Abstract
Purpose
To validate the utility and performance of a T2* correction method for hepatic fat quantification in an animal model of both steatosis and iron overload.
Materials and Methods
Mice with low (n=6), medium (n=6) and high (n=8) levels of steatosis were sedated and imaged using a chemical shift-based fat-water separation method to obtain MRI fat-fraction measurements. Imaging was performed before and after each of two superparamagnetic iron oxide injections to create hepatic iron overload. Fat-fraction maps were reconstructed with and without T2* correction. Fat-fraction with and without T2* correction, and T2* measurements were compared after each injection. Liver tissue was harvested and imaging results were compared to triglyceride extraction and histology grading.
Results
Excellent correlation was seen between MRI fat-fraction and tissue-based fat quantification. Injections of SPIOs led to increases in R2* (=1/T2*). Measured fat-fraction was unaffected by the presence of iron when T2* correction was used, whereas measured fat-fraction dramatically increased without T2* correction.
Conclusion
Hepatic fat-fraction measured using a T2*-corrected chemical shift-based fat-water separation method was validated in an animal model of steatosis and iron overload. T2* correction enables robust fat-fraction estimation in both the presence and absence of iron, and is necessary for accurate hepatic fat quantification.
Keywords: Hepatic steatosis, iron overload, IDEAL, chemical shift, mice, T2* correction, SPIO
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease(1), paralleling the obesity and diabetes epidemics in the United States and other Western societies(2). Biopsy, the current gold standard for quantitative assessment of hepatic steatosis is limited by sampling variability(3) and subjective semi-quantitative grading(4), as well as the cost and morbidity associated with biopsy. For these reasons, there is a growing and unmet need for non-invasive, quantitative biomarkers of the disease features of NAFLD, including steatosis.
Magnetic resonance (MR) is highly sensitive to signal differences between water and fat, and extensive recent technical development has led to methods with great potential to quantify fat accurately and noninvasively(5-8). MR imaging (MRI) has been shown to quantify fat noninvasively, and has been proven to be more accurate for quantifying fat than other radiological techniques, such as ultrasound and CT(9). Further, MRI, unlike MR spectroscopy (MRS), can assess fat over the entire volume of the liver, which is advantageous because steatosis commonly has a heterogeneous distribution(10). Therefore, quantitative MRI methods may be a viable adjunct to biopsy for accurate quantification of liver fat.
In order for an MRI fat-water separation technique to provide quantitative estimates of fat, corrections for several known confounding factors must be performed(11, 12). Such confounding factors include the spectral complexity of fat(13, 14), noise bias and T1 bias(15), extraneous phase shifts such as those caused by eddy currents(16), and T2* decay(13, 14, 17). Methods to avoid or correct for these confounding factors have been extensively studied in phantoms(8, 14), animal models(6, 18) and in human studies that use MRS as the reference standard(5, 7, 11, 12, 19). Unfortunately, there has been a relative lack of in vivo data demonstrating the importance of T2* correction, particularly in the presence of iron overload, which is well known to accelerate T2* decay. Iron overload is known to occur concomitantly with NAFLD in many patients(20, 21). While the role of iron in the pathogenesis of NASH remains uncertain, its presence in this disease is highly relevant to MRI methods attempting to quantify fat. Iron has a profound impact on signal decay, characterized by the exponential time constant T2* of the MRI signal(22). While steatosis and iron overload can occur simultaneously in patients with NAFLD(20), few studies have reported simultaneous fat-fraction and R2* (=1/T2*) measurements in vivo(5, 23, 24).
Comprehensive histology grading and tissue triglyceride extraction is possible in animals(6), allowing for complete validation of MRI with known methods of fat quantification(11). Although triglycerides are solely responsible for MRI-visible fat signal, few studies have compared MRI fat-fraction with extracted triglycerides. Few human studies exist that use biopsy as the reference standard and none exist that use chemical extraction of triglycerides as the reference. In patient studies, triglyceride extraction is rarely performed due to the destruction of the limited sample size, and as discussed previously, the sample size of biopsy may not accurately represent the entire liver. While different measures of fat exist (MRI proton density fat fraction, histology grading, lipid extraction), no single study has thoroughly compared multiple measures of fat to each other.
