Abstract
Background
The loss of Ikaros is associated with the development of B and T cell leukemia. Data on Ikaros function, including its role as a tumor suppressor and a regulator of cell cycle progression, come almost exclusively from murine studies; little is known of the mechanisms that regulate human Ikaros function. Our studies are the first to examine the function and regulation of human Ikaros isoforms during the cell cycle in human ALL.
Procedures
Electromobility shift assay (EMSA), confocal microscopy, and phosphopeptide mapping were used to study Ikaros function during different stages of the cell cycle.
Results
The DNA-binding activity of human Ikaros complexes undergoes dynamic changes as the cell cycle progresses. In S phase, Ikaros DNA-binding affinity for regulatory regions of its target genes decreases, while its binding to pericentromeric heterochromatin is preserved and correlates with Ikaros pericentromeric localization. These S phase-specific changes in Ikaros function are controlled by phosphorylation via the CK2 kinase pathway. During cell cycle progression, the subcellular pericentromeric localization of the largest human Ikaros isoforms is different from that in mouse cells, suggesting unique functions for human Ikaros.
Conclusions
Our results demonstrate that the function of Ikaros is cell cycle-specific and controlled by CK2-mediated phosphorylation during S phase of the cell cycle in human T-cell and B-cell ALL. The differences we observe in murine and human Ikaros function highlight the importance of using human cells in studies of ALL. These data identify the CK2 pathway as a target for therapies in ALL.
Keywords: Ikaros, leukemia, cell cycle, phosphorylation, casein kinase 2, isoforms
INTRODUCTION
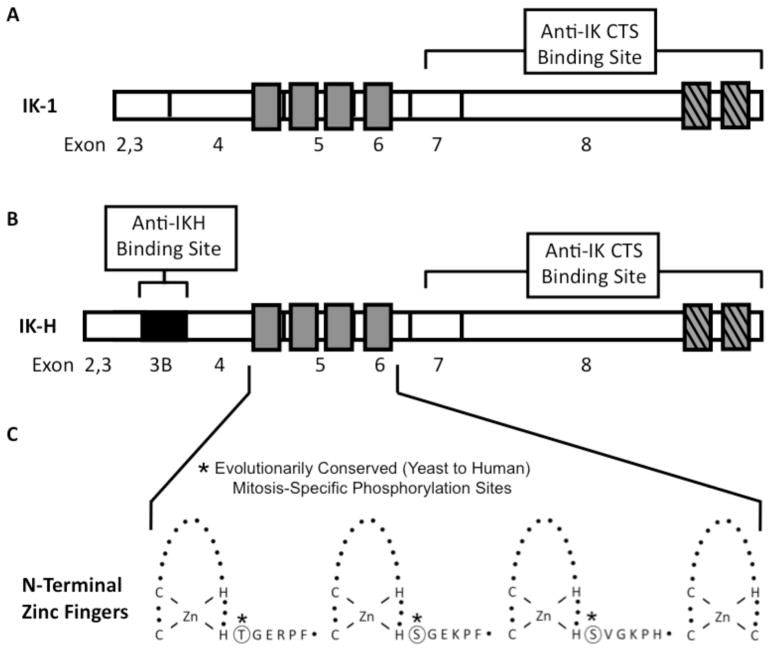
The IKAROS gene encodes DNA-binding zinc finger proteins that play essential roles in normal hematopoiesis and tumor suppression [1–8]. The two largest and most abundantly expressed Ikaros isoforms in human lymphoid cells are IK-1 and IK-H (Figure 1). IK-H contains 20 additional amino acids (compared to IK-1) that are encoded by exon 3B, making it the longest Ikaros isoform [9–11]. IK-H is essentially absent from mouse but abundantly expressed in human hematopoietic cells [9]. Coordinated expression of IK-1 and IK-H regulate DNA-binding of Ikaros complexes [9] and expression of Ikaros target genes [9,12]. The DNA-binding zinc fingers of Ikaros isoforms are connected by an evolutionarily conserved linker. The sequence of this linker is identical in murine and human Ikaros and is highly conserved among all C2H2 zinc finger proteins. This linker undergoes mitosis-specific phosphorylation (Figure 1C – circled residues) and this phosphorylation regulates detachment of C2H2 zinc finger proteins from DNA [13]. Additional DNA-binding Ikaros isoforms have been described (e.g., IK-X, IK-2) [10,11,14, 15] as well as splice variants that contain the C terminal zinc fingers responsible for protein interactions but which lack the DNA-binding domains. These can function as dominant-negatives and inhibit the activity of the DNA-binding Ikaros isoforms [10,11,14–16] Ikaros complexes bind to the upstream regulatory elements (URE) of Ikaros target genes and aid in their recruitment to pericentromeric heterochromatin (PC-HC) where they are transcriptionally activated or silence 0d [17,18]. Thus, Ikaros function depends on its DNA-binding activity and pericentromeric localization, both of which are regulated by phosphorylation [19–21] and by the expression of the IK-H isoform in human cells [12,15].
