Abstract
Bacteria colonizing chronic wounds often exist as biofilms, yet their role in chronic wound pathogenesis remains unclear. Staphylococcus aureus biofilms induce apoptosis in dermal keratinocytes, and given that chronic wound biofilms also colonize dermal tissue, it is important to investigate the effects of bacterial biofilms on dermal fibroblasts. The effects of a predominant wound pathogen, methicillin-resistant S. aureus, on normal, human, dermal fibroblasts were examined in vitro. Cell culture medium was conditioned with equivalent numbers of either planktonic or biofilm methicillin-resistant S. aureus, and then fed to fibroblast cultures. Fibroblast response was evaluated using scratch, viability, and apoptosis assays. The results suggested that fibroblasts experience the same fate when exposed to the soluble products of either planktonic or biofilm methicillin-resistant S. aureus, namely limited migration followed by death. Enzyme-linked immunosorbent assays demonstrated that fibroblast production of cytokines, growth factors, and proteases were differentially affected by planktonic and biofilm-conditioned medium. Planktonic-conditioned medium induced more interleukin-6, interleukin-8, vascular endothelial growth factor, transforming growth factor-β1, heparin-bound epidermal growth factor, matrix metalloproteinase-1, and metalloproteinase-3 production in fibroblasts than the biofilm-conditioned medium. Biofilm-conditioned medium induced more tumor-necrosis factor-α production in fibroblasts compared to planktonic-conditioned medium, and suppressed metalloproteinase-3 production compared to controls.
Keywords: biofilms, chronic wounds, dermal fibroblast, Staphylococcus aureus
INTRODUCTION
Biofilms are surface-attached microbial communities. These communities secrete an extracellular polymer matrix and form complex structures. The bacteria within biofilms are phenotypically distinct from their planktonic counterparts (1) and are orders of magnitude more resistant to antibiotics and biocides than planktonic bacteria (2). Chronic wounds are an ideal environment for bacterial infection and biofilm formation. The wound remains open for a prolonged period of time, increasing the odds of bacterial infection. The wound bed provides a surface for growth and nutrients, while poor blood flow and hypoxia discourage host defenses (3). It has been demonstrated that bacteria colonizing chronic wounds exist as biofilms (4), and using animal models, studies have shown that wounds inoculated with bacteria eventually form biofilms (5) that can delay healing (6). Despite the prevalence of biofilms in chronic wounds, their role in chronic wound pathogenesis remains unclear.
The microbial flora of chronic wound biofilms is often diverse. Using molecular techniques, Dowd et al demonstrated that most prevalent species include Staphylococcus, Pseudomonas, Peptoniphilus, Enterobacter, Stenotrophomonas, Finegoldia, and Serratia spp (7). While most chronic wounds are colonized by multiple species of microorganisms, there is no common wound biofilm and the constituents of the biofilm can vary by individual and type of wound (7). Furthermore, the complexity of a multispecies biofilm increases the difficultly of identifying the root cause of a specific host response. Therefore, to further understand the role of biofilms in chronic wound pathogenesis, a single, prevalent pathogen, MRSA, was selected. S. aureus, in addition to being a predominant component of the chronic wound biofilm (7), has one of the highest incidence rates in traumatic, surgical, burn, and other wound infections (8). Recently, it was demonstrated that the soluble products from planktonic and MRSA biofilms have differential effects on human keratinocytes (HK) (9, 10). Soluble products from S. aureus biofilms induced dramatic HK morphological changes, reduced HK viability, and increased HK apoptosis compared to soluble products from planktonic S. aureus (9). Furthermore, biofilm-conditioned and planktonic-conditioned media induced different temporal patters of cytokine production by keratinocytes (10).
The chronic wound biofilm also likely colonizes dermal tissue, and it is therefore important to investigate the effects of bacterial biofilms on the dermal fibroblast as well in order to improve understanding of wound biofilms and wound chronicity. Thus, in this investigation, the effects of a predominant wound pathogen, MRSA, on normal human, dermal fibroblasts (HF) were examined.
