Abstract
The content of prospective memory is comprised of representations of an action to perform in the future. When people form prospective memories, they temporarily put the memory representation in an inactive state while engaging in other activities, and then activate the representation in the future. Ultimately, successful activation of the memory representation yields an action at an appropriate, but temporally distant, time. A hallmark of prospective memory is that activation of the memory representation has a deleterious effect on current ongoing activity. Recent evidence suggests that scrub jays and non-human primates, but not other species, are capable of future planning. We hypothesized that prospective memory produces a selective deficit in performance at the time when rats access a memory representation but not when the memory representation is inactive. Rats were trained in a temporal bisection task (90 min/day). Immediately after the bisection task, half of the rats received an 8-g meal (meal group) and the other rats received no additional food (no-meal group). Sensitivity to time in the bisection task was reduced as the 90-min interval elapsed for the meal group but not for the no-meal group. This time-based prospective-memory effect was not based on response competition, an attentional limit, anticipatory contrast, or fatigue. Our results suggest that rats form prospective memories, which produces a negative side effect on ongoing activity.
Keywords: prospective memory, prospection, comparative cognition, animal model, rat
Introduction
Prospective memory refers to the ability to remember to do something in the future (McDaniel and Einstein 2007). In prospective memory, the representation of the future action is inactive for a period of time while one is engaged in other activities. Time-based prospective memory is the ability to retrieve the representation at an appropriate time in the future (McDaniel and Einstein 2007; Einstein and McDaniel 1990); that is “remembering to remember” (Harris 1984). The hallmark of prospective memory is that a deleterious effect on ongoing behavior appears as the time to execute draws near due to greater attentional resources being diverted to the now active prospective memory (Hicks et al. 2005; Marsh et al. 2006; Smith 2003; Smith et al. 2007). This concept is introduced with an example that outlines several basic features of prospective memory (Marsh et al. 1998). Frequently, important actions cannot always be acted upon now, but rather only at some temporally distant point. For example, when a parent must remember to pick up his child from daycare at the end of the day, the intention to act is inactive throughout the day (i.e., it is not actively rehearsed). Yet the intention needs to be activated at a future time to perform the action. As the appropriate time approaches, it becomes difficult to continue to engage in ongoing activities presumably because more cognitive resources are diverted to processing the prospective memory representation at the appropriate time. For example, Kliegel, Martin, McDaniel, and Einstein (2001) asked participants to perform an action (pressing a key) in the future while engaged in an ongoing task (rating words along a number of dimensions). An analysis of errors on the ongoing task suggested that errors occurred more frequently near the time at which an imminent prospective response was required, particularly if the prospective task was important. The participants also engaged in a prospective-monitoring behavior more frequently during time periods that were closer to the time at which the prospective response was required, particularly if the prospective task was important. Kliegel et al. concluded that increasing the importance of the prospective memory task reduced the attentional resources allocated towards the ongoing task, which thereby decreased ongoing-task performance.
We hypothesized that if rats possess prospective memory then a selective deficit in performance is expected at the time when anticipation of a future event is greatest. Our approach was to determine if anticipating a future event would produce a deleterious effect on ongoing activity, specifically at a later time when the representation of the event is most likely to be active. Whether animals plan for the future is controversial (Roberts and Feeney 2009; Suddendorf and Corballis 1997; Suddendorf and Corballis 2007). Recent evidence suggests that scrub jays (Correia et al. 2007; Raby et al. 2007) and non-human primates (Mulcahy and Call 2006; Naqshbandi and Roberts 2006; Osvath 2009; Osvath and Osvath 2008), but not rats (Naqshbandi and Roberts 2006), are capable of future planning. Although the current study did not directly investigate whether rats plan for the future, we instead asked whether an animal’s on-going behavior is affected by its representation of a future event, and if this effect is exacerbated as the time to that event draws near. This ability need not be planning per se, but may instead be a precursor to planning that may be broadly distributed across species.
To this end, rats were trained in a standard temporal bisection (Church and Deluty 1977) task (90 min/day). In each daily behavioral test session, each trial began with the presentation of two 50-ms noise pulses separated by an interval that ranged from 2 to 8 s. Next, two response levers were inserted into the test chamber, and the rat was required to press one of the levers. A small reward was delivered if the rat pressed one lever (e.g., left) after a 2-s interval or the other lever (e.g., right) after an 8-s interval. Immediately after the bisection task each day, half of the rats received an 8-g meal (meal group) whereas other rats received no additional food (no-meal group). The meal was earned by breaking a photobeam in the food trough, but photobeam breaks were only effective 90-min after the start of the session. We hypothesized that the representation of the event is initially inactive and only becomes active at an appropriate later time (i.e. immediately before the start of the meal). Hence, we measured sensitivity to time in the bisection task at early and late time points. If rats have prospective memory, then we should observe a negative side effect on ongoing activity at the later time point, which is when the representation is most likely to be activated.
We hypothesized that if rats possess prospective memory, then they will both anticipate the start of the meal and show a selective deficit in performance at the time when anticipation of the meal is greatest. Of course, there are many examples of timing intervals in the literature. Because timing a fixed interval is retrospective with respect to the event that initiated the timing episode (Church 1978), a demonstration of fixed-interval timing alone (in this study or in many studies from the literature) would not be a sufficient basis to propose prospective memory. By contrast, prospective memory would be documented by a selective deficit in performance when a future event is anticipated.
