Abstract
Objective
Stem cells from human exfoliated deciduous teeth (SHED) are a unique postnatal stem cell population capable of regenerating mineralized tissue and treating immune disorders. However, the mechanism that controls SHED differentiation is not fully understood. Here, we showed that basic fibroblast growth factor (bFGF) treatment attenuated SHED-mediated mineralized tissue regeneration through activation of the extracellular signal-regulated kinase (ERK) 1/2 pathway.
Material and Method
The level of mineralized nodule formation was assessed by alizarin red staining. Expression levels of osteogenic genes, OCN and Runx2, were examined by RT-PCR. Subcutaneous implantation approach was used to assess in vivo bone formation. Downstream signaling pathways of bFGF were examined by Western blotting.
Result
Activation of ERK1/2 signaling by bFGF treatment inhibited WNT/β-catenin pathway, leading to osteogenic deficiency of SHED. ERK1/2 inhibitor treatment rescued bFGF-induced osteogenic differentiation deficiency.
Conclusion
These data suggest that bFGF inhibits osteogenic differentiation of SHED via ERK1/2 pathway. Blockade ERK1/2 signaling by small molecular inhibitor-treatment improves bone formation of SHED after bFGF treatment.
Keywords: SHED, ERK, differentiation, tissue regeneration
INTRODUCTION
Stem cell-based craniofacial tissue engineering is a promising approach for craniofacial structure reconstruction. Stem cells from human exfoliated deciduous teeth (SHED) are capable of differentiating into osteogenic/odontogenic cells, adipocytes, and neural cells (Miura et al., 2003). When transplanted subcutaneously into immunocompromised mice, SHED induced robust bone formation in vivo (Miura et al., 2003; Seo et al., 2008). Additionally, SHED effectively repaired critical-size orofacial defects both in mouse and minipig models (Seo et al., 2008; Zheng et al., 2009), indicating that SHED are feasible cell source for orofacial regeneration. However, the mechanism of SHED-based tissue regeneration is not fully understood.
Multiple signaling pathways contribute to the osteogenic differentiation of mesenchymal stem cells. Basic fibroblast growth factor is a broad-spectrum mitogenic, angiogenic, and neurotrophic factor that is expressed in many tissues and cell types (Ortega et al., 1998). bFGF plays an important role in bone remodeling (Hurley, et al., 1993; Hurley, et al., 1994). However, the effects of bFGF on the osteogenic differentiation of mesenchymal stem cells are still controversial. Previous study indicated that bFGF stimulated osteogenic differentiation of bone marrow-derived mesenchymal cells (Pitaru, et al., 1993) by enhanced alkaline phosphatase (ALP) activity, osteocalcin production, and bone nodule formation. However, other study suggested that bFGF treatment promoted proliferation rate, but inhibited osteogenic differentiation of bone marrow derived osteoblastic progenitors (Majors, et al., 1996). It was also known that bFGF treatment induced a dose dependent response on proliferation and differentiation of bone marrow mesenchymal cells (Varkey, et al., 2006). To understand whether bFGF treatment affects differentiation of SHED, we examine signaling alteration in bFGF-treated SHED in vitro. In our study, we found that bFGF inhibited osteogenesis of SHED via ERK1/2-mediated down regulation of WNT/β-catenin.
MATERIALS AND METHODS
Mice
Beige nude/nude XID (III) mice (Female, 8- to 12-week-old) were purchased from Harlan (Indianapolis, IN, USA). All animal experiments were performed under an institutionally approved protocol for the use of animal research (University of Southern California protocol #10874 and #10941). All animals were maintained in a temperature-controlled room with a 12-h alternating light-dark cycle and fed sufficient diet and water ad libitum throughout the experimental period.
Antibodies and Reagents
p44/42 (ERK1/2), phospho-p44/42 (ERK1/2), p38, phospho-p38, JNK and phospho-JNK antibodies were purchased from Cell Signaling (Danvers, MA, USA). β-catenin, active β-catenin, Runx2 and OCN antibodies were used from Milipore (Billerica, MA). β-actin were purchased from SIGMA-Aldrich (St. Louis, MO). bFGF (Peprotech, Rocky Hill, NJ), ERK inhibitor—PD325901, P38 inhibitor—SB203580, JNK inhibitor—SP600125 (Calbiochem) were used for cell treatment. ERK siRNA and control siRNA were purchased from Cell signaling (Danvers, MA, USA) and Santa Cruz (Santa Cruz, CA, USA), respectively. Lipofectamine RNAiMAX Transfection Reagent was used for siRNA transfection.