Animal studies are very helpful, allowing rigorous testing and validation of imaging findings using multiple metrics that may not have been possible to perform in humans. In addition, the amount of steatosis and iron can be carefully controlled to create simultaneous hepatic steatosis and iron overload. Unlike humans, larger sample sizes of tissue can be used for analysis, multiple analyses can be performed, and large numbers of animals in a shorter time frame than human subjects can be used. Further, almost all human studies have relied upon MR spectroscopy as the reference standard, where one magnetic resonance technique is used to validate another.
Therefore, the purpose of this work is to use an animal model of steatosis and iron overload to evaluate the utility and performance of a T2* correction algorithm used as part of a chemical shift-based MRI fat quantification method. First, we describe an animal model of both steatosis and iron overload, and test the performance of MRI fat quantification without and with T2* correction, and thereby demonstrate the necessity of T2* correction. Further, quantitative MRI fat-fraction measurements are compared to triglyceride extraction and histology grading, which to our knowledge, has not been reported in a single study.
Materials and Methods
Animal Preparation
Animal research protocols were approved by our institution's research animal resource center.
The ob/ob mouse is a well-established model of NAFLD and the metabolic syndrome(25). Ob/ob mice are leptin-deficient, causing hyperphagia and subsequent obesity, diabetes, and hepatic steatosis that worsen with age(26). Thus, ob/ob mice can be manipulated to vary of the severity of steatosis based on age, making it an excellent model for in vivo hepatic fat quantification using MRI.
Twenty male mice fed standard chow ad libitum were used for this study, which has 14% of the calories from fat (Diet #8604 Harlan Teklad, Madison, WI). Mice were divided into three groups to create different levels of steatosis: “low”, “medium”, and “high” fat. The low fat mice (n=6) were wild-type mice (C57BL6/J, Harlan Sprague Dawley, Madison, WI) serving as controls, as wild-type mice do not develop hepatic steatosis when fed standard chow. The medium-fat mice (n=6) were four-week-old ob/ob mice, and the high-fat mice (n=8) were eight-week old ob/ob mice.
Prior to imaging, mice were sedated with an intraperitoneal injection of 40 mg/kg sodium pentobarbital (Nembutal, Ovation Pharmaceuticals, Inc., Deerfield, IL). A catheter constructed from a 30 gauge needle, P1 tubing, and heparinized saline was inserted into a mouse tail vein for injection of SPIOs (Superparamagnetic Iron Oxide, Feridex, Berlex Healthcare, Wayne, NJ).
Immediately after imaging, mice were euthanized with CO2 and tissues perfused with saline. The caudate lobe was frozen at -80°C, and the left lateral lobe was placed in formalin for histology grading.
MR Imaging
One chemical shift-based MRI method that has been developed for hepatic fat quantification is IDEAL (Iterative Decomposition of fat and water with Echo Asymmetry and Least squares estimation). IDEAL is a chemical shift-based fat water separation method that generates separate, perfectly registered fat and water images that can be acquired in a single clinically-feasible breath-hold(5, 27, 28). Methods have been developed and applied to address confounding factors including the complex spectrum of fat, T2* decay, noise and T1 bias, and eddy currents when using fat-water separation techniques such as IDEAL(13, 15-17). In this manner, complete fat-water separation is performed, making the technique a quantitative measure of hepatic triglyceride concentration over a dynamic range of 0-100%.
From the separated fat (Sf) and water (Sw) signals, a fat-fraction map (η) can be calculated, where the concentration of fat in the tissue is expressed on a pixel-by-pixel basis in the following manner:
| (1) |
When all corrections for confounding factors have been performed, the fat-fraction represents the proton density fat-fraction (PDFF), which is equivalent to the ratio of the unconfounded signal from all MRI-visible protons of fat to the sum of the unconfounded signal from all MRI-visible protons of fat and water. The fat-fraction map provides a normalized metric of fat from 0-100% fat avoiding variations in coil receive sensitivity (B1 inhomogeneities).
The IDEAL reconstruction also generates estimates of R2* (=1/T2*) as part of the T2* correction algorithm. Iron shortens the T2* decay of tissue, and hence increases R2*. Changes in R2* have been well-validated as a biomarker of hepatic iron content(22). Similar to the fat-fraction map, R2*-maps provide estimates of R2* on a pixel-by-pixel basis.