Materials and methods
Animals
Twenty male Sprague Dawley rats (Rattus norvegicus; Harlan, Madison, WI; 74 days old and 291 g, on average, at the start of the experiment) were individually housed with light offset and onset in the colony at 7 a.m. and 7 p.m. EST, respectively. They received 45-mg pellets (F0165; Bio-Serv, Frenchtown, NJ) during experimental sessions and 5001-Rodent-Diet (Lab Diet, Brentwood, MO) approximately 2 h after completing each session, adjusted to maintain total daily consumption at 15 g. Water was available ad libitum. All procedures were approved by the University of Georgia institutional animal care and use committee and followed the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals.
Apparatus
The behavioral testing chambers, described elsewhere (Crystal and Baramidze 2007; Crystal et al. 2003), had two retractable levers. A 45-mg pellet dispenser positioned outside the chamber was attached to a food trough, and each pellet delivery was accompanied by an audible click. A photobeam located 1 cm inside the food trough (1.5 cm from the trough bottom) detected head entries. A response was registered at the time that the photobeam was interrupted. The photobeam interruption had to end before the next response was registered; the duration of photobeam breaks was not recorded. An audio generator delivered white noise (70 dB) to a speaker. A water bottle was placed outside of the chamber with the tube inserted across from the food cup. In a nearby room, two computers running Med-PC for Windows (Version 4.0) controlled experimental events and recorded the time at which each event occurred with 10-ms accuracy.
Procedure
The rats were tested once per day beginning at approximately 9:00 a.m. and 11:00 a.m. for subsets of animals, 5 days per week. Initial pretraining consisted of one 30-min session in which food was delivered every 60 s, followed by three sessions in which each food pellet was delivered contingent on a single lever press. During lever training, the left lever was inserted until 10 responses occurred, at which point the left lever was retracted and the right lever was inserted. Left and right levers alternated after each additional 10 lever presses until 60 pellets had been earned or 60 min had elapsed. Rats were randomly assigned to testing chambers and meal (n = 10) and no-meal (n = 10) groups prior to the start of the experiment.
In the bisection procedure (90 min per day), a trial consisted of presentation of two 50-ms white noise pulses separated by an interval, insertion of levers, a lever press response, feedback, and an intertrial interval. The interval was initially 2 or 8 s (randomly determined on each trial) for the first 13 daily sessions. One lever (e.g., left) was designated as the correct response after a 2-s interval, and the other lever (e.g., right) was designated as the correct response after an 8-s interval (this arrangement was randomly assigned to each rat prior to the start of the experiment with the constraint that half of the rats had 2–8 short-long assignments and the remaining rats had 2–8 long-short assignments). After levers were inserted, the rat was required to press one lever to obtain feedback. If the rat pressed the correct lever, a single 45-mg pellet was delivered to the food trough. If the rat pressed the incorrect lever, no pellet was delivered. On subsequent sessions (105 days), the first 10 trials were 2 or 8 s (randomly determined) to provide daily practice; in the remaining trials, the interval duration was randomly selected to be 2.00, 2.52, 3.27, 4.00, 5.04, 6.35, or 8.00 s with probabilities of occurrence 0.25, 0.10, 0.10, 0.10, 0.10, 0.10, and 0.25, respectively. Trials with the five intermediate interval values terminated without the delivery of a pellet after either a left or right lever press. Intertrial intervals were randomly selected integers between 45 and 65 s. The bisection procedure terminated 90 min after the start of the daily session, but the rats remained in the box for 30 min as described below.
Meal group
After 90 min of the bisection procedure described above, rats in the meal group had 30 min of access to earn up to 8 g (178 pellets) of food each day. During the meal, each pellet was obtained by interrupting the photobeam in the food trough after a variable interval (exponentially distributed with a mean of 10 s) had elapsed. Breaking the photobeam before 90 min did not produce pellets. The levers were retracted during this period of time, and noise pulses were not presented. Total daily food consumption was 15 g, which was achieved by adjusting the supplemental ration of food in the home cage based on the amount obtained in the chamber.
No-meal group
After 90-min of the bisection procedure described above, rats in the no-meal group remained in the chamber for an additional 30-min, during which no food was delivered. The levers were retracted during this period of time, and noise pulses were not presented. Total daily food consumption was 15 g, which was achieved by adjusting the supplemental ration of food in the home cage based on the amount obtained in the chamber.
Data analysis
Bisection performance was estimated by the probability of a “long” classification response as a function of interval durations. Early and late time points correspond to the first and fourth quartiles (each 20 min) of daily sessions after the initial 10-trials of daily practice. Temporal sensitivity was measured by calculating the slope of the psychophysical function relating the probability of a “long” classification response as a function of interval duration, using durations 3.27, 4.00, and 5.04 s. Latencies were estimated by the median of each distribution. Meal anticipation was estimated by the number of photobeam breaks as a function of time during the first 90-min of each session. Paired scores (number of photobeam responses and slope described above) were obtained from each individual session to calculate a correlation. All analyses were conducted on the last 80 sessions. An effect size is reported for each test that is critical to documenting prospective memory and is measured by η2p (partial eta squared) for analysis of variance by ω2p (partial omega squared) for repeated-measures t-test (Sheskin 2004); each reported value is considered a large effect based on Cohen’s (1988) criteria.