SHED isolation and culture expansion
Mononuclear cells isolated from the remnant dental pulp tissue of the deciduous incisors were cultured as reported previously. SHED used in this study were frozen cells which were derived from three donors (Miura et al., 2003).
BrdU Incorporation assay
100 ng/ml bFGF was added to P1 SHED culture medium for 48 h and then re-seeded the SHED to 2-well chamber slides (Nunc, Rochester, NY, 10×103 per well). P2 cells were cultured for 1–2 days, incubated with BrdU reagent (1:100, Invitrogen) for 24 hours, and stained with the BrdU staining kit (Invitrogen), according to the manufacture’s instruction. Cells were lightly stained with hematoxylin solution (Invitrogen). To quantify cell proliferation rate, ten representative images were used to calculate BrdU positive nuclei number. Cell proliferation was shown as a percentage of BrdU positive nuclei over total nucleated cells. The BrdU assay was repeated in five independent isolated cells for each experimental group.
Immunophenotype analysis
100 ng/ml bFGF was added to P1 SHED culture medium for 48 h. P2 SHED were stained with stem cell surface markers and analyzed by flow cytometry as described previously (Yamaza et al., 2010). Briefly, single-cell suspensions (2×105/100 μl) were incubated with mouse monoclonal antibodies specific to cell surface markers (1 μg/100 μl each) for 45 min on ice, followed by reaction with R-phycoerythrin (PE) conjugated goat antibodies against mouse IgM or IgG (each 1 μg/100 μl, Southern Biotechnology) for 30 min on ice. As negative controls, isotype-matched mouse immunoglobulins (IgG1, IgG2a and IgM) (1 μg/100 μl each, Southern Biotechnology) were incubated instead of the primary antibodies. The cells were analyzed using a FACS Calibur flow cytometer (BD Bioscience).Positive cells were identified by comparison with the corresponding control cells in which a false-positive rate of less than 1% was accepted.
In vitro osteogenic induction assay
Osteogenic differentiation of SHED was performed according to previous publications (Miura et al., 2003; Yamaza et al., 2008). Briefly, bFGF was added to culture medium at the concentration of 100 ng/ml. PD325901 (5 μM), SB203580 (1 μM), or SP600125 (5 μM) were added to the medium with bFGF, respectively. After 72-hour exposure to these drugs, the culture medium was replaced with osteogenic induction medium. ERK siRNA and control siRNA were added at 24 hours prior to bFGF treatment for in vitro osteogenic induction experiment, then bFGF was added with siRNA for additional 72 hours before osteogenic induction. During the osteogenic induction, bFGF, inhibitors or siRNA were not added to the medium. MSCs cultured in osteogenic induction medium for two weeks were washed three times with PBS and collected RNA. Alizarin red-S staining and calcium level test were performed at 4 weeks post induction. Mineralized nodule formation and calcium level were assessed as described previously (Shi et al., 2002; Yamaza et al., 2008). Calcium concentration was quantified relative to the cell number of each well.
In vitro adipogenic induction assay
Adipogenic differentiation of SHED was performed according to previous publications (Miura et al., 2003; Yamaza et al., 2008). Briefly, bFGF was added to culture medium at the concentration of 100 ng/ml for 72h before adipogenic induction. During the adipogenic induction, bFGF was not added to the medium. MSCs cultured in adipogenic induction medium for two weeks were washed three times with PBS and collected RNA. Oil red O staining was performed at 4 weeks post induction. Adipogenic markers and lipid formation were also assessed as previous published papers (Miura et al., 2003; Yamaza et al., 2008).
In vivo osteogenic differentiation
Xenogeneic transplantation was performed using immunocompromised mice as described (Miura et al., 2003). Briefly, the culture medium of SHED was replaced with medium containing bFGF (100 ng/ml) or bFGF (100 ng/ml) plus PD325901 (5 μM). After 72-hour exposure to the drugs, SHED (4×106 cells) were subcutaneously transplanted into beige nude/nude XID (III) mice with hydroxyapatite tricalcium phosphate (HA/TCP, Zymer, Warsaw, IN) as a carrier. Eight weeks post-transplantation, the transplants were harvested. The transplants were fixed with 4 % paraformaldehyde and demineralized with 5% EDTA for 4 weeks prior to histological analysis.