Imaging was performed at 3T (MR750, GE Healthcare) using a designated, commercial eight-channel wrist coil. Images were acquired using a multi-echo 3D-spoiled gradient-echo (SPGR) method combined with an investigational version of IDEAL(28). Standard imaging gradients (slew rate = 200 T/m/s, maximum strength = 50 mT/m) were used for all imaging. Imaging parameters included the following: TEminimum = 2.6ms, TR = 41.4ms, BW = ±100kHz, 1 signal average, flip angle = 5° to minimize T1 bias(15), 256×256 matrix, FOV = 12x7.2cm, and 28 slices (0.8mm thick) for a total scan time of 10:13 minutes, and true spatial resolution of 0.47x0.28x0.8mm3. A total of 15 echoes were acquired with 1.4ms echo spacing by using three interleaved echo-trains with five-echoes per TR(6).
Mice were imaged prior to SPIO administration (“Time 0”). SPIOs (Feridex, Bayer Healthcare Pharmaceuticals) were injected over 15 minutes, followed by 30 minutes to allow uptake of the agent into the Kupffer cells in the liver. To prepare the contrast agent injection, SPIOs were diluted in 5% dextrose, and a final concentration of 0.56mg/kg was administered for each dose, per the manufacturer's recommendation, using a 0.5mL insulin syringe. During injection and uptake, mice were removed from the scanner and placed on a warming pad for visual monitoring, although no heating was provided during the scan, which was only approximately 10 minutes.. After uptake, MRI was repeated (“Time 1”). The injection and scanning were repeated (“Time 2”) such that imaging was acquired before, and then after single and double doses of SPIOs. Of note, while Feridex has limited commercial availability, a similar product is available (e.g. FeraHeme, Biopal, Inc.).
Imaging for each mouse took approximately two hours, and was performed in one imaging session. It was important that all imaging for a single mouse occur within a single session, because the fat-content of the liver should not fluctuate during this time. However, the addition of SPIOs may affect the ability of the imaging method to measure fat accurately when T2* correction is not used, as SPIOs shorten T2* decay and may confound fat-fraction measurements. The fact that fat concentration is constant between SPIO injections acts as an internal control; if the apparent fat-fraction changes after injection of SPIO, then T2* correction has not been effective at removing the confounding effects of iron.
Image Reconstruction
As discussed earlier, T2* decay is shortened in the presence of iron, and can affect apparent fat-fraction measurements. Yu et al suggested that a minimum echo-train of six echoes provides adequate estimation of T2*, while balancing scan time constraints(17). However, the optimal echo-train length that balances accuracy of T2* measurements and scan time is unknown.
Using the same set of complex images (“source images”), reconstructions can be performed using different reconstruction parameters. First, source images were post-processed to correct for T2* decay using an echo-train length of six. Data reconstructed using all 15 echoes will be used for future studies. Next, images were reconstructed without T2* correction (“no T2*”) to demonstrate the effects of SPIOs on measured fat-fraction, also using an echo-train length of six. Both image reconstruction combinations were performed with accurate spectral modeling(13) using the spectrum described by Hamilton et al(29), and correction for noise bias(15), residual T1 bias(15, 30), and eddy currents(16).
Measurement of Fat-Fraction and R2*
A region of interest (ROI) was placed on fat-fraction maps and R2*-maps in each of the five major lobes of the mouse liver. ROIs were placed on one fat-fraction map and copied onto the same location on all fat-fraction and R2*-map from the four different image reconstructions. The size of the ROIs was chosen to include as much as the lobe as possible while avoiding blood vessels. As individual lobes and mice can differ greatly in size, ROI size varied between lobes and individual mice (range, 46-509 pixels). However, between time points for individual mice, ROIs were placed in the same areas of the lobes when possible. The average and standard deviation weighted by ROI size were calculated using the average, standard deviation, and ROI size of each lobe to obtain an average fat-fraction and R2* across the liver (“MRI fat-fraction”).