Results and discussion
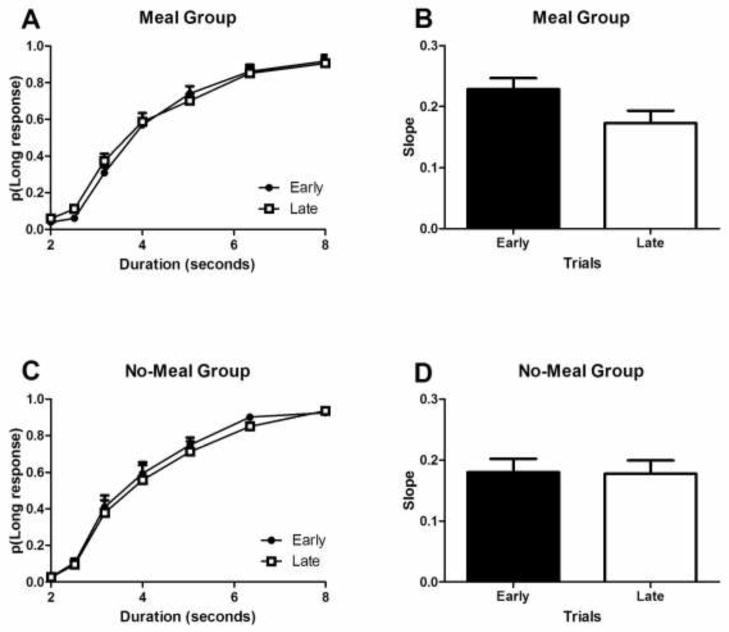
Sensitivity to time in the ongoing task declined near the meal time in the meal group but not in the no-meal group. Fig. 1A shows performance in the ongoing task for the meal group at early and late time points. As expected, the probability of long responses increased as a function of increasing durations (F(6, 54) = 197.0, p < 0.001), and did not differ between early and late time points (F(1, 9) = 2.9, p = 0.1). Importantly, there was also an interaction between the early-late and duration variables (F(6, 54) = 4.7, p < 0.001, η2p = 0.341), which suggests a difference in temporal sensitivity. The slope of the psychophysical function (Fig. 1B) was smaller at the late relative to early time points (t(9) = 3.34, p = 0.009, ω2p = 0.336), indicating a decline in performance as the meal approached (i.e., when it was more likely that the representation of the event was active). To show that the sensitivity decline in the meal group was produced by the approaching meal, we examined bisection performance in the no-meal group. Unlike the meal group, no significant interaction in the proportion long functions (Fig. 1C) was observed (F(6, 54) = 1.9 p = 0.1) in the no-meal group, and the slopes (Fig. 1D) were not significantly different in the no-meal group (t(9) = 0.2, p = 0.8). These observations are consistent with the proposal that sensitivity to time is relatively constant throughout the session when a representation of a meal is absent. Moreover, the magnitude of the difference in early and late slopes was larger in the meal group than in the no-meal group, as documented by a significant interaction of the data shown in Fig. 1B and 1D (F(1,18) = 5.68, p = 0.03, η2p = 0.240). The same conclusion was reached when the probability of long responses was analyzed using data shown in Fig. 1A and 1C; there was a significant three-way interaction between group, early-late, and duration variables (F(6,108) = 3.0, p = 0.009, η2p = 0.145). These data suggest that the approaching meal produced the decline in performance in ongoing activity in the meal group. For the no- meal group, the proportion of long responses also increased as a function of increasing durations (F(6, 54) = 108.1, p < 0.001) but did not differ between early and late time points (F(1,9) = 4.8, p = 0.06) as expected1.
Fig. 1.
Anticipation of a meal reduced sensitivity to time in the ongoing interval-duration classification task near the meal time. Sensitivity to time in the ongoing task declined near the meal time in the meal group but not in the no-meal group. The probability of judging an interval as long (A and C) increased as a function of the interval duration. Sensitivity to time, as measured by the slope of the probability function (B and D) declined immediately before the end of the daily session in the meal group (A and B) but not in the no-meal group (C and D). (A-D) Error bars indicate SEM.
The meal group anticipated the arrival of the meal, as shown in Fig. 2. Food- trough responses increased more as a function of time in the meal group than in the no- meal group (F(17, 306) = 22.0, p < 0.001). This interaction was produced by an increase in food trough responses in the meal group (F(17, 153) = 21.5, p < 0.001) but not in the no-meal group (F(17, 153) = 1.2, p = 0.3). Thus, the meal produced the increase in food trough responses.
Fig. 2.
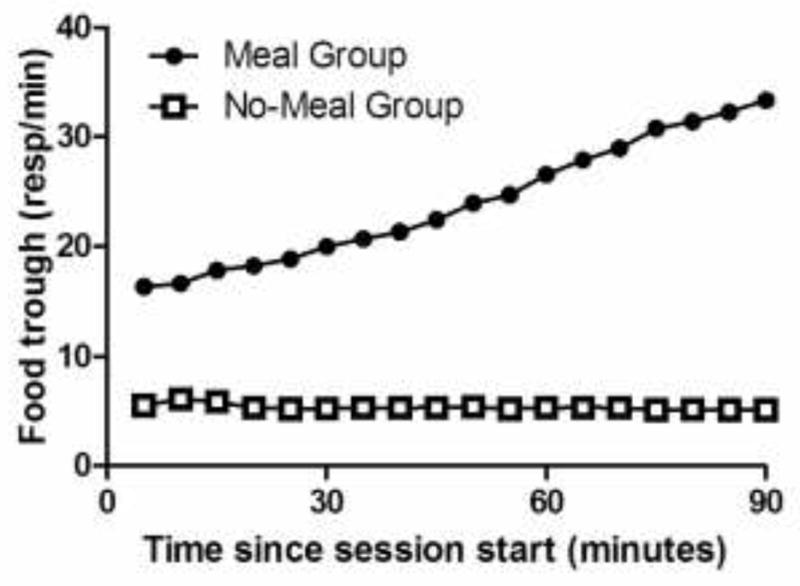
The meal group anticipated the arrival of the meal, as shown by the increase in food-trough responses before the meal. The increase in food-trough responses was absent in the no-meal group. Error bars indicate SEM but are too small to be seen in the figure (mean = 0.4, range: 0.2 – 0.7).