MATERIALS and METHODS
Cell Culture
HF were isolated from newborn foreskin using methods previously described (11) and in accordance with the University of Washington Institutional Review Board. Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA) with 10% Newborn Calf Serum (NCS, Sigma, St. Louis, MO), and penicillin and streptomycin (P/S), 100 U/mL and 100 μg/mL, respectively (Hyclone, Logan, UT). All cultures were kept in a humidified 5% CO2 incubator at 37°C. Experiments were conducted with DMEM with 10% NCS without P/S, unless noted otherwise.
Biofilm-conditioned Medium
Biofilm-conditioned medium (BCM) was produced by using methods similar to those previously described (9). Briefly, tissue culture inserts (25 mm diameter, pore size 0.2 μm; Nalge Nunc International, Rochester, New York) were inoculated with five individual 10 μl drops of a 106 CFU/mL culture of S. aureus in tryptic soy broth (TSB). The inserts were then placed in a 6-well plate, each well containing 1.0 mL of TSB, and incubated at 37°C, resulting in the growth of five, individual biofilms/insert (Figure 1). The insert-supported biofilms were transferred to a new 6-well plate with fresh TSB every 24 hours for a total of 72 hours of incubation. Afterwards, the insert-supported biofilms (referred to as Day 3 biofilms) were placed in wells containing 1.5 mL of PBS for one hour to remove excess TSB and then used to collect BCM. BCM was obtained by placing Day 3 biofilms in 6-well plates containing 1.5 mL/well with DMEM with 10% NCS (no P/S) and incubated. Every 24 hours the medium was collected and replaced with fresh medium. A total of seven 24-hour collections were pooled, stored at −20°C, and used for experiments as BCM.
Figure 1.
Photograph of the MRSA biofilms grown on tissue culture inserts. The insert (25 mm in diameter) is placed in a well of a 6-well plate.
The number of viable bacterial cells in the initial S. aureus inoculum and the biofilms were determined using the spread plate technique. Briefly, biofilm samples were vortexed, sonicated, serially diluted in PBS, plated on tryptic soy agar (TSA), and incubated at 37°C overnight. Afterwards, the plates were counted and the number of colony forming units (CFU) per inoculum or insert was calculated. The collected BCM was also plated to ensure its sterility. If any bacterial growth was detected, the medium was not used for experiments.
Planktonic-conditioned Medium
Planktonic-conditioned medium (PCM) was prepared to give a similar proportion of bacteria per unit fluid volume to that of the BCM, also using previously described methods (9). An overnight culture of S. aureus was grown in TSB at 37°C with agitation. The cell suspension was then centrifuged at 3000 x g, and the cells were washed in PBS to remove excess TSB. The suspension was centrifuged again at 3000 × g, and the cells were resuspended in DMEM with 10% NCS (no P/S) at a cell density equivalent to a biofilm (2×109 CFU/mL). The final suspension was incubated at 37°C with agitation for 24 hours. Afterwards, the suspension was filtered through a 0.22 μm filter, stored at −20°C, and used for experiments as PCM.
Migration/Scratch Assay
HF cultures were grown in 24-well plates (30,000 cells/well) for two days, after which 80–90% confluence was reached. The cultures were then scratched with a 200 μl plastic pipette tip, washed twice with HEPES buffered saline (HBS), and resupplied with 300 μl of cell culture medium. The scratched cultures were imaged, obtaining the initial scratch area. Afterwards, the the HF cultures were incubated in humidified 5% CO2 incubator at 37°C. Every 24 hours, the cells were rinsed with HBS, imaged, replenished with fresh conditioned medium, and returned to the incubator. The assay was terminated after 72 hours. Control HF cultures were exposed standard culture medium without P/S. Triplicate cultures were used for all conditions.
All scratch images were taken using a 4x objective on a Nikon Eclipse E800 epi-fluorescent microscope. Images were analyzed and percent scratch area closed was calculated for each time-point using the Metamorph® image analysis program.