Triglyceride Quantification
Samples of the caudate lobe were processed for quantification of triglycerides using a colorimetric assay (“triglyceride fat-fraction” or “triglyceride mass percent”), (AniLytics Inc., Gaithersburg, MD). Results are expressed as a mass percent, where the ratio of the mass of extracted triglycerides to the mass of the tissue subject for analysis is multiplied by 100. Tissues were frozen at -80°C and thawed on-site for processing. Maximum sample size for the assays was 300mg.
Histology Grading
The left lateral lobe was submitted for H&E, trichrome, and Prussian blue staining. A pathologist graded the slides for inflammation, fibrosis, ballooning degeneration, and iron. Grading of fat was performed according to Brunt et al, where the percentage of cells affected by fat vacuoles is assessed in 5% increments (“histology grading”) and further separated by grade: grade 0 (<5%), grade 1 (5-33%), grade 2 (33-66%), grade 3 (>66%)(4). The total percent of cells affected by steatosis was further separated into cells affected by microvesicular and macrovesicular steatosis.
Statistical Analysis
Linear regression was performed between MRI fat-fractions and triglyceride fat-fractions. The linear regression was repeated for MRI fat-fraction versus histology grading, MRI fat-fraction versus histology area fraction, and finally for MRI fat-fraction versus the number of SPIO injections. Lastly, linear regression was performed between R2* values and MRI fat-fraction. Statistical analyses were performed using Excel (v6.1.7600, Microsoft, Redmond, WA).
A mixed model ANOVA was used to test for differences in fat-fractions in low fat mice versus time, with time as a fixed effect and a random term for mouse to account for repeated measures within the same animal. P<0.05 (two-sided) was used as the criterion for statistical significance. Analysis was performed using R (v2.10.0, R Development Core Team).
Results
Imaging Results
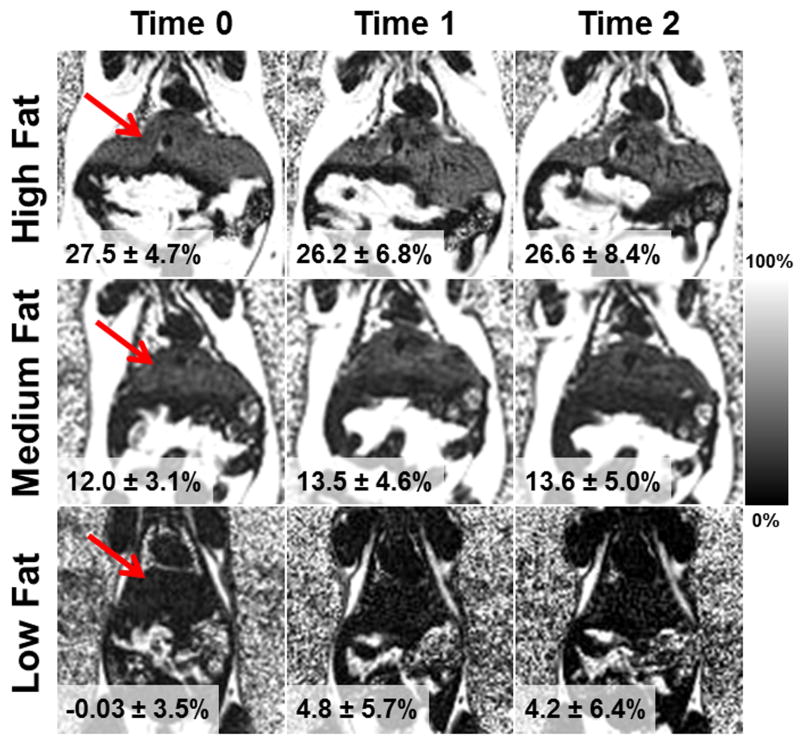
Figure 1 shows example fat-fraction maps in the coronal plane, of low, medium and high fat mice. (bottom, middle, and top row, respectively). Images are shown prior to SPIO administration, after one dose of SPIOs, and after a second dose of SPIOs (left, middle, and right column, respectively). T2* correction was used for these images; note that the fat-fraction remains relatively constant in the presence of increasing iron in high, medium, and low fat mice. Figure 2 displays the corresponding R2*-maps at the same location and in the same mice shown in Figure 1. Average R2* values across the liver are also shown. As expected, R2* values increase substantially, indicating that iron deposition has been successfully induced with injections of SPIOs.