As outlined above, we documented that sensitivity to time declined as a meal approached. One potential explanation of these data is prospective memory. According to this hypothesis, the rats formed a representation of the meal which was inactive early, and as the expectation of the meal grew, more attentional resources were recruited to maintain the representation of the forthcoming meal, thereby impairing temporal sensitivity on the bisection task. As predicted by this hypothesis, slope for the meal group declined as the meal approached whereas the no-meal group’s slope remained constant throughout the session. It is noteworthy that the two groups appear to have different slopes at the start of the session (although this difference was not statistically significant, t(18) = 1.69, p = 0.1). Indeed, prospective memory would predict, if all other variables are equated, that the groups would have the same levels of accuracy at the start of the session. However, all other variables were likely not equated because the meal group received substantially more food (approximately 10 g vs. the no-meal group’s 2-g average) over the course of a session. We proposed that there are a number of potential explanations by which more food in the chamber may have produced an overall higher baseline sensitivity to time in the meal group, from which prospective memory produced a subsequent decline in sensitivity. First, the associative properties of the test chamber were likely different for meal and no-meal rats. The valance of the chamber was likely higher for rats in the meal group because they received substantially more food over the course of a session. The higher valance may have supported a higher baseline level of sensitivity to time (i.e., higher slope) overall in the meal group, from which prospective memory produced a subsequent decline in sensitivity. Second, the meal group received a higher percentage of its food ration in the chamber compared to the no-meal group; because the meal group’s survival depended to a greater extent on food in the chamber, these rats may have devoted more effort in the bisection task to maximize food in the chamber; the increased pressure to earn food in the chamber may have supported a higher baseline level of sensitivity, from which prospective memory produced a subsequent decline. Third, the meal group likely produced a lever press at the end of 90 min, which was often immediately followed by a large number of pellets (i.e., the meal); thus, it is possible that the meal functioned to reinforce lever pressing. This reinforcement may have increased the likelihood that the rat was engaged in the lever pressing task, thereby increasing the percentage of trials in which the animals were attending to the lever-press task; the reinforcement of the lever-press task may have supported a higher baseline level of sensitivity, from which prospective memory produced a subsequent decline. Experiments that directly manipulate and assess the reward contingencies will provide a more direct test of these proposals.
Before accepting the representational account, it is important to evaluate simpler, alternative hypotheses. In the sections that follow we first consider two broadly defined categories, namely an attentional limit and response competition. Then we discuss two specific alternative hypotheses that focus on the potential effect of anticipatory contrast and fatigue on temporal sensitivity.
Attentional Limit
The first alternative hypothesis focuses on the observation that the rats in the meal group processed two types of information (judging intervals and anticipating the meal) whereas in the no-meal group, rats processed only one type (judging intervals); we refer to this proposal as limited attention. Indeed, food-trough responses increased as a function of time in the meal group; perhaps the increase in food-trough responses corresponds to more attention to the meal. Importantly, engaging in two processes may require more processing resources (e.g., attention) than engaging in one process, thereby reducing the processing resources available for judging intervals in the meal group. However, it is important to note that the rats in the meal group were engaged in two tasks throughout the entire pre-meal window. Consequently, the limited-attention hypothesis predicts that performance would be worse throughout the entire pre-meal window. Contrary to this prediction, our data show that performance is worse immediately before the meal relative to an early time point (Fig. 1A-1B). For example, consider application of a general information processing theory of timing (Church 1978; Gibbon et al. 1984) to the 90-min interval2. According to this proposal, the rat compares a current estimate of elapsed time since the start of the session to a memory of the pre-meal interval on a moment to moment basis. At some point prior to the meal, the elapsed and remembered durations are judged to be similar enough to expect the meal soon. Importantly, because the comparison between elapsed and remembered times occurs throughout the pre-meal interval, this account may predict a decline in performance but the decline would be equivalent early and late in the pre-meal interval. Therefore, application of a general information processing theory of time suggests that food-trough responses may increase as the meal approaches without a corresponding increase in attention.
Response Competition
A second alternative hypothesis focuses on the observation that the rats in the meal group are engaged in two behaviors (pressing levers to express a judgment of intervals and investigating the food trough in anticipation of the meal) whereas rats in the no-meal group are engaged in only one behavior (pressing levers). Indeed, the general information processing theory of timing described above predicts that the mean rate of investigating the food trough will increase throughout the 90-min interval for the meal group (but not for the no-meal group), which we observed (Fig. 2). Perhaps rats in the meal group are less likely to hear the noise pulses, process the interval duration, or remember the to-be-selected duration classification response while they are investigating the food trough. Each version of this response-competition hypothesis assumes that engaging in food-trough exploration causes the observed decline in temporal sensitivity.
Because rats in the meal group produced robust behavior at the food trough as the meal approached, we asked whether food-trough responses are responsible for the decrease in sensitivity to time; we refer to this proposal as response competition. To test the response-competition hypothesis, we examined the slope of the psychophysical functions and the number of food-trough responses on individual days. There was variability in number of food-trough responses (42 ± 8, mean ± SEM) and slopes (0.3 ± 0.01, mean ± SEM). A response competition hypothesis predicts that a high number of responses are expected to produce low slopes and a small number of responses are expected to produce high slopes. Thus, response competition predicts a significant negative correlation between slope and number of food-trough responses in the meal group immediately before the meal. Contrary to this prediction, the observed correlation was −0.006 ± 0.061 (mean ± SEM), which was not significantly different from zero (t(9) = −0.02, p = 1); the proportion of variance explained by response competition is 0.00004 (i.e., less than one hundredth of one percent). In supplementary materials, we report a Bayesian analysis which suggests that the null hypothesis (Gallistel 2009) of a zero correlation is a reasonably safe bet (see Online Resource 1).