Reverse-Transcription PCR (RT-PCR) analysis
Total RNA isolation, first-strand cDNA synthesis and PCR processes were performed as described previously (Yamaza et al., 2009). All the primers used as follows: RUNX2 sense 5’CAGTTCCCAAGCATTTCATCC3’ and antisense 5’TCAATATGGTCGCCAAACAG3’; OCN sense 5’CATGAGAGCCCTCACA3’ and antisense 5’AGAGCGACACCCTAGAC3’; LPL sense 5’ATGGAGAGCAAAGCCCTGCTC3’ and antisense 5’GTTAGGTCCAGCTGGATCGAG3’; PPARγ2 sense 5’CTCCTATTGACCCAGAAAGC3’ and antisense 5’GTAGAGCTGAGTCTTCTCAG-3; GAPDH sense 5’AGCCGCATCTTCTTTTGCGTC3’ and antisense 5’ TCATATTTGGCAGGTTTTTCT3’.
Western blot analysis
Cells were lysed in M-PER extraction reagent (Thermo Scientific, Rockford, IL) and protein concentrations were measured using Bio-Rad Protein Assay kit (Bio-Rad Laboratories Inc. Hercules, CA). Ten micrograms of protein were applied to each lane and separated on Tris-Glycine SDS-PAGE gel (Novex; Invitrogen Co.). The proteins were then transferred onto nitrocellulose membranes (Millipor) and blocked for 1 hour at room temperature in blocking solution (TBS with 0.1% Tween 20, and 5% BSA). Following immunolabeling, membranes were washed and reacted with Super Signal chemiluminescence HRP substrate (Pierce Chemical Co., Rockford, IL) and then visualized on Kodak BioMax film (Eastman Kodak Co., Los Angeles, CA).
Statistics
All data were expressed as the mean ± SD of, at least, triplicate determinations. Statistical difference between the values was examined by Student’s t-test. The p values lower than 0.05 were considered statistically significant.
RESULTS
bFGF inhibits SHED osteogenic differentiation
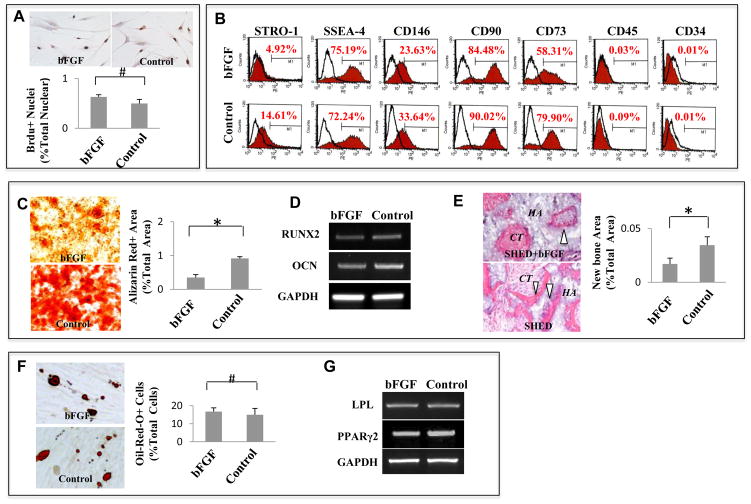
To identify the role of bFGF in regulating stem cell properties of SHED, we investigate whether bFGF alters the proliferation rate and surface molecule expression of SHED. bFGF treatment did not alter the proliferation rate of SHED (Fig. 1A), but expression of some stem cell surface markers, including STRO-1, CD146, CD90 and CD73, were slightly decreased in the bFGF-treated group (Fig. 1B). Alizarin red S staining showed that treating SHED with bFGF resulted in a reduced mineralized nodule formation compared to the untreated control group (Fig. 1C, *P<0.05). RT-PCR analysis determined that expression of runt-related transcription factor 2 (Runx 2) and osteocalcin (OCN) were decreased in bFGF-treated SHED compared to the control group (Fig. 1D). Furthermore, in vivo stem cell implantation system was then used, in which 4×106 bFGF-treated SHED with carrier HA/TCP particles were subcutaneously implanted into immunocompromised mice. This experiment confirmed that bFGF treatment inhibited osteogenesis of SHED at 8 weeks post-implantation (Fig. 1E, *P<0.05). Oil red O staining demonstrated that the numbers of lipid-laden adipocytes were not significantly different between the bFGF-treated group and the control group (Fig 1F). Moreover, bFGF treatment did not affect the expression of the adipogenic markers lipoprotein lipase (LPL) and peroxisome proliferator-activated receptor 2 (PPARγ2), as determined by RT-PCR analysis (Fig. 1G).