Figure 1.
Example coronal fat-fraction maps of high, medium and low fat mice (top, middle, bottom) prior to SPIO administration, after a single dose of SPIOs, and after a second dose of SPIOs (left, middle, right). Shown are the average fat-fractions across the liver, and fat-fraction remains relatively constant. Red arrow points to the liver. Images were reconstructed with T2* correction.
Figure 2.
R2*-maps correspond to example mice shown in Figure 1, and shown are average measured R2* values across the liver. Red arrow points to the liver. Images were reconstructed with T2* correction. R2* values increase with increasing amounts of iron.
All imaging studies had excellent image quality except one mouse, where breathing artifacts related to under-sedation led to inadequate image quality at Time 0. This individual mouse at Time 0 was excluded from the analysis. For Time 0, analysis was performed on 19 mice, and for Time 1 and Time 2, analysis was performed on all 20 mice.
Figure 3 displays average MRI fat-fractions measured without T2* correction (dashed lines) and with T2* correction (solid lines) plotted against the number of SPIO injections for each group of mice. Since the entire experiment for each mouse required less than two hours, the degree of steatosis is expected to remain constant. Therefore, MRI fat-fraction acts as its own control and should remain constant between time points, if T2* correction has been effective. Without T2* correction, fat-fractions increases after injection of SPIO, indicating that shortening T2* (increasing R2*) corrupts the ability of MRI to quantify fat unless T2* correction is performed. This effect was most dramatic in the low fat mice. Importantly, when T2* correction is used, fat-fractions remain relatively constant in the presence of increasing iron. For low fat mice, a slight increase in fat-fraction was seen with increasing injections of SPIOs. However, we failed to find differences in fat-fractions of low fat mice due to time (p=0.91).
Figure 3.
Average measured fat-fractions for mouse groups versus SPIO injection number. Data with (solid lines) and without T2* correction (dashed lines) are shown. Low fat mice are shown in blue, medium fat in red, and high fat in black. Without T2* correction, measured fat-fractions increase in the presence of iron, particularly for low fat mice, and are elevated at Time 0. When T2* correction is included in the image reconstruction, fat-fractions remain constant with increasing amounts of iron. Error bars represent standard deviation.
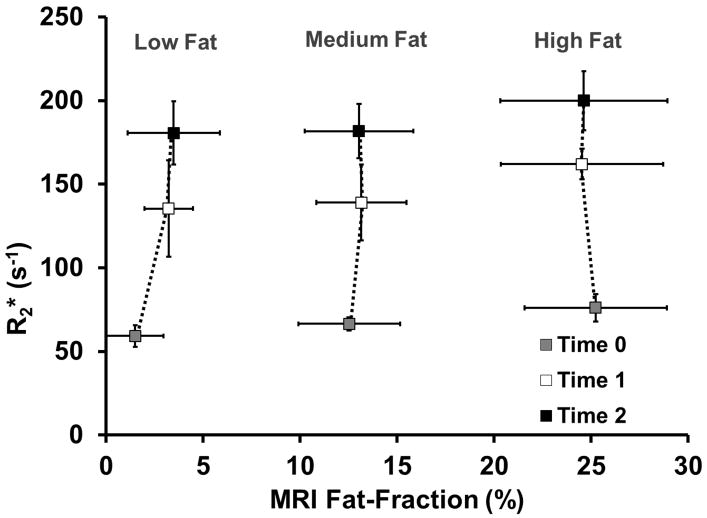
Figure 4 shows average R2* values for each group of mice. As expected, R2* values increase with increasing SPIOs, which confirms the progressive uptake of SPIOs into the liver.
Figure 4.
Average measured R2* values for mouse groups versus MRI fat-fraction. R2* values increase with increasing amounts of iron, although no differences in the pattern of uptake between groups of mice is seen. Error bars represent standard deviation.
Triglyceride Quantification Results
The average triglyceride concentration for low, medium, and high fat groups of mice were 12.4±1.2mg/g, 100.3±22.9mg/g, and 152.4±12.6mg/g, respectively, or 1.2±0.1%, 10.0±2.3%, and 15.2±1.3% fat by mass (“mass percent”), respectively.