Contrast
The rats in the meal group received an abrupt increase in the rate of food delivery (i.e. the meal) near the end of each session. Therefore, the subjective value of food earned late in the bisection task may have decreased in the meal group but not in the no-meal group. According to this anticipatory contrast hypothesis (Flaherty and Checke 1982; Flaherty 1996) devalued rewards late in the bisection task may produce a decline in accuracy (i.e. temporal sensitivity) before the meal. If a subjective decrease in the value of reward occurred before the meal, then other variables that measure reward properties (i.e., variables affected by motivation) would also be expected to decline before the meal.
If the value of reward decreased in the meal group, then the latency to respond with a lever press should increase as the meal approached. Fig. 3A shows the latency to press a lever in the meal group. Unlike the contrast prediction outlined above, latencies do not appear to increase from early to late time points for the meal group (F(1,9) = 0.86, p = 0.4). As expected (Meck 1983), latencies were higher for intermediate durations (F(6,54) = 6.03, p < 0.001), and there was no interaction of these two variables (F(6,54) = 1.17, p = 0.3).
Fig. 3.
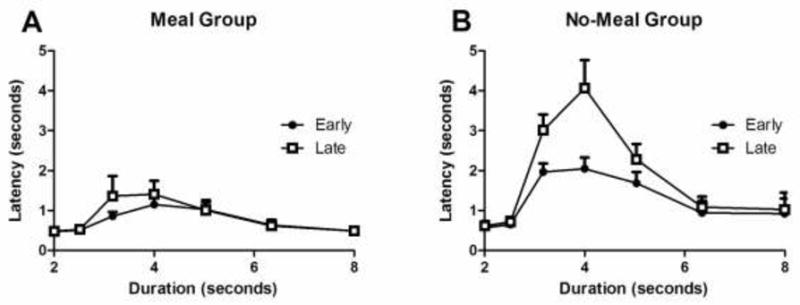
The median latency to press a lever in the interval-duration classification task did not increase from early to late time points in the meal group and was lower, overall, in the meal group relative to the no-meal group. In the no-meal group, latencies increased from early to late time points. In both groups, latencies were larger for intermediate durations. Error bars indicate SEM.
Although the meal group’s latency to respond did not increase from early to late time points (Fig. 3A) contrary to a contrast account, the no-meal group (Fig. 3B) produced longer latencies at late relative to early time points (F(1,9) = 19.57, p= 0.002). The no-meal group also had longer latencies for intermediate durations (F(6,54) = 12.91, p < 0.001), and these two variables interacted (F(6,54) = 7.55, p < 0.001), suggesting that the increase was more pronounced for intermediate durations.
Moreover, devalued reward in the meal group predicts longer latencies in the meal group relative to the no-meal group. A comparison of Fig. 3A and 3B suggests that the opposite pattern was observed. Importantly, latencies were longer in the no-meal group relative to the meal group (F(1,18) = 12.95, p = 0.002). Moreover, the three-way interaction was significant (F(6,108)= 3.6, p = 0.003), suggesting that the magnitude of the interaction of early-late and durations was larger in the no-meal group relative to the meal group. In addition, the test for each other variable in this analysis was also statistically significant (all ps < 0.02).
Although the latency data described above do not suggest that reward value declined as the meal approached, we examined a final contrast prediction. There was variability in latencies (15 ± 3, mean ± SEM) and slopes (0.3 ± 0.01, mean ± SEM). A contrast hypothesis predicts that when contrast in high, temporal sensitivity should be low (small slopes) and reward value should be low (large latencies). Similarly, when contrast is low, temporal sensitivity should be high (large slopes) and reward value should be high (small latencies). Thus, contrast predicts a significant negative correlation between slope and latency. The correlation between slope and latency was −0.06 ± 0.04 (mean ± SEM), which was not significantly different from zero (t(9) = −1.48, p = 0.2). Indeed, the contrast hypothesis explains only 0.0036 of the variance in slope and latencies (i.e. less than one half of one percent of the variance). In supplementary materials, we report a Bayesian analysis which suggests that the null hypothesis of a zero correlation is a reasonably safe bet (see Online Resource 2). We examined three predictions of a contrast hypothesis. In each case, the data did not conform to the contrast hypothesis. Therefore, we suggest that it is unlikely that contrast is responsible for the decrease in slope observed in the meal group (Fig. 1A-1D).
Fatigue
The meal group engaged in the bisection task and anticipated the arrival of the meal whereas the no-meal group only engaged in one task. Indeed food trough responses increased as a function of time in the meal group but not in the no-meal group (Fig. 2). Therefore, the added activity could produce fatigue at a later time point, which could potentially produce a decline in accuracy (i.e. reduced temporal sensitivity and slope late relative early) in the meal group. The fatigue hypothesis predicts that the meal group would have longer latencies to press the lever relative to the no-meal group, particularly at a late time point. As described above, the data are not consistent with these predictions (see Fig. 3A and 3B).
Summary
We propose that rats possess prospective memory. According to this view, anticipation of a forthcoming meal produced a temporally-specific impairment in the rats’ ongoing activity. This impairment was specific to the time at which the representation of the future was most likely accessed via prospective memory but not earlier. Importantly, the meal group (but not the no-meal group) produced a time-dependent decline in temporal sensitivity in the bisection task. Correspondingly, the meal group (but not the no-meal group) anticipated the arrival of the 90-min time point, which was the point at which the meal group began to obtain access to the meal. We believe that time-based prospective memory in the rat is a potential explanation for these observations. We suggest that our procedure is sufficient to produce a deleterious effect on ongoing performance. It will be valuable to identify the necessary conditions to produce a deleterious effect in future experiments (e.g., by identifying the contributing roles of location of food, response requirement to obtain food, temporal separation between ongoing and prospective tasks, cueing the need for temporal monitoring, and other factors).