Figure 1. Structure of the IK-1 and IK-H Ikaros isoforms.
(A–B) Exon one is untranslated (not shown), exons 2 through 8 and 3B (black) are indicated. Hatched gray bars represent C-terminal zinc fingers that participate in dimerization and higher order complex formation. Open gray bars represent N-terminal DNA-binding zinc fingers. The segments of Ikaros protein that were used to generate specific anti-IK-H antibodies and anti-IK-CTS antibodies are indicated. C. Expansion of the zinc finger region to show the zinc fingers (loops) and the amino acid sequence of evolutionarily conserved linkers. Evolutionarily conserved phosphorylation sites are indicated.
In humans, the loss of Ikaros function has been associated with childhood and adult ALL, and deletion of an IKAROS allele has been identified as a poor prognostic marker for childhood ALL [22–34]. These data established Ikaros as a major tumor suppressor in human leukemia. The mechanisms by which Ikaros regulates cellular proliferation in human cells remains unknown. Data on Ikaros tumor suppressor activity come from studies of murine Ikaros–very few studies have examined the function of human Ikaros proteins in leukemia.
Here we report the first functional studies of human Ikaros proteins during the different stages of the cell cycle. Our results demonstrate that the DNA-binding activity of Ikaros is cell cycle-specific and controlled by CK2-mediated phosphorylation during S phase of the cell cycle. We show that the subcellular pericentromeric localization of the IK-1 and IK-H isoforms during the cell cycle is different from that observed in mouse cells. These data provide the first evidence that the cell cycle-specific activities of Ikaros in human leukemia are regulated by the CK2 pathway, and are distinct from those of murine Ikaros. Our results provide a foundation for determining the role of Ikaros in controlling cell cycle progression in human leukemia and identify CK2 as a potential target for treatment of ALL.
METHODS
Antibodies
Antibodies against the C-terminus of Ikaros (IK-CTS) and the IK-H protein, and secondary antibodies for visualization are as described previously [9,35] and in more detail in Supplemental Appendix I.
Cells, Plasmids, and Retroviral Transduction
MOLT-4 and CCRF-CEM (CEM) cells are early human T leukemia cell lines while Nalm6 is a human B-ALL cell line. Retroviral constructs containing IK-H, IK-1 or HA-tagged IK-1, as well as transduction were as described previously [9] and in more detail in Supplemental Appendix I.
Flow Cytometry
MOLT-4 and CEM cells were treated with mimosine (0.3 mM), hydroxyurea (1 mM) followed by three-hour release, or vinblastine (0.2 μM) to arrest the cells in G1, S or G2/M phase respectively. DNA content was determined by propidium iodide (PI) staining. Cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA).
Confocal Microscopy
CEM cells were infected with amphotropic retrovirus and analyzed by confocal microscopy as described previously [19] and in more detail in Supplemental Appendix I.
Biochemical Experiments
Nuclear extractions, Western blots, gel-shift experiments, and two-dimensional phosphopeptide mapping were performed as described previously [9,13,19,20,36]. Gel shift probes CENP-B, γ sat 8, α Sat, VPAC-1, Granzyme B, and IKCa1 have been described previously [9] and additional details are in Results and in Supplemental Appendix I.
RESULTS
DNA-Binding Ability of Human Ikaros is Cell Cycle Specific
We have previously shown that the DNA-binding of murine Ikaros is abolished during mitosis [13]. Neither the DNA-binding ability of murine Ikaros during S phase, nor the cell cycle-specific DNA binding of Ikaros in human leukemia have been studied. Here, we examine the ability of human Ikaros to bind DNA during different phases of the cell cycle using human T- and B-ALL cell lines.