Figure 1. bFGF inhibits SHED osteogenic differentiation.
(A) bFGF treatment did not alter the proliferation rate of SHED as assessed by BrdU incorporation assay. The proliferation rate indicated as a percentage of BrdU+ nuclei to the total nuclear cells and averaged from five independent assays. (B) Flow cytometry showed that bFGF treatment reduced expression levels of STRO-1, CD 73, CD 90, and CD 146, but had no significant effect on expression of SSEA-4. SHED are negative to CD 45 and CD 34 antibody staining. (C–E) After osteogenic inductive culture for 4 weeks, bFGF-treated SHED showed a decreased mineralized nodule formation than untreated control group as assessed by alizarin red staining. Alizarin red-positive (Alizarin Red+) area corresponding to total area was averaged from five independent groups (C). After 2 weeks osteogenic induction, expression levels of osteogenic genes Runx2 and osteocalcin (OCN) were reduced in SHED treated with bFGF compared to untreated control group as assessed by RT-PCR. GAPDH was used as an internal control (D). SHED regenerated de novo bone (B white triangle) and connective tissue (CT) around HA/TCP carrier (HA) when transplanted subcutaneously into immunocompromised mice. bFGF-treated SHED formed less bone than untreated SHED. Newly formed bone area was calculated as a percentage of total area and averaged from three independent transplant assays (E). (F, G) Oil-red O staining showed that lipid accumulation in bFGF-treated SHED was similar to untreated SHED at 4 weeks adipogenic induction. Number of oil-red O-positive (Oil-Red-O+) cells was calculated as a percentage to total cells and averaged from five independent cultures (F). RT-PCR analysis indicated that expression levels of adipocyte-specific molecules LPL and PPARγ2 were no difference between bFGF-treated SHED and untreated SHED at 4 weeks post adipogenic culture. GAPDH was used as an internal control (G). (*P<0.05)
bFGF attenuats SHED osteogenesis through ERK1/2
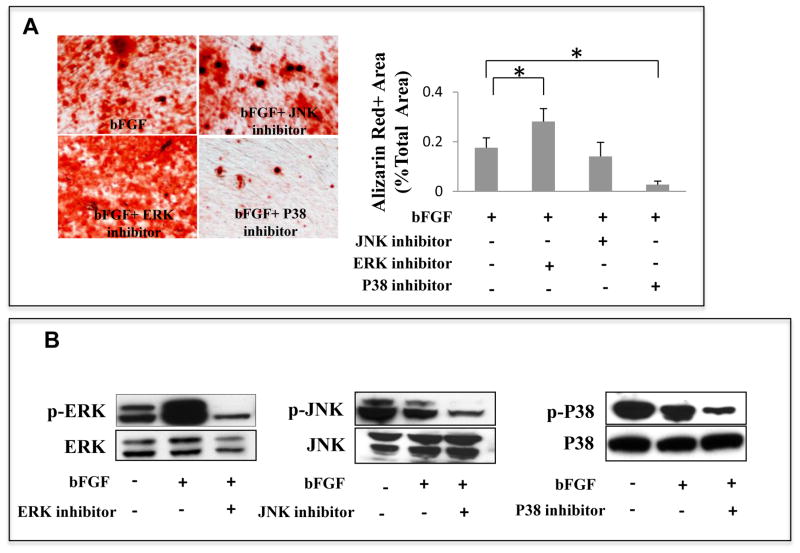
Three canonical pathways are downstream of bFGF signaling: ERK1/2, JNK, and P38. To identify the pathway involved in bFGF-induced inhibition of SHED osteogenesis, we tested whether blocking these three pathways with chemical inhibitors abolished bFGF-induced inhibition of SHED osteogenesis. From our dose-dependent experiment that inhibitors rescue bFGF-induced osteogenic deficiency of SHED (Supplementary Figure 1), we chose the most effective dose for our experiments, including 5 μM PD325901 (ERK1/2 inhibitor), 5 μM SP600125 (JNK inhibitor), and 1 μM SB203580 (P38 inhibitor). PD325901, SP600125 or SB203580 were added with bFGF to the cultured SHED for 72h. After an osteogenic induction of 4 weeks, Alizarin Red S staining showed that the inhibition of ERK1/2 restored the mineralized nodule formation that was inhibited by bFGF (Fig. 2A, *P<0.05). However, inhibition of the JNK pathway had no effect on mineralized nodule formation in SHED treated with bFGF. As expected, inhibition of P38 signaling completely suppressed the osteogenesis of SHED (Fig. 2A; Greenblatt et al., 2010). To further confirm the effects of bFGF on these signaling pathways, we conducted western blot analysis, which showed that bFGF activated ERK1/2 by increasing phosphorylated ERK1/2 levels. In contrast, inhibition of ERK1/2 abrogated ERK phosphorylation (Fig. 2B). Western blot analysis also showed that phosphorylation of JNK and P38 was not significantly affected by bFGF treatment (Fig. 2B). However, treatment with the JNK inhibitor SP600125 and the P38 inhibitor SB203580 reduced phosphorylation of both JNK and P38 (Fig. 2B). These data indicate that ERK1/2, but not JNK and P38, contribute to bFGF-induced inhibition of SHED osteogenesis.
Figure 2. ERK1/2 is down stream target of bFGF-induced osteogenesis deficiency in SHED.
(A) After 4 weeks osteogenic culture induction, bFGF-treated SHED showed a reduction in mineralized nodule formation, which was rescued by the ERK1/2 inhibitor treatment as assessed by alizarin red staining. However, the JNK and P38 inhibitors did not show any restoration of bFGF-induced reduction of mineralized nodule formation in SHED. Alizarin red-positive (Alizarin Red+) area corresponding to total area was averaged from five independent groups. (B) Western blot analysis showed that bFGF treatment increased phosphorylated ERK1/2 (p-ERK) and the ERK1/2 inhibitor treatment reduced level of phosphorylated ERK1/2 (p-ERK). bFGF appeared to decrease phosphorylated JNK (pJNK) and P38 (pP38). The JNK and P38 inhibitor treatment reduced levels of phosphorylated JNK (pJNK) and P38 (p-P38).(*P<0.05).
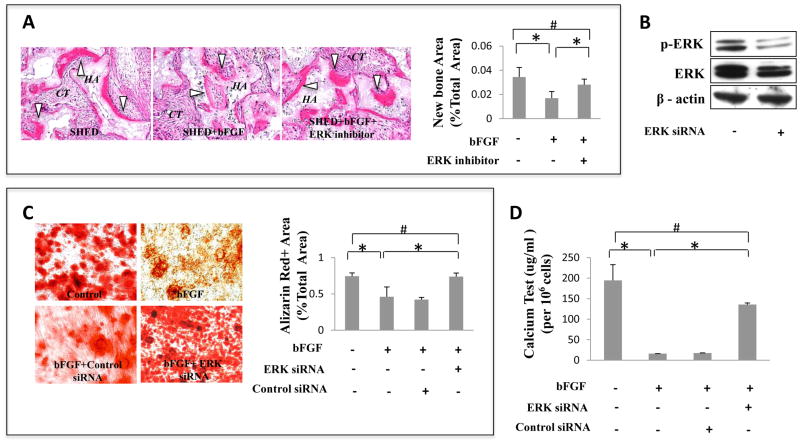
To further confirm the role of the ERK pathway in SHED osteogenic differentiation, we transplanted bFGF-treated and bFGF plus ERK inhibitor treated SHED subcutaneously into immunocompromised mice using HA/TCP (HA) as carrier. Newly formed bone area was calculated as a percentage of total area and averaged from three independent transplant assays. After bFGF treatment, SHED generated much less bone in vivo compared with control group (Fig. 3A). Abrogation of bFGF-mediated ERK signaling with ERK inhibitors increased bone formation (B white triangle) to the level observed in untreated SHED group (Fig. 3A, *P<0.05). Additionally, treatment of SHED with ERK1/2 siRNA blocked ERK1/2 signaling and restored osteogenesis to the level observed in untreated SHED group (Fig. 3C, *P<0.05). Calcium levels were also measured after acid treatment (Fig. 3D, *P<0.05). bFGF treatment decreased calcium level of SHED after osteogenic induction, but ERK siRNA treatment reversed this effect. The effect of ERK1/2 siRNA was confirmed with Western blot analysis (Fig. 3B). These data further confirm that bFGF-induced inhibition of SHED osteogenesis is mediated through ERK1/2 signaling.