We evaluated two broad categories of alternatives (attentional limit and response competition) and two specific alternatives (contrast and fatigue) that we believe are unlikely to account for our data. We believe that any potential alternative explanation of our data falls into one of these categories of potential alternative explanations. We propose that prospective memory should only be considered after alternative accounts have been applied to the data. We developed a number of credible alternative accounts, but they do not appear to explain the pattern of observed data. Part of our argument for prospective memory relies upon testing alternative accounts to determine if they accord with the data. It is always difficult to interpret failures of a phenomenon to reveal itself in data (a classic problem of null effects). However, this concern is unlikely to apply to the rejection of alternative accounts described above. First, in some cases, the data showed a significant difference in the opposite direction predicted by alternative explanations (e.g., contrast and fatigue; figure 4). It seems unlikely that insufficient sensitivity of our measures is responsible for the failure of an alternative explanation to reveal itself in data when a significant difference is detected in the opposite direction. Second, in other cases, alternative hypotheses predicted a negative correlation, and the data were consistent with a zero correlation. Although closer to the classic problem with null hypotheses, we took extra steps in these cases. As shown in supplemental materials (online resources 1 and 2), we used a Bayesian analysis to show that the hypothesis of a zero correlation is a reasonably safe bet given a number of plausible prior hypotheses. Moreover, the evidence in favor of a negative correlation explained almost none of the variability in the data (from less than one hundredth of one percent to less than one half of one percent of the observed variance); this vanishingly small amount of variability does not support the alternative explanations. We propose that prospective memory is a reasonable account for our data.
Implications
Recently, considerable interest has focused on the question of whether animals can plan for the future (Clayton et al. 2003; Correia et al. 2007; Mulcahy and Call 2006; Naqshbandi and Roberts 2006; Raby et al. 2007; Raby and Clayton 2009; Roberts 2002; Roberts and Feeney 2009; Suddendorf and Busby 2003, 2005; Suddendorf and Corballis 1997; Suddendorf and Corballis 2007; Tulving 2001, 2005; Zentall 2005, 2006). One perspective that has dominated this research is the hypothesis of mental time travel (Roberts and Feeney 2009; Tulving 2001; Suddendorf and Corballis 1997), which focuses on the idea that one can envision oneself in a future scenario. This approach has led to efforts to document that animals plan for a future event that is outside its current motivational state (Suddendorf and Corballis 2007; Tulving 2005). Although considerable progress has been made within this framework (e.g., Correia et al. 2007; Raby et al. 2007), it has recently been argued that future-oriented abilities should also be examined outside of the mental-time-travel framework (Raby and Clayton 2009; Zentall 2006). Our approach has attempted to model prospective memory in rats outside the mental-time-travel framework. One advantage of our approach is that it may provide insight into the evolution of planning to act in the future by focusing on a readily specified operational definition (the deleterious side-effect of a prospective memory representation is a reduced ability to process information in an ongoing task) that may be evaluated across a wide array of species. Thus, we emphasize that our model does not require rats to engage in mental time travel. We also note that our work differs from other work on prospective cognition in animals (Cook et al. 1985; Gipson et al. 2008) that sought to identify the content of a memory representation as the to-be-selected stimulus or response. In contrast, we propose that rats produced a behaviorally observable side effect of anticipating a future event (deleterious performance on an ongoing task). Prospective memory proposes that the deleterious effect should occur at a time specific to when the representation of the future is accessed.
Prospective memory in the rat may represent a precursor to planning, and the precursor may have a more wide-spread evolutionary distribution than previously proposed. We note that this precursor to planning may exist in the absence of other aspects of planning. For example, it is not known if a precursor to planning would support mental time travel (i.e., envision specific future scenarios) or more flexible or creative deployment of a plan. Nonetheless, our data suggest that prospective memory in the rat affects on-going activity, a phenomenon which may be used to develop a more complete profile of how representational abilities vary across animals (Crystal 2009).
Future Directions
We view the current work as an initial attempt to develop a rodent model of prospective memory. The evaluation of alternative explanations above suggests that the model has some potential. Next we consider some limitations in the current model and offer some ideas about future directions. A central feature of prospective memory is distinguishing “remembering to remember” from “remembering.” Our experiment used one target interval (90 min) and distinguished between early and late time points in ongoing-task performance. Future work is needed to show that the rats remember to do an action at a specific point in time in the future, which would enhance the case for remembering to remember (i.e., “get food in 20 min” rather than “get food when it’s available”). Similarly, the hypothesis that rats show a precursor to planning could be better evaluated in future studies. In particular, our experiment used two temporally based tasks. It remains to be determined if the observation of deleterious effects is limited to situations that use related tasks; for example, timing two targets may be more cognitively demanding that timing a single target. Future research should explore this issue by using a non-temporal ongoing task. In our experiment, the rats had continuous access to the site at which the future action is needed (i.e., the food trough in our experiment). However, in many cases of prospective memory in people, the future action is not available for some period of time. For example, when a parent must remember to pick up his child from daycare at the end of the day, he does not actually go and attempt to pick up the child for some period of time. Would a deleterious effect in an ongoing task occur in the absence of actions that attempt to obtain the future event (i.e., in conditions that prevent access to the target response)? Finally, future research should evaluate the possibility that the animal can be instructed to remember to do (or omit) the target behavior. Developing instructions of this type (i.e., stimulus control) would evaluate the possibility of prospective memory being deployed “on demand.” Ultimately, we expect that rats may show some elements of prospective memory, and future research will be needed to develop a more complete profile of the similarities and differences to human prospective memory.