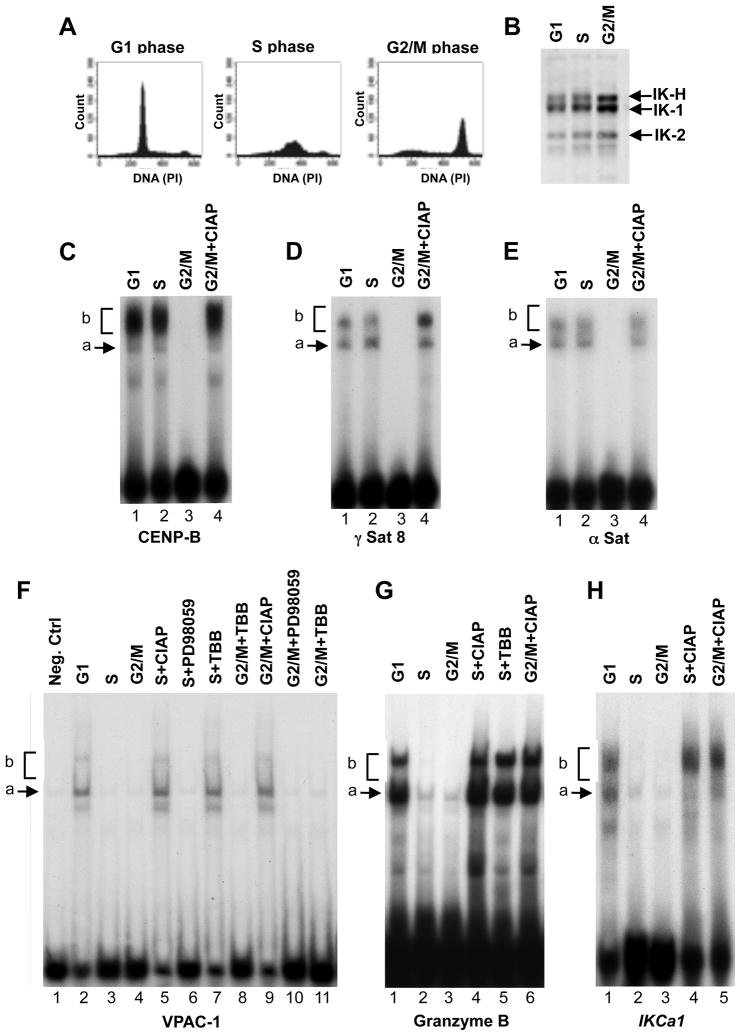
Cells were arrested in G1, S, or G2/M phase of the cell cycle, and nuclear extracts were normalized for the amount of expressed Ikaros protein by Western blot (Figure 2A–B). The DNA-binding ability of Ikaros was studied by EMSA (Figures 2C–H). Probes derived from repetitive sequences that are located at human PC-HC (CENP-B, γ sat 8, and α Sat) were used to asses pericentromeric binding of Ikaros proteins (Figures 2C–E). Probes derived from the URE [9] of previously identified Ikaros target genes (VPAC-1, Granzyme B, and IKCa1) were used to asses the ability of Ikaros protein to bind to regulatory regions upstream of Ikaros target genes (Figures 2F–H). [9,37,38]
Figure 2. DNA binding of Ikaros during cell cycle.
(A) MOLT-4 cells were arrested in G1, in S, or in G2/M phase of the cell cycle, and assessed for DNA content by flow cytometry. (B) Nuclear extracts were obtained from cells at each phase, and normalized for Ikaros content by Western blot. (C–H) DNA binding of nuclear extracts from cells at indicated stages of cell cycle with probes derived from PC-HC (B–D) or the URE of indicated genes (E–G). Ikaros DNA-binding complexes are indicated as a) arrows indicating dimers that contain Ikaros isoforms and b) brackets indicating tetramers/higher order complexes of Ikaros isoforms.
Results from MOLT-4 T-ALL cells in Figure 2 show that Ikaros’ DNA-binding is strongest during G1 phase of the cell cycle (Figures 2C–H). As predicted based on murine studies, the ability of human Ikaros to bind DNA is almost abolished during mitosis (G2/M) (Figures 2C–H).
During S phase, the Ikaros DNA-binding varied depending on the DNA probe. With PC-HC probes, Ikaros was able to bind equally well in G1 and S phases (Figures 2C–E lane 2 compared to lane 1). However, with URE probes, Ikaros binding was diminished in S phase as compared to G1 phase of the cell cycle (Figure 2F lane 3 compared to lane 2, and Figures 2G, H lane 2 compared to lane 1). These results demonstrate that the ability of human Ikaros to bind DNA is cell cycle-regulated and varies based on the target sequence.
These data suggest that during S phase of the cell cycle, Ikaros loses its ability to bind the URE of its target genes, while retaining its DNA binding activity toward PC-HC.
Phosphorylation Regulates the Cell Cycle-Specific DNA-Binding Ability of Human Ikaros
Phosphorylation is a major regulatory mechanism of Ikaros DNA-binding activity [13,19,20]. We tested whether phosphorylation of Ikaros is responsible for the loss of its DNA-binding activity toward the URE of its target genes during S phase of the cell cycle. To dephosphorylate Ikaros proteins, nuclear extracts from MOLT-4 cells in G1 and S phase were treated with calf intestinal alkaline phosphatase (CIAP) followed by EMSA. Dephosphorylation of Ikaros proteins in S phase by CIAP, restored the ability of Ikaros to bind the DNA probes derived from URE of Ikaros target genes (Figure 2F lane 5 and Figures 2G–H lanes 4). These data provide evidence that the phosphorylation of Ikaros is responsible for the cell cycle-specific loss of Ikaros binding to the URE of its target genes in S phase of the cell cycle in human cells.
CK2 kinase phosphorylates Ikaros in vivo [19]. We tested whether CK2-mediated phosphorylation of Ikaros regulates its S phase-specific DNA-binding. Inhibition of CK2 kinase by a specific inhibitor (TBB) in S phase resulted in the restoration of Ikaros DNA-binding (Figure 2F lane 7, and Figure 2G lane 5). Treatment with the ERK2 kinase inhibitor, PD98059, did not restore Ikaros binding activity (Figure 2F lane 6), confirming that CK2-specific inhibition is required to restore Ikaros binding activity during S phase of the cell cycle. These results suggest that the phosphorylation of Ikaros by CK2 causes the decreased Ikaros binding to the UREs of Ikaros target genes observed during S phase of the cell cycle.