Figure 3. Blockage of ERK1/2 signaling rescued bFGF-induced osteogenic deficiency of SHED.
(A) The ERK1/2 inhibitor treatment rescued bFGF-induced reduction of SHED-mediated bone formation (B white triangle) as assessed by subcutaneously implantation into immunocompromised mice using HA/TCP (HA) as carrier. Newly formed bone area was calculated as a percentage of total area and averaged from three independent transplant assays. (B) Western blot analysis showed that ERK1/2 siRNA blocked phosphorylated ERK1/2 (p-ERK). β-actin was used as an internal control. Three independent assays showed similar results. (C) After 4 weeks culture induction in osteogenic medium, bFGF-treated SHED showed decreased mineralized nodule formation than control group as assessed by alizarin red staining. ERK1/2 siRNA rescued bFGF-induced reduction of mineralized nodule formation, but control siRNA didn’t rescue the reduction. Alizarin red-positive (Alizarin Red+) area corresponding to total area was averaged from five independent groups (*P<0.05). (D) After 4 weeks culture induction in osteogenic medium, bFGF-treated SHED showed decreased calcium level of extracellular matrix than control group. ERK1/2 siRNA rescued bFGF-induced reduction of calcium level, but control siRNA didn’t rescue the reduction (*P<0.05).
ERK1/2 signaling regulates WNT pathway in SHED
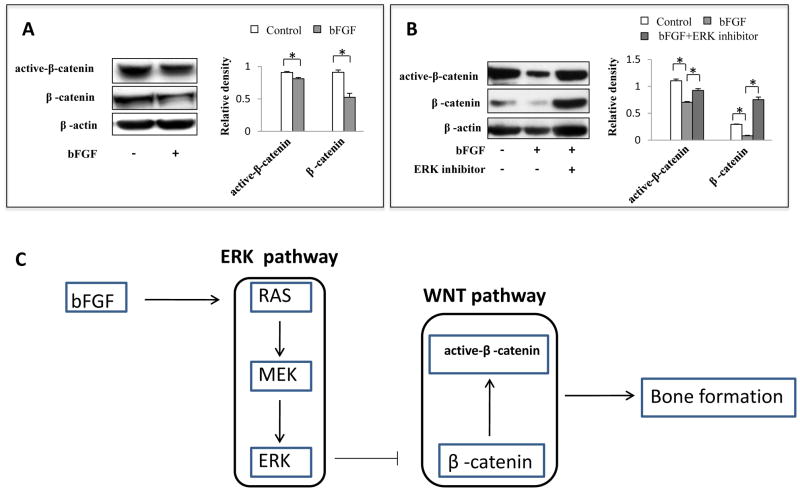
Since bFGF decreased osteogenesis of SHED by activating ERK1/2 signaling, we next investigated the downstream targets of the ERK1/2. It was reported that ERK may regulate WNT/β-catenin pathway in cancer cells (Kim et al., 2007), here we found that bFGF-induced ERK1/2 signaling inhibited both total and activated β-catenin expression (Fig. 4A, *P<0.05), suggesting that ERK1/2 signaling may regulate Wnt canonical pathway. Further, we revealed that the ERK1/2 inhibitor PD325901 abolished bFGF-induced reduction of β-catenin expression (Fig. 4B, *P<0.05), indicating that bFGF regulates WNT pathway through ERK1/2 signaling.
Figure 4. ERK1/2 signaling affects osteogenesis via regulating WNT/β-catenin pathway.