The ability to think about the future develops in children by three to five years of age (Atance and O’Neill 2001; Russell et al. 2010). Failures of prospective memory (i.e., forgetting to act on an intention at an appropriate time in the future) is a common feature of aging (Aberle et al. 2010; d’Ydewalle et al. 2001; Driscoll et al. 2005; Henry et al. 2004; Craik 1986). Moreover, prospective memory is impaired in a number of patient populations, including mild cognitive impairment (Schmitter-Edgecombe et al. 2009; Troyer and Murphy 2007), Alzheimer’s disease (Blanco-Campal et al. 2009; Jones et al. 2006; Troyer and Murphy 2007), Parkinson disease (Foster et al. 2009; Raskin et al. in press), and traumatic brain injury (Henry et al. 2007; McCauley et al. 2009). Thus, the development of a rodent model of prospective memory may open new opportunities to explore the neuroanatomical, neurochemical, neurophysiological, and molecular mechanisms of normal and impaired prospective memory.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health grant R01MH080052 to JDC. We thank our colleague, the late Rich Marsh–scholar of prospective memory–for encouraging this work. We thank four anonymous reviewers for constructive criticism.
Footnotes
The no-meal group appears to show a slight rightward, although not significant, shift in the psychophysical function late in the session. A rightward shift suggests a slight, although not significant, bias to judge all durations as short.
We outline an account based on timing a 90-min interval. However, a similar account could be developed for time of day (Gallistel 1990); the meals occurred at approximately a constant time of day because the daily sessions began at an approximately constant time of day. Other cues available to the animal include the number of food pellets, physiological signals of satiation, number of trials (e.g., noises, lever insertions), and delay since last lever insertion. Additional experiments would be needed to determine if anticipation of the meal was based on interval, circadian, and/or other cues.
Conflict of interest
The experiments complied with the current laws of country in which they were performed. The authors declare no conflict of interests.
References
- Aberle I, Rendell PG, Rose NS, McDaniel MA, Kliegel M. The age prospective memory paradox: Young adults may not give their best outside of the lab. Dev Psychol. 2010;46 (6):1444–1453. doi: 10.1037/a0020718. doi:10.1037/a0020718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atance C, O’Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5 (12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Blanco-Campal A, Coen RF, Lawlor BA, Walsh JB, Burke TE. Detection of prospective memory deficits in mild cognitive impairment of suspected Alzheimer?s disease etiology using a novel event-based prospective memory task. J Int Neuropsychol Soc. 2009;15 (01):154–159. doi: 10.1017/S1355617708090127. [DOI] [PubMed] [Google Scholar]
- Church RM. The internal clock. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive Processes in Animal Behavior. Erlbaum; Hillsdale, NJ: 1978. pp. 277–310. [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. J Exp Psychol Anim Behav Process. 1977;3 (3):216–228. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nat Rev Neurosci. 2003;4 (8):685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cook RG, Brown MF, Riley DA. Flexible memory processing by rats: Use of prospective and retrospective information in the radial maze. J Exp Psychol Anim Behav Process. 1985;11 (3):453–469. doi: 10.1037/0097-7403.11.3.453. [DOI] [PubMed] [Google Scholar]
- Correia SPC, Dickinson A, Clayton NS. Western scrub-jays anticipate future needs independently of their current motivational state. Curr Biol. 2007;17 (10):856–861. doi: 10.1016/j.cub.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Hagendorf FKH, editor. Human memory and cognitive capabilities: Mechanisms and performances. Elsevier; North Holland: 1986. pp. 409–422. [Google Scholar]
- Crystal JD. Elements of episodic-like memory in animal models. Behav Process. 2009;80 (3):269–277. doi: 10.1016/j.beproc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Baramidze GT. Endogenous oscillations in short-interval timing. Behav Process. 2007;74 (2):152–158. doi: 10.1016/j.beproc.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Maxwell KW, Hohmann AG. Cannabinoid modulation of sensitivity to time. Behav Brain Res. 2003;144 (1):57–66. doi: 10.1016/s0166-4328(03)00062-7. [DOI] [PubMed] [Google Scholar]
- d’Ydewalle G, Bouckaert D, Brunfaut E. Age-related differences and complexity of ongoing activities in time- and event-based prospective memory. Am J Psychol. 2001;114 (3):411–423. [PubMed] [Google Scholar]
- Driscoll I, McDaniel MA, Guynn MJ. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19 (1):28–34. doi: 10.1037/0894–4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. J Exp Psychol-Learn Mem Cogn. 1990;16 (4):717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Problems in the Behavioural Sciences. Cambridge University Press; Cambridge: 1996. Incentive Relativity. [Google Scholar]
- Flaherty CF, Checke S. Anticipation of incentive gain. Anim Learn Behav. 1982;10 (2):177–182. doi: 10.3758/bf03212267. [DOI] [Google Scholar]
- Foster ER, McDaniel MA, Repovš G, Hershey T. Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology. 2009;23 (3):347–358. doi: 10.1037/a0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Gallistel CR. The importance of proving the null. Psychol Rev. 2009;116 (2):439–453. doi: 10.1037/a0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. In: Gibbon J, Allan L, editors. Annals of the New York Academy of Sciences: Timing and time perception. Vol. 423. New York Academy of Sciences; New York: 1984. pp. 52–77. [DOI] [PubMed] [Google Scholar]