We previously showed that phosphorylation at an evolutionarily conserved zinc finger linker is responsible for the lack of Ikaros DNA binding during mitosis in mouse cells [13]. To confirm that this is also the case in human leukemia, nuclear extracts from cells arrested in G2/M phase of the cell cycle were treated with CIAP. Dephosphorylation of Ikaros following CIAP-treatment restored the ability of Ikaros to bind both PC-HC and URE probes in G2/M phase (Figures 2C–E, lane 4; Figure 2F, lane 9; Figure 2G lane 6; Figure 2H, lane 5). This confirms that phosphorylation is responsible for the lack of Ikaros DNA-binding activity during G2/M phase of the cell cycle. Treatment with TBB or PD598059 failed to restore Ikaros DNA-binding activity during G2/M phase, providing evidence that a kinase other than CK2 or ERK2 is responsible for the absence of Ikaros binding during G2/M phase.
Ikaros acts as a tumor suppressor in both T-cell and B-cell ALL. To determine if Ikaros function during the cell cycle in B-ALL is regulated in the same way as we observed for T-ALL, the DNA-binding experiments described above were performed using the human Nalm6 B-ALL cell line. The results obtained for Nalm6 B-ALL were the same as those obtained for T-ALL cells (Supplemental Figure 1). These data demonstrate that the cell cycle-specific DNA-binding of Ikaros and its regulation by CK2 are the same in T- and B-ALL cells.
Human IK-H Exhibits a Cell Cycle Phosphorylation Pattern that is Distinct from that of IK-1
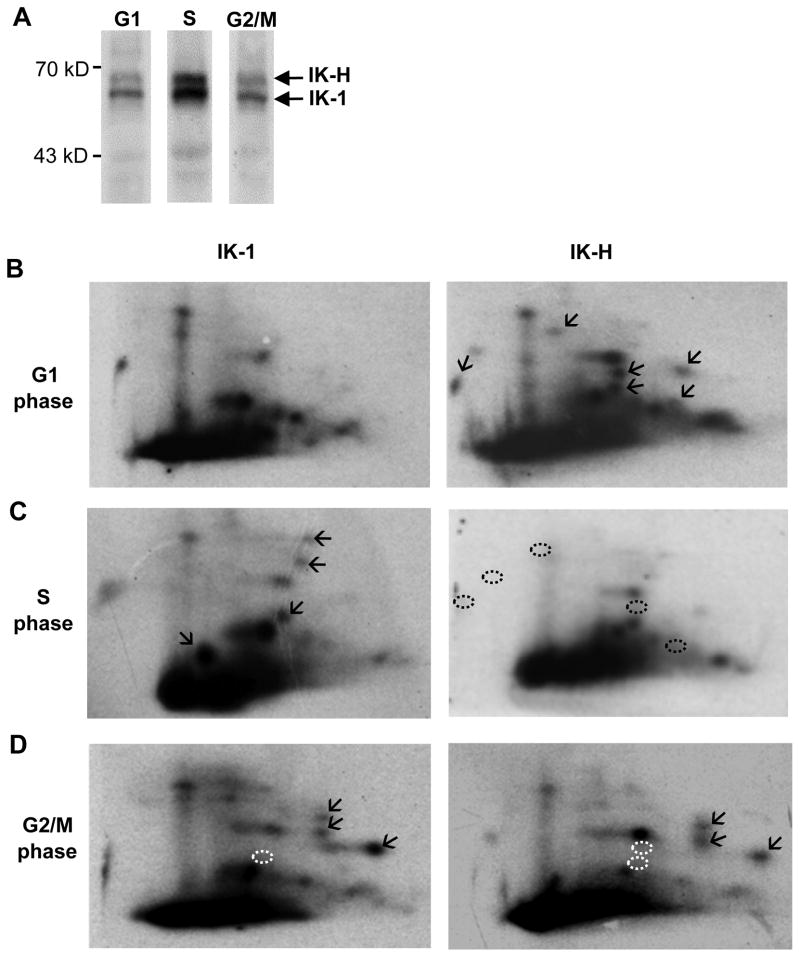
The above data suggest that the cell cycle-specific DNA binding of Ikaros is regulated by phosphorylation. To determine if the pattern of Ikaros phosphorylation changes during the cell cycle, phosphopeptide mapping was performed on IK-1 and IK-H from MOLT-4 cells arrested in G1, S, or G2/M phase of the cell cycle (Figure 3A).
Figure 3. Phosphopeptide mapping of human Ikaros isoforms during cell cycle.
(A) MOLT-4 cells were grown in the presence of 32P labeled orthophosphate and arrested in G1, S, or G2/M phase, and Ikaros isoforms were immunoprecipitated. (B–D) Phosphopeptide maps were generated for IK-1 and IK-H obtained at indicated phases of cell cycle. (B) Arrows mark phosphorylated sites detected only in the right panel (C) Arrows indicate phosphorylated sites not detected in panel above. Black dashed circles mark the absence of phosphorylated sites detected in panel above. (D) Arrows mark previously described mitosis-specific phosphorylation of the evolutionarily conserved linker. White dashed circles mark the absence of phosphorylation sites detected in either G1 or S phase.
In G1 phase Ikaros isoforms had distinct phosphorylation patterns (Figure 3B). IK-H showed additional phosphorylation sites (marked by arrows in Figure 3B right panel) that were not observed for IK-1 (left panel).
During S phase, each Ikaros isoform again exhibited a unique phosphorylation pattern (Figure 3C). IK-1 was hyperphosphorylated at multiple sites compared to G1 phase of the cell cycle (marked by arrows in Figure 3C left panel as compared to Figure 3B left panel), while IK-H was hypophosphorylated in S phase as compared to G1 phase (dephosphorylated peptides marked with dashed black circles in Figure 3C right panel as compared to Figure 3B right panel).
During mitosis (G2/M), both isoforms showed mitosis-specific phosphorylated sites (marked with arrows, Figure 3D). This mitosis-specific phosphopeptide pattern of human Ikaros is identical to that reported for murine Ikaros [13]. These phosphopeptides are due to evolutionarily conserved phosphorylation sites [13] in the three linkers that connect zinc finger motifs in murine and human Ikaros which share identical sequence (Figure. 1C).
Taken together, phosphopeptide maps of IK-1 and IK-H during the cell cycle revealed dynamic changes in phosphorylation status that are unique for each protein suggesting a distinct function for each isoform.
Human Ikaros Isoforms Exhibit a Unique Cell Cycle Localization Pattern
In murine cells, Ikaros complexes are reorganized during the cell cycle [17]. The above data demonstrate that, during the cell cycle, the DNA-binding ability of human Ikaros isoforms varies and the phosphorylation status of IK-1 and IK-H are differentially regulated. Given that these features are likely to be major determinants of Ikaros localization, we examined the localization of IK-1 and IK-H proteins during the cell cycle. [17]
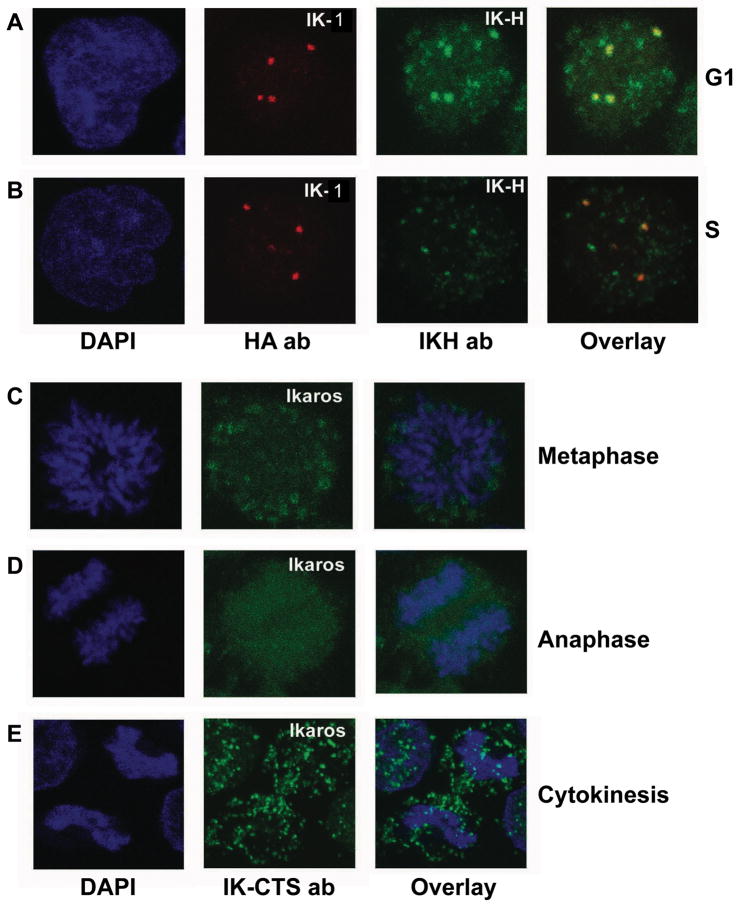
To distinguish the localization of IK-1 and IK-H, isoforms, CEM cells were transduced to express HA-tagged IK-1 and cells were arrested at G1 or S phase of the cell cycle (Figures 4A and B respectively). HA-antibodies were used to detect IK-1, while IK-H antibodies were used to detect the K-H isoform. In both G1 and S phases, IK-1 localizes primarily to PC-HC, while endogenous IK-H has both pericentromeric and non-pericentromeric localization (Figures 4A and B), as seen previously [9]. In S phase, the non-pericentromeric localization of IK-H appears less prominent when compared to G1 phase (Figure 4B), possibly due to the dephosphorylation of this isoform that we observed during S phase (Figureure 3C, right panel).
Figure 4. Subcellular localization of human Ikaros isoforms during cell cycle.
(A–B) CEM T-ALL cells infected with amphotrophic retrovirus expressing HA-tagged hIK-VI. Cells were arrested in G1 or S phase as described previously and stained for confocal microscopy. DAPI stain shows nuclear DNA (left panels). HA-IK-VI was detected with anti-HA antibody (red) and endogenous hIK-H with IK-H antibody (green). The combined image from anti-HA and IK-H staining is shown in the right panel. (C–E) Cycling CEM cells at various stages of cell cycle were selected based on DAPI staining (blue). Staining with IK-CTS antibody (green) detects all Ikaros isoforms. The combined image from DAPI and IK-CTS staining is shown in the right panel. Data shown are representative of cells at each stage.
In mouse cells, Ikaros localization changes markedly from G1 to S phase where Ikaros loses its pericentromeric localization and becomes diffusely distributed throughout the nucleus [17]. In contrast, S phase pericentromeric localization of Ikaros was retained in human cells and this correlated with S phase binding of Ikaros to PC-HC probes as shown in Figures 2C–E and Supplemental Figure 1B–D. In addition, diffuse, non-pericentromeric Ikaros localization was simultaneously reduced during S phase as compared to G1 phase (Figure 4B vs. 4A right panels). These data underscore the functional differences in Ikaros proteins in human vs. murine hematopoietic cells.
We examined the subcellular localization of human Ikaros by confocal microscopy during mitosis. Since, during mitosis, both IK-1 and IK-H show identical hyperphosphorylation at an evolutionarily conserved site in linkers that connect DNA-biding zinc fingers (Figure 1C), and this phosphorylation pattern is consistent with loss of DNA binding and pericentromeric localization [13], we used only IK-CTS antibodies to detect endogenous Ikaros in uninfected cells. Confocal microscopy showed that human Ikaros is dissociated from compacted chromosomes during all stages of mitosis–metaphase, late anaphase, and cytokinesis (Figures ure 4C–E). This subcellular localization of human Ikaros during mitosis resembles that observed in mouse cells [13]. This is most likely due to mitosis-specific phosphorylation of the evolutionarily conserved linker sequence that connects zinc finger motifs (Figure 1).
DISCUSSION
Ikaros acts as a tumor suppressor in human leukemia. The loss of Ikaros function due to deletion or mutation has been detected in 30% of B-ALL and 5% of T-ALL [25]. The mechanisms by which Ikaros function is regulated are still unknown, and almost all previous studies of Ikaros function in leukemia and normal hematopoiesis were performed in murine cells. CK2 kinase has been reported to control Ikaros function in mouse cells, but no studies in human leukemia cells have been performed. Overexpression of CK2 kinase is associated with various types of human cancer including AML [39,40], where increased expression of CK2 correlates with negative clinical outcome [40]. The pro-oncogenic activity of CK2 kinase has been documented in an animal model of T-cell leukemia [41–43]. Inhibitors of CK2 kinase have been tested as a potential treatment of CLL [44]. Our data provide the first evidence that: 1) CK2 kinase regulates Ikaros function in human T- and B-ALL; 2) Ikaros function is cell cycle-specific and regulated by CK2-mediated phosphorylation in S phase of the cell cycle; 3) Human Ikaros isoforms function differently from their murine counterparts during the cell cycle suggesting that the use of human hematopoietic cells will be essential for studies of Ikaros function in human leukemia. These studies identify CK2 kinase as a potential target for treatment of ALL.
Our results revealed that human Ikaros proteins have unique cell cycle-specific functions that are different from those observed for murine Ikaros. The most striking observation is that during S phase, Ikaros has high DNA-binding affinity toward PC-HC, while its ability to bind the URE of its target genes is simultaneously diminished (Figure 2) as compared to G1 phase. This observed DNA binding affinity correlates with data from confocal microscopy, which showed the retention of pericentromeric Ikaros localization (Figure 4). This is quite different from what has been observed in the mouse where Ikaros is distributed diffusely in the nucleus during S phase [17]. Our results provide evidence that CK2-mediated phosphorylation of Ikaros regulates S phase-specific alteration in the DNA-binding affinity of human Ikaros.
Although CK2-mediated phosphorylation of Ikaros has been observed previously [19,45], our data demonstrate for the first time that CK2-mediated phosphorylation inhibits Ikaros binding to the URE of its target genes during S phase of the cell cycle. A possible explanation for these results is that Ikaros localization to PC-HC is essential for proper chromatin organization during DNA replication in S phase in human cells. The difference in Ikaros pericentromeric localization during S phase in human and mouse cells might reflect unique organization of human PC-HC [12].