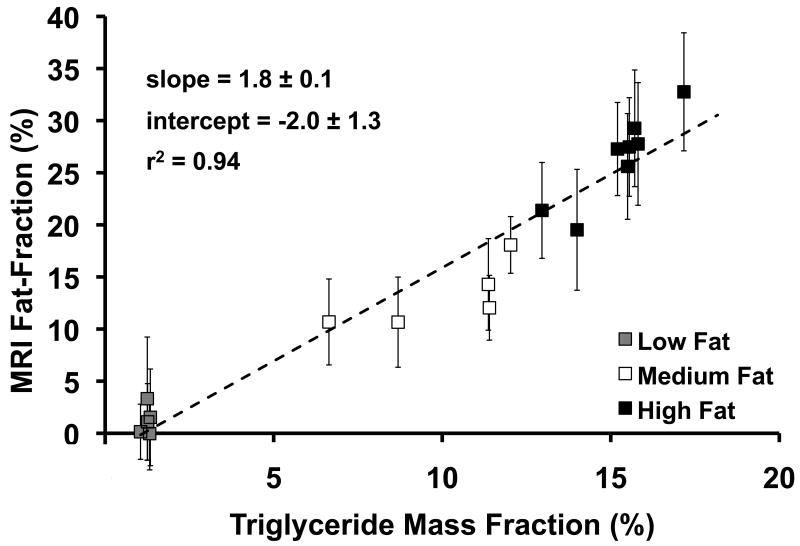
Figure 5 plots MRI fat-fraction in the caudate lobe at Time 0 versus triglyceride mass percent, which was extracted from the caudate lobe. Data were separated into low, medium, and high fat groups of mice. Excellent agreement is seen between the two techniques (r2=0.94). The line of best fit has a slope of 1.8±0.1 and intercept of -2.0±1.3, although the intercept is not statistically different from 0.0 (p=0.13). While PDFF measured from MRI and triglyceride fat-fraction are both fundamental tissue properties and should correlate well, they measure closely related, but different parameters (signal versus mass). Therefore, close, but not exact, agreement between these two values is expected, even when measured perfectly.
Figure 5.
MRI fat-fraction versus triglyceride mass fraction. MRI fat-fraction is predictive of the amount of triglycerides present in tissue. Clustering of each group of mice is seen, so low fat mice are displayed in gray, medium fat mice in white, and high fat mice in black. Dashed line is unity, and error bars represent standard deviation.
The triglyceride extraction result from one mouse was highly discordant from both histology grading and from the rest of the group. Triglyceride extraction reported 1.0% for a medium fat mouse; the range of this group excluding this mouse was (6.6%, 12.0%). Further, this value was highly discordant with histology grading, which showed 55% of cells affected by steatosis. After performing a q-test, this point was confirmed to be an outlier and was excluded from analysis. The triglyceride quantification analysis was performed on the remaining 19 mice.
Histology Grading Results
Figure 6 plots MRI fat-fraction at Time 0 over the entire liver versus total percent of cells affected by steatosis. Data were separated into low, medium, and high fat groups of mice. The large scatter in histology grade and relatively tight clustering of MRI fat-fraction for group, but with wider scatter for histology (particularly the medium fat mice) suggests that MRI may be a more precise measure of liver fat content than histology grading. Linear regression showed agreement between MRI and histology fat-fractions (r2=0.63), although the data does not appear to be necessarily linear. Obtained slope and intercept were 0.2±0.04 and 5.8±0.4, respectively.
Figure 6.
MRI fat-fraction versus histology fat-fraction, or percent of cells affected by steatosis. Clustering of each group of mice is seen, so low fat mice are displayed in gray, medium fat mice in white, and high fat mice in black. Good correlation (r2 = 0.63) seen between MRI and Histology, although the trend is not necessarily linear.
From conventional histology analysis the total percentage of cells affected by fat vacuoles for the low, medium, and high fat mice are 1.7±2.6%, 28.7±33.4%, and 73.8±14.1%, respectively. Histology analysis also showed no evidence of inflammation and fibrosis in any mice, as assessed with H&E and trichrome staining, respectively. Further, a grade of zero was reported for Prussian blue staining in all mice. No hepatocytes displayed ballooning degeneration in the low fat mice, an average of 18±11% (range, 1-30%) of cells displayed ballooning degeneration in the medium fat mice, and an average of 12±11% (range, 1-30%) of cells displayed ballooning degeneration in the high fat mice.