- Gipson CD, DiGian KA, Miller HC, Zentall TR. Radial maze analog for pigeons: Evidence for flexible coding strategies may result from faulty assumptions. Learn Motiv. 2008;39 (4):285–295. doi: 10.1016/j.lmot.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE. Remembering to do things: A forgotten topic. In: Harris JE, Morris PE, editors. Everyday memory, actions, and absent-mindedness. Academic Press; London: 1984. pp. 71–92. [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychol Aging. 2004;19 (1):27–39. doi: 10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Kliegel M, Theodorou G, Summers F. Traumatic brain injury and prospective memory: Influence of task complexity. J Clin Exp Neuropsychol. 2007;29 (5):457–466. doi: 10.1080/13803390600762717. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Marsh RL, Cook GI. Task interference in time-based, event-based, and dual intention prospective memory conditions. J Mem Lang. 2005;53 (3):430–444. doi: 10.1016/j.jml.2005.04.001. [DOI] [Google Scholar]
- Jones S, Livner Å, Bäckman L. Patterns of prospective and retrospective memory impairment in preclinical Alzheimer’s disease. Neuropsychology. 2006;20 (2):144–152. doi: 10.1037/0894-4105.20.2.144. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO. Varying the importance of a prospective memory task: Differential effects across time - and event-based prospective memory. Memory. 2001;9 (1):1–11. doi: 10.1080/09658210042000003. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI. Task interference from prospective memories covaries with contextual associations of fulfilling them. Mem Cognit. 2006;34 (5):1037–1045. doi: 10.3758/bf03193250. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Landau JD. An investigation of everyday prospective memory. Mem Cognit. 1998;26 (4):633–643. doi: 10.3758/bf03211383. [DOI] [PubMed] [Google Scholar]
- McCauley SR, McDaniel MA, Pedroza C, Chapman SB, Levin HS. Incentive effects on event-based prospective memory performance in children and adolescents with traumatic brain injury. Neuropsychology. 2009;23 (2):201–209. doi: 10.1037/a0014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Sage Publications; Thousand Oaks, CA: 2007. [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9 (2):171–201. [PubMed] [Google Scholar]
- Mulcahy NJ, Call J. Apes Save Tools for Future Use. Science. 2006;312 (5776):1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- Naqshbandi M, Roberts WA. Anticipation of Future Events in Squirrel Monkeys (Saimiri sciureus) and Rats (Rattus norvegicus): Tests of the Bischof-Kohler Hypothesis. J Comp Psychol. 2006;120 (4):345–357. doi: 10.1037/0735-7036.120.4.34. doi:10.103710735-7036. 20.4.345. [DOI] [PubMed] [Google Scholar]
- Osvath M. Spontaneous planning for future stone throwing by a male chimpanzee. Curr Biol. 2009;19 (5):R190–R191. doi: 10.1016/j.cub.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Osvath M, Osvath H. Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: self-control and pre-experience in the face of future tool use. Anim Cogn. 2008;11 (4):661–674. doi: 10.1007/s10071-008-0157-0. [DOI] [PubMed] [Google Scholar]
- Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western scrub-jays. Nature. 2007;445 (7130):919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- Raby CR, Clayton NS. Prospective cognition in animals. Behav Process. 2009;80 (3):314–324. doi: 10.1016/j.beproc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, Tröster AI. A differential deficit in time- versus event-based prospective memory in Parkinson’s disease. Neuropsychology. doi: 10.1037/a0020999. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA. Are animals stuck in time? Psychol Bull. 2002;128 (3):473–489. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC. The comparative study of mental time travel. Trends Cogn Sci. 2009;13 (6):271–277. doi: 10.1016/j.tics.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Russell J, Alexis D, Clayton N. Episodic future thinking in 3- to 5-year-old children: The ability to think of what will be needed from a different point of view. Cognition. 2010;114 (1):56–71. doi: 10.1016/j.cognition.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23 (2):168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 3. Chapman & Hall/CRC; New York: 2004. [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: investigating the capacity demands of delayed intention performance. J Exp Psychol-Learn Mem Cogn. 2003;29 (3):347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE, Hunt RR, McVay JC, McConnell MD. The cost of event-based prospective memory: Salient target events. J Exp Psychol-Learn Mem Cogn. 2007;33 (4):734–746. doi: 10.1037/0278-7393.33.4.734. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Mental time travel in animals? Trends Cogn Sci. 2003;7 (9):391–396. doi: 10.1016/s1364-6613(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Making decisions with the future in mind: Developmental and comparative identification of mental time travel. Learn Motiv. 2005;36:110–125. [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genet Soc Gen Psychol Monogr. 1997;123 (2):133–167. [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30 (3):299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ. Memory for intentions in amnestic mild cognitive impairment: Time- and event-based prospective memory. J Int Neuropsychol Soc. 2007;13 (2):365–369. doi: 10.1017/s1355617707070452. [DOI] [PubMed] [Google Scholar]
- Tulving E. Chronesthesia: Awareness of subjective time. In: Stuss DT, Knight RC, editors. The Age of the Frontal Lobes. Oxford University Press; New York: 2001. pp. 311–325. [Google Scholar]
- Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace H, Metcalfe J, editors. The Missing Link in Cognition: Origins of Self-Reflective Consciousness. Oxford University Press; New York: 2005. pp. 3–56. [Google Scholar]
- Zentall TR. Animals may not be stuck in time. Learn Motiv. 2005;36:208–225. [Google Scholar]
- Zentall TR. Mental time travel in animals: A challenging question. Behav Process. 2006;72 (2):173–183. doi: 10.1016/j.beproc.2006.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.