Normal Ikaros DNA-binding function is essential for its pericentromeric localization in human cells. Deletion and/or mutation of one of the IKAROS alleles results in loss of its pericentromeric localization in B-cell ALL [46], T cells [36] and T-cell ALL [25]. This is believed to be due to the dominant negative effect of IKAROS alleles with deletions or mutations. The phosphorylation of specific amino acids, including those that are distant from the DNA-binding zinc fingers of Ikaros, have been shown to alter Ikaros’ DNA-binding ability, pericentromeric localization, and its ability to control cell cycle progression [13,19,20,45]. Thus, the unique phosphorylation patterns that we observed for IK-1 and IK-H are likely to be functionally significant. [9]
In summary, we present the first functional analysis of the largest Ikaros isoforms (IK-1 and IK-H) during the different phases of the cell cycle in human leukemia. Our results suggest that Ikaros function in regulating gene expression and in chromatin remodeling is cell cycle-specific, and controlled by direct phosphorylation via the CK2 kinase signal transduction pathway (Figure 5). Presented data underscore the critical role of CK2 kinase in regulating Ikaros function during S phase of the cell cycle. The distinct subcellular localization, along with the differential expression of IK-H in human and mouse hematopoiesis, strongly suggests that the mechanisms by which Ikaros regulates hematopoiesis and cellular proliferation in human cells are distinct and more complex than in mouse cells. Our data suggest that the use of human cells is essential for studies of Ikaros in human leukemia and identify CK2 kinase as a potential therapeutic target for ALL.
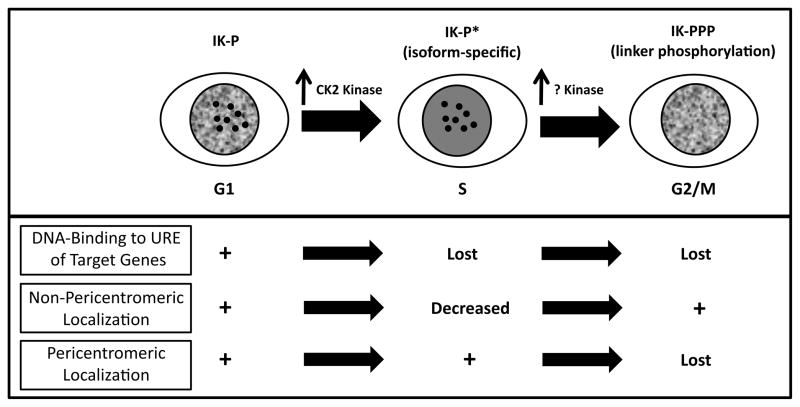
Figure 5. Summary of the cell cycle-specific changes in Ikaros phosphorylation and function in human cells.
During G1-S transition, Ikaros undergoes changes in phosphorylation (marked P*). Increased phosphorylation of IK-1 by CK2 kinase, results in the loss of DNA binding to the URE of Ikaros target genes. During mitosis, Ikaros undergoes hyperphosphorylation at the linker sequence by an unknown kinase resulting in the loss of pericentromeric localization.
Supplementary Material
Acknowledgments
This work was supported by R01 HL095120, a St. Baldrick’s Foundation Career Development Award, the Four Diamonds Fund of Pennsylvania State University, College of Medicine, and the John Wawrynovic Leukemia Research Scholar Endowment (SD). This work was also supported by the Center for Health Disparities and Molecular Medicine and the Division of Anatomy, Loma Linda University School of Medicine (KJP).
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
References
- 1.Nichogiannopoulou A, et al. Defects in hemopoietic stem cell activity in Ikaros mutant mice. J Exp Med. 1999;190(9):1201–14. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JH, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5(6):537–49. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 3.Lopez RA, et al. Multiple hematopoietic defects and delayed globin switching in Ikaros null mice. Proc Natl Acad Sci U S A. 2002;99(2):602–7. doi: 10.1073/pnas.022412699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papathanasiou P, et al. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;19(1):131–44. doi: 10.1016/s1074-7613(03)00168-7. [DOI] [PubMed] [Google Scholar]
- 5.Dumortier A, et al. Ikaros regulates neutrophil differentiation. Blood. 2003;101(6):2219–26. doi: 10.1182/blood-2002-05-1336. [DOI] [PubMed] [Google Scholar]
- 6.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–99. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 7.Okano H, et al. Homozygous deletions and point mutations of the Ikaros gene in gamma- ray-induced mouse thymic lymphomas. Oncogene. 1999;18(48):6677–83. doi: 10.1038/sj.onc.1203100. [DOI] [PubMed] [Google Scholar]
- 8.Rebollo A, Schmitt C. Ikaros, Aiolos and Helios: transcription regulators and lymphoid malignancies. Immunol Cell Biol. 2003;81(3):171–5. doi: 10.1046/j.1440-1711.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- 9.Ronni T, et al. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282(4):2538–47. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- 10.Payne KJ, et al. Cutting edge: predominant expression of a novel Ikaros isoform in normal human hemopoiesis. J Immunol. 2001;167(4):1867–70. doi: 10.4049/jimmunol.167.4.1867. [DOI] [PubMed] [Google Scholar]
- 11.Payne KJ, et al. Ikaros isoform x is selectively expressed in myeloid differentiation. J Immunol. 2003;170(6):3091–8. doi: 10.4049/jimmunol.170.6.3091. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, et al. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 2009;19(4):533–44. doi: 10.1101/gr.086496.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dovat S, et al. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 2002;16(23):2985–90. doi: 10.1101/gad.1040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis OL, et al. Regulator of myeloid differentiation and function: The secret life of Ikaros. World J Biol Chem. 2011;2(6):119–25. doi: 10.4331/wjbc.v2.i6.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, et al. Ikaros isoforms: The saga continues. World J Biol Chem. 2011;2(6):140–5. doi: 10.4331/wjbc.v2.i6.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. Embo J. 1996;15(19):5358–69. [PMC free article] [PubMed] [Google Scholar]