Fibroblast Viability
HF viability was assessed using 2,3-bis[2-Methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT; Sigma, St. Louis, MO). To begin, 30,000 cells/well were seeded in 24-well plates and cultured for two days. Once 80–90% confluence was reached, HF cultures were scratched to ensure an actively migrating or “healing” culture and exposed to 300 μl BCM or PCM. After 4, 24, 48, and 72 hours of exposure, the conditioned medium was removed and replaced with 300 μl of fresh DMEM medium (without phenol red, Invitrogen) + 60 μl of XTT. After 4 hours, the medium solution was sampled and the absorbance at 450 nm was measured. Afterwards, fresh BCM or PCM was added to the wells, and the plate was returned to incubator until the next time point. Control HK cultures were exposed to standard culture medium. Blank samples, containing no cells, only cell culture medium and the XTT solution were also used as a control. Triplicate cultures were used for all conditions.
Apoptosis
TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining was used to investigate the induction of apoptosis. HF were cultured in 96-well plates (5000 cells/well) two days. Once 80–90% confluence was reached, HF cultures were exposed to BCM or PCM. After 4, 8,12, 24, 48, or 72 hours of exposure, the conditioned medium was removed, and the cultures were fixed in 4% paraformaldehyde in PBS for 15 minutes at 37°C followed by three 5 minute washes in PBS. Ethanol (70%) was then added to the cultures, which were then stored at −20°C until assayed. The APO-BrdU TUNEL Assay Kit (Invitrogen) was used, and the manufacturer’s staining protocol was adapted for fluorescence microscopy. All enzyme solutions were made in the same proportions suggested by the manufacturer, but were added directly to the culture plate. The cultures were then imaged using a Nikon Eclipse E800 epi-fluorescent microscope using a 10x objective, and the percentage of cells staining positive for TUNEL was enumerated. Control cultures were also stained and consisted of HF exposed to standard culture medium (no P/S). Triplicate cultures were used for all conditions.
Enzyme-linked Immunosorbent Assays
The production of several inflammatory cytokines, growth factors, and proteases in response to BCM and PCM exposure was quantified using enzyme-linked immunosorbent assays (ELISA, all purchased from R&D Systems, Minneapolis, MN). To begin, HF were seeded in 6-well plate (150,000 cells/well) and grown to 80–90% confluence. Afterwards, HF cultures were scratched to ensure an actively migration or “healing” culture and exposed to 1.5 mL BCM or PCM. After 4, 8,12, 24, 48, or 72 hours of exposure, the conditioned medium was removed, diluted two-fold with ELISA reagent diluent, divided into 250 μl aliquots, and stored at −80°C until assayed. Control cultures consisted of HF exposed to standard culture medium (no P/S). Triplicate cultures were used for all conditions. ELISAs for interleukin-6 (IL-6), IL-8, tumor necrosis factor-α, (TNF-α), vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TFG-β1), heparin-bound epidermal growth factor (HB-EGF), matrix metalloproteinase-1 (MMP-1), and MMP-3 were all conducted.
Statistical Analysis
Data are presented as the mean ± standard deviation (s.d.). Statistical analysis for significance was determined using an ANOVA with a Tukey’s HSD post-hoc test with α=0.5 and p≤0.05 considered to be significant.
RESULTS
Conditioned Medium
Multiple MRSA biofilms were grown on tissue culture inserts and used to produce BCM. The initial inoculum was 1.02×104 CFU/biofilm, and at the end of the BCM-collection period the biofilms had grown to 6.13±1.68×108 CFU/biofilm. The biofilms were cultured with 1.50 mL cell culture medium, resulting in average density of 2.04±0.56×109 CFU/mL. The pH of the BCM rose to 8.26 during the culture period and was adjusted to 7.5 prior to sterile filtration. The BCM was stored at −20°C.
Planktonic S. aureