1:137–143. doi: 10.1007/s11910-001-0009-7. [DOI] [PubMed] [Google Scholar]
- 48.Rivera-Luna R, Medina-Sanson A, Leal-Leal C, Pantoja-Guillen F, Zapata-Tarres M, Cardenas-Cardos R, Barrera-Gómez R, Rueda-Franco F. Brain tumors in children under 1 year of age: emphasis on the relationship of prognostic factors. Childs Nerv Syst. 2003;19:311–314. doi: 10.1007/s00381-003-0738-9. [DOI] [PubMed] [Google Scholar]
- 49.Rivera-Luna R, Zapata-Tarres M, Medina-Sanson A, Lopez-Aguilar E, Niembro-Zuniga A, Amador Zarco J, Marhx-Bracho A, Rueda-Franco F, Bornstein-Quevedo L. Longterm survival in children under 3 years of age with low-grade astrocytoma. Childs Nerv Syst. 2007;23:543–547. doi: 10.1007/s00381-006-0287-0. [DOI] [PubMed] [Google Scholar]
- 50.Rutka JT, Kuo JS. Pediatric surgical neuro-oncology: current best care practices and strategies. J Neurooncol. 2004;69:139–150. doi: 10.1023/b:neon.0000041877.14749.b6. [DOI] [PubMed] [Google Scholar]
- 51.Sabet LM. Congenital glioblastoma multiforme associated with congestive heart failure. Arch Pathol Lab Med. 1982;106:31–34. [PubMed] [Google Scholar]
- 52.Sacrez R, Juif JG, Friederich E. [Cerebral tumor in infant.] Arch Fr Pediatr. 1954;11:261–263. [PubMed] [Google Scholar]
- 53.Sakamoto K, Kobayashi N, Ohtsubo H, Tanaka Y. Intracranial tumors in the first year of life. Childs Nerv Syst. 1986;2:126–129. doi: 10.1007/BF00270839. [DOI] [PubMed] [Google Scholar]
- 54.Sato O, Tamura A, Sano K. Brain tumors of early infants. Childs Brain. 1975;1:121–125. doi: 10.1159/000119560. [DOI] [PubMed] [Google Scholar]
- 55.Serowka K, Chiu Y, Gonzalez I, Gilles F, McComb G, Krieger M, et al. Central nervous system (CNS) tumors in the first six months of life: the Children's Hospital Los Angeles experience, 1979–2005. Pediatr Hematol Oncol. 27:90–102. doi: 10.3109/08880010903447342. [DOI] [PubMed] [Google Scholar]
- 56.Severino M, Schwartz ES, Thurnher MM, Rydland J, Nikas I, Rossi A. Congenital tumors of the central nervous system. Neuroradiology. 52:531–548. doi: 10.1007/s00234-010-0699-0. [DOI] [PubMed] [Google Scholar]
- 57.Shamji MF, Vassilyadi M, Lam CH, Montes JL, Farmer JP. Congenital tumors of the central nervous system: the MCH experience. Pediatr Neurosurg. 2009;45:368–374. doi: 10.1159/000257526. [DOI] [PubMed] [Google Scholar]
- 58.Shimamura N, Asano K, Ogane K, Yagihashi A, Ohkuma H, Suzuki S. A case of definitely congenital glioblastoma manifested by intratumoral hemorrhage. Childs Nerv Syst. 2003;19:778–781. doi: 10.1007/s00381-003-0807-0. [DOI] [PubMed] [Google Scholar]
- 59.Singhal RM, Jain M, Tanwar RK, Raman NV. Congenital glioblastoma. Indian J Pediatr. 1994;61:733–736. doi: 10.1007/BF02751992. [DOI] [PubMed] [Google Scholar]
- 60.Skowronska-Gardas A. Evaluation of radiotherapy for pediatric CNS tumors. Expert Rev Neurother. 2003;3:491–500. doi: 10.1586/14737175.3.4.491. [DOI] [PubMed] [Google Scholar]
- 61.Takaku A, Kodama N, Ohara H, Hori S. Brain tumor in newborn babies. Childs Brain. 1978;4:365–375. doi: 10.1159/000119793. [DOI] [PubMed] [Google Scholar]
- 62.Thiele RL, Dimmick MJ. Intracranial malignant neoplasm with onset before birth. J Pediatr. 1951;39:611–615. doi: 10.1016/s0022-3476(51)80184-7. [DOI] [PubMed] [Google Scholar]
- 63.Turner CD, Rey-Casserly C, Liptak CC, Chordas C. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24:1455–1463. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- 64.Ward C, Phipps K, de Sousa C, Butler S, Gumley D. Treatment factors associated with outcomes in children less than 3 years of age with CNS tumours. Childs Nerv Syst. 2009;25:663–668. doi: 10.1007/s00381-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 65.Xue H, Horwitz JR, Smith MB, Lally KP, Black CT, Cangir A, Takahashi H, Andrassy RJ. Malignant solid tumors in neonates: a 40-year review. J Pediatr Surg. 1995;30:543–545. doi: 10.1016/0022-3468(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 66.Yang C-P, Hung I-J, Jaing T-H, Shih L-Y, Chang W-H. Cancer in infants: a review of 82 cases. Pediatric Hematology & Oncology. 2005;22:463–481. doi: 10.1080/08880010591002233. [DOI] [PubMed] [Google Scholar]
- 67.Young HK, Johnston H. Intracranial tumors in infants. J Child Neurol. 2004;19:424–430. doi: 10.1177/088307380401900605. [DOI] [PubMed] [Google Scholar]