- 17.Brown KE, et al. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91(6):845–54. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 18.Liberg D, Smale ST, Merkenschlager M. Upstream of Ikaros. Trends Immunol. 2003;24(11):567–70. doi: 10.1016/j.it.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Gurel Z, et al. Recruitment of ikaros to pericentromeric heterochromatin is regulated by phosphorylation. J Biol Chem. 2008;283(13):8291–300. doi: 10.1074/jbc.M707906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popescu M, et al. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284(20):13869–80. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song C, et al. Regulation of Ikaros function by casein kinase 2 and protein phosphatase 1. World J Biol Chem. 2011;2(6):126–31. doi: 10.4331/wjbc.v2.i6.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–64. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 23.Mullighan CG, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 24.Kuiper RP, et al. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–66. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- 25.Marcais A, et al. Genetic inactivation of Ikaros is a rare event in human T-ALL. Leuk Res. 2010;34(4):426–9. doi: 10.1016/j.leukres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Dovat S, Payne KJ. Tumor suppression in T cell leukemia--the role of Ikaros. Leuk Res. 2010;34(4):416–7. doi: 10.1016/j.leukres.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunger SP, et al. Improving outcomes for high-risk ALL: translating new discoveries into clinical care. Pediatr Blood Cancer. 56(6):984–93. doi: 10.1002/pbc.22996. [DOI] [PubMed] [Google Scholar]
- 28.Mullighan CG. Genetic variation and the risk of acute lymphoblastic leukemia. Leuk Res. 34(10):1269–70. doi: 10.1016/j.leukres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Mullighan CG. New strategies in acute lymphoblastic leukemia: translating advances in genomics into clinical practice. Clin Cancer Res. 17(3):396–400. doi: 10.1158/1078-0432.CCR-10-1203. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, et al. Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1999;96(2):680–5. doi: 10.1073/pnas.96.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakase K, et al. Dominant negative isoform of the Ikaros gene in patients with adult B- cell acute lymphoblastic leukemia. Cancer Res. 2000;60(15):4062–5. [PubMed] [Google Scholar]
- 32.Mullighan CG, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastner P, Chan S. Role of Ikaros in T-cell acute lymphoblastic leukemia. World J Biol Chem. 2011;2(6):108–14. doi: 10.4331/wjbc.v2.i6.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dovat S, et al. Ikaros, CK2 kinase, and the road to leukemia. Mol Cell Biochem. 2011 doi: 10.1007/s11010-011-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahm K, et al. The lymphoid transcription factor LyF-1 is encoded by specific, alternatively spliced mRNAs derived from the Ikaros gene. Mol Cell Biol. 1994;14(11):7111–23. doi: 10.1128/mcb.14.11.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman FD, et al. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the ikaros gene. Pediatr Blood Cancer. doi: 10.1002/pbc.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wargnier A, et al. Down-regulation of human granzyme B expression by glucocorticoids. Dexamethasone inhibits binding to the Ikaros and AP-1 regulatory elements of the granzyme B promoter. J Biol Chem. 1998;273(52):35326–31. doi: 10.1074/jbc.273.52.35326. [DOI] [PubMed] [Google Scholar]
- 38.Ghanshani S, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275(47):37137–49. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad KA, et al. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16(10):1037–43. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Kim JS, et al. Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res. 2007;13(3):1019–28. doi: 10.1158/1078-0432.CCR-06-1602. [DOI] [PubMed] [Google Scholar]
- 41.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21(34):5280–8. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 42.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. Embo J. 1996;15(19):5160–6. [PMC free article] [PubMed] [Google Scholar]
- 43.Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267(5199):894–7. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 44.Martins LR, et al. Targeting CK2 overexpression and hyperactivation as a novel therapeutic tool in chronic lymphocytic leukemia. Blood. 2010;116(15):2724–31. doi: 10.1182/blood-2010-04-277947. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-del Arco P, Maki K, Georgopoulos K. Phosphorylation controls Ikaros’s ability to negatively regulate the G(1)-S transition. Mol Cell Biol. 2004;24(7):2797–807. doi: 10.1128/MCB.24.7.2797-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun L, et al. Expression of aberrantly spliced oncogenic ikaros isoforms in childhood acute lymphoblastic leukemia. J Clin Oncol. 1999;17(12):3753–66. doi: 10.1200/JCO.1999.17.12.3753. [DOI] [PubMed] [Google Scholar]
- 47.Cobb BS, et al. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes and Development. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kathrein KL, et al. Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol Cell Biol. 2005;25(5):1645–54. doi: 10.1128/MCB.25.5.1645-1654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.