(A) Western blot analysis showed that bFGF reduced expression level of total β-catenin and active- β-catenin. β-actin was used as an internal control. Three independent assays showed similar results (*P<0.05). (B) bFGF treatment decreased β-catenin expression level and ERK1/2 inhibitor treatment increased β-catenin levels. β-actin was used as an internal control. Three independent assays showed similar results (*P<0.05). (C) bFGF activates ERK1/2 through RAS and MEK. Phosphorylated ERK1/2 inhibited β-catenin expression and then bone formation was reduced.
DISCUSSION
Autogenous bone grafts, allogeneic materials, and prosthetic compounds are currently used to reconstruct craniofacial bone defects (Marchac, 1982; Shenaq, 1988; Goodrich et al., 1992; Nicholson, 1998; Rah, 2000; Bruens et al., 2003; Cowan et al., 2004). Despite some clinical success, each of these strategies has limitations. Stem cell-based tissue engineering provides a promising alternative for craniofacial bone regeneration. Understanding the mechanisms by which stem cells regulate signaling pathways may provide tools to improve cell-based tissue regeneration.
To investigate the mechanism of SHED-mediated mineralized tissue formation, we treated SHED with high dose of bFGF in vitro and transplanted high dose of bFGF-treated SHED subcutaneously into immunocompromised mice. Our results indicated that high dose bFGF treatment reduced expression level of mesenchymal stem cell markers STRO-1, CD146, CD90 and CD73 and osteogenic differentiation of SHED. However, the proliferation rate and adipogenic differentiation were not affected by bFGF treatment, suggesting that bFGF treatment partially attenuates SHED differentiation.
The canonical downstream cascades of bFGF signaling, in terms of Ras-MAP kinase pathway that includes ERK1/2, p38, and JNK kinase (Schlessinger et al., 2000), were considered in the bone formation process. The MAP kinase is a family of proteins that regulate the activity of downstream kinase or transcription factors. The Proteins of this family share many structural similarities, in which ERK1/2 promotes the mitogenic response, while the p38 and JNK kinase are usually associated with inflammatory and stress-responses (Johnson et al., 2002). bFGF activated MAP kinase pathway in a dose dependent manner to affect proliferation and differentiation of mouse myoblast cells (Tortorella et al., 2001). In order to fully activate MAP kinase/ERK pathway, we chose a high dose of bFGF (100 ng/ml) to treat SHED (Supplementary Figure 2). According to the above reports, we tested these three downstream pathway and found that high dose of bFGF activated ERK1/2, but not P38 and JNK. ERK1/2 pathway mediates bFGF-induced osteogenesis deficiency and inhibition of ERK1/2 signaling restored SHED-based mineralized tissue regeneration. In contrast, inhibition of the p38 pathway decreased SHED osteogenesis, which is consistent with a previous report (Greenblatt et al., 2010).
Wnt/β-catenin or canonical pathway play an important role in bone biology (Westendorf et al., 2004; Rawadi et al., 2004). Activation of Wnt/β-catenin signaling occurs upon binding of Wnt to the receptors, thus causing hypophosphorylation of its substrate, β-catenin (Hay, et al., 2004). Stabilized β-catenin then accumulates in the cytosol and translocates to the nucleus, where this transcriptional coactivator interacts with osteogenic transcription factors and stimulates osteogenesis. It has been previously shown that there is a crosstalk between ERK1/2 signaling and WNT pathway (Kim et al., 2007). In this study, we found that over-activated bFGF could reduce both β-catenin and active- β-catenin production. ERK inhibitor treatment not only reversed osteogenic deficiency but also recovered the expression level of β-catenin and active- β-catenin compared with control group.
ERK signaling is involved in a number of biological processes. It may affect cell adhesion, migration or proliferation (Zohrabian et al., 2009). In this study, we only focused on its effect on osteogenesis of SHED. In summary, we conclude that over-activated bFGF signaling activates RAS-RAF-ERK pathway. Then referring to the crosstalk between ERK and WNT pathway, ERK regulates WNT and causes the bone formation change of SHED (Fig. 4C).
Supplementary Material
Acknowledgments
This work was supported by grants from National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449 R01 DE017449, R01 DE019932, and R01 DE019413 to S.S.) and California Institute for Regenerative Medicine (RN1-00572 to S.S.).
Abbreviations
- SHED
Stem cell from human exfoliated deciduous teeth
- bFGF
Basic fibroblast growth factor
- HA/TCP
hydroxyapatite/tricalcium phosphate
Footnotes
All the authors have no conflicts of interest to declare.