When the total percent of cells affected by steatosis was divided into microvesicular and macrovesicular amounts of steatosis, the group averages for the low, medium, and high fat mice for microvesicular (macrovesicular) steatosis were 1.7±2.6% (0±0%), 25.8±31.2% (2.8±2.5%), and 61.9±16.2% (11.9±5.3%), respectively. Microvesicular steatosis was the dominant pattern of overall steatosis deposition observed in this model.
Discussion
In this work, we investigated and validated the utility of T2* correction for quantification of hepatic steatosis using a quantitative MRI method in a combined animal model of hepatic steatosis and iron overload. Our results demonstrated the importance of T2* correction for accurate quantification of fat even without iron overload, but particularly at higher concentrations of iron. Further, we demonstrated excellent correlation between MRI measures of fat and extracted tissue triglyceride concentrations. Histology grading of steatosis also demonstrated good correlation with T2* corrected MRI, although results suggest that MRI may be a more sensitive biomarker of early steatosis than biopsy.
Further, this work demonstrates the feasibility of a combined animal model of hepatic steatosis and iron overload. Although the ob/ob mouse is a well established model of obesity, metabolic syndrome and fatty liver disease, its use with injected SPIOs to create hepatic iron overload has not been described previously. The use of SPIOs to accelerate MRI signal decay served as an “iron challenge”, providing a means to rigorously test the robustness of T2* correction methods. The excellent correlation between T2* corrected MRI fat-fraction and triglyceride extraction and the observation that fat-fraction was independent of liver iron only when T2* correction was used, demonstrate that the MRI method tested in this study was robust to the presence of iron overload. Thus, while the imaging technique and reconstruction methods have been previously reported, their application in this animal model is highly novel.
MRI fat-fraction measurements without T2* correction were consistently higher than fat-fractions with T2* correction, including at Time 0 before injection of SPIOs. These results are consistent with previous work(5, 8), that has shown in patients and phantoms that fat-fraction measurements without T2* correction are falsely elevated, particularly at low fat-fractions, using spectroscopy and known fat-fractions as references, respectively. Thus, T2* correction, in conjunction with correction for other confounding factors, is necessary for accurate fat quantification in both the presence and absence of iron overload. However, previous studies have shown that T2* and fat-fraction have no relationship to each other(5).
As discussed above, it is important to stress that the methods used to assess the severity of steatosis (MRI fat-fraction, histology grading, histology area-fraction, and triglyceride extraction) are fundamentally different metrics of fat concentration. While perfect agreement between these biomarkers is not expected, correlation between these methods should be seen.
An unexpected result was that the low fat mice displayed a slight increase in the apparent fat-fraction with increasing amounts of iron, although we failed to find differences in fat-fractions due to time (p=0.91). A second unexpected result was that no SPIO deposition was seen with Prussian blue staining. As suggested by our pathologist, if the SPIOs were present in a dispersed form, rather than discrete granules, they would not be detected by Prussian blue staining. It is also likely more time may have been needed for the tissues to uptake SPIOs after imaging in order to be detected. Finally, it is possible, that the perfusion preparation, which is not performed for clinical biopsy cores, may have washed SPIO-containing Kupffer cells from the tissue In this study, the presence of SPIOs in the liver was confirmed by the large increases in R2*, which was the intended purpose of SPIO injection in this animal model.
Accurate spectral modeling was performed using a published fat spectral model obtained in humans(29). The assumption that mice and humans have the same spectral model is a potential limitation of this work. However, mice and humans have remarkably similar metabolic physiology, making mice an excellent model organism, and we do not expect that major differences in the fat spectral model of mice and humans exist. However, verification of the similarities between mouse and human fat spectral models would be worthwhile. Unfortunately, it is challenging to perform in vivo spectroscopy on mouse livers because of their small, irregular size. A valid study would require ex vivo NMR in liver samples taken from the mice, although such resources were not available to us for this study.
In summary, MRI fat-fraction measured using a T2*-corrected chemical shift-based fat-water method is an accurate method for quantifying liver triglyceride concentration. Further, T2* correction is necessary, particularly at high iron concentrations to provide accurate measures of liver fat. This work also shows that MRI fat-fractions are highly predicative of liver triglyceride concentration, and may be more sensitive than subjective histology grading at early stages of steatosis.
Acknowledgments
Grant support: We gratefully acknowledge support from the NIH (R01-DK083380, R01-DK088925, RC1-EB010384-01), The Coulter Foundation, The University of Wisconsin IEDR, and GE Healthcare.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50(1):171–180. doi: 10.1007/s10620-005-1267-z. [DOI] [PubMed] [Google Scholar]
- 3.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 4.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 5.Meisamy S, Hines CD, Hamilton G et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258(3):767–75. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hines CD, Yu H, Shimakawa A, et al. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 2010;254(1):119–128. doi: 10.1148/radiol.09090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoo T, Bydder M, Hamilton G, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251(1):67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hines CD, Yu H, Shimakawa A, et al. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J J Magn Reson Imaging. 2009;30(5):1215–1222. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and (1)H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21(1):87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26(6):1637–1653. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 11.Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18(3):337–357. ix. doi: 10.1016/j.mric.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SS, Lee Y, Kim N, et al. Hepatic fat quantification using chemical shift MR imaging and MR spectroscopy in the presence of hepatic iron deposition: validation in phantoms and in patients with chronic liver disease. J Magn Reson Imaging. 2011;33(6):1390–8. doi: 10.1002/jmri.22583. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho Water-Fat Separation and Simultaneous R2* Estimation with Multifrequency Fat Spectrum Modeling. Magn Reson Med. 2008;60(5):1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26(3):347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58(2):354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Shimakawa A, Reeder SB, McKenzie CA, Brittain JH. Magnitude fitting following phase sensitive water-fat separation to remove effects of phase errors. Proceedings of the 17th Annual Meeting of ISMRM; Honolulu. 2009. abstract 462. [Google Scholar]
- 17.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26(4):1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 18.Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of Fat-Water MRI and Single-voxel MRS in the Assessment of Hepatic and Pancreatic Fat Fractions in Humans. Obesity (Silver Spring) 2010;18(4):841–7. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258(3):749–59. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonkovsky HL, Jawaid Q, Tortorelli K, et al. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol. 1999;31(3):421–429. doi: 10.1016/s0168-8278(99)80032-4. [DOI] [PubMed] [Google Scholar]
- 21.Sirlin CB, Reeder SB. Magnetic resonance imaging quantification of liver iron. Magn Reson Imaging Clin N Am. 2010;18(3):359–81. ix. doi: 10.1016/j.mric.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guiu B, Petit JM, Loffroy R, et al. Liver methylene fraction by dual- and triple-echo gradient-echo imaging at 3.0T: Correlation with proton MR spectroscopy and estimation of robustness after SPIO administration. J Magn Reson Imaging. 2011;33(1):119–127. doi: 10.1002/jmri.22390. [DOI] [PubMed] [Google Scholar]
- 24.Bydder M, Shiehmorteza M, Yokoo T, et al. Assessment of liver fat quantification in the presence of iron. Magn Reson Imaging. 2010;28(6):767–76. doi: 10.1016/j.mri.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanji AA. Animal models of nonalcoholic fatty liver disease and steatohepatitis. Clin Liver Dis. 2004;8(3):559–574. ix. doi: 10.1016/j.cld.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Menahan LA. Age-related changes in lipid and carbohydrate metabolism of the genetically obese mouse. Metabolism. 1983;32(2):172–178. doi: 10.1016/0026-0495(83)90225-1. [DOI] [PubMed] [Google Scholar]
- 27.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54(3):636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 28.Reeder SB, McKenzie CA, Pineda AR, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25(3):644–652. doi: 10.1002/jmri.20831. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2010 doi: 10.1002/nbm.1622. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hines CD, Yokoo T, Bydder M, Sirlin CB, Reeder SB. Optimization of flip angle to allow tradeoffs in T1 bias and SNR performance for fat quantification. Proceedings of the 18th Annual Meeting of ISMRM; Stockholm. 2010. abstract 2927. [Google Scholar]