References
- Bruens ML, Pieterman H, de Wijn JR, Vaandrager JM. Porous polymethylmethacrylate as bone substitute in the craniofacial area. J Craniofac Surg. 2003;14(1):63–8. doi: 10.1097/00001665-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22(5):560–7. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- Galderisi U, Helmbold H, Squillaro T, Alessio N, Komm N, Khadang B, et al. In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes. Stem Cells Dev. 2009;18(7):1033–42. doi: 10.1089/scd.2008.0324. [DOI] [PubMed] [Google Scholar]
- Goodrich JT, Argamaso R, Hall CD. Split-thickness bone grafts in complex craniofacial reconstructions. Pediatr Neurosurg. 1992;18(4):195–201. doi: 10.1159/000120662. [DOI] [PubMed] [Google Scholar]
- Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. 2010;120(7):2457–73. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E, Faucheu C, Suc-Royer I, Touitou R, Stiot V, Vayssiere B, et al. Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J Biol Chem. 2005;280(14):13616–23. doi: 10.1074/jbc.M411999200. [DOI] [PubMed] [Google Scholar]
- Hurley MM, Abreu C, Harrison JR, Lichtler AC, Raisz LG, Kream BE. Basic fibroblast growth factor inhibits type I collagen gene expression in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268(8):5588–93. [PubMed] [Google Scholar]
- Hurley MM, Abreu C, Gronowicz G, Kawaguchi H, Lorenzo J. Expression and regulation of basic fibroblast growth factor mRNA levels in mouse osteoblastic MC3T3-E1 cells. J Biol Chem. 1994;269(12):9392–6. [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kim D, Rath O, Kolch W, Cho KH. A hidden oncogenic positive feedback loop caused by crosstalk between Wnt and ERK pathways. Oncogene. 2007;26(31):4571–9. doi: 10.1038/sj.onc.1210230. [DOI] [PubMed] [Google Scholar]
- Marchac D. Split-rib grafts in craniofacial surgery. Plast Reconstr Surg. 1982;69(3):566–7. doi: 10.1097/00006534-198203000-00044. [DOI] [PubMed] [Google Scholar]
- Majors AE, Muschler GF. Basic FGF enhances proliferation and reversively inhibits differentiation of osteoblastic progenitors. Trans Orth Res Soc. 1996;21:113. [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JW. Glass-ionomers in medicine and dentistry. Proc Inst Mech Eng H. 1998;212(2):121–6. doi: 10.1243/0954411981533890. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A. 1998;95(10):5672–7. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaru S, Kotev-Emeth S, Noff D, Kaffuler S, Savion N. Effect of basic fibroblast growth factor on the growth and differentiation of adult stromal bone marrow cells: Enhanced development of mineralized bone-like tissue in culture. J Bone Miner Res. 1993;8:919–29. doi: 10.1002/jbmr.5650080804. [DOI] [PubMed] [Google Scholar]
- Rah DK. Art of replacing craniofacial bone defects. Yonsei Med J. 2000;41(6):756–65. doi: 10.3349/ymj.2000.41.6.756. [DOI] [PubMed] [Google Scholar]
- Rawadi G, Roman-Roman S. Wnt signalling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets. 2005;9(5):1063–77. doi: 10.1517/14728222.9.5.1063. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34. doi: 10.1111/j.1601-0825.2007.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587–91. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- Shenaq SM. Reconstruction of complex cranial and craniofacial defects utilizing iliac crest-internal oblique microsurgical free flap. Microsurgery. 1988;9(2):154–8. doi: 10.1002/micr.1920090218. [DOI] [PubMed] [Google Scholar]
- Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276(17):13709–17. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- Varkey M, Kucharski C, Haque T, Sebald W, Uludag H. In vitro osteogenic response of rat bone marrow cells to bFGF and BMP-2 treatments. Clin Orthop Relat Res. 2006;443:113–23. doi: 10.1097/01.blo.0000200236.84189.87. [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5. doi: 10.1186/scrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza T, Miura Y, Akiyama K, Bi Y, Sonoyama W, Gronthos S, et al. Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood. 2009;113(11):2595–604. doi: 10.1182/blood-2008-10-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 2008;3(7):e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, et al. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88(3):249–54. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M. Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res. 2009;29(1):119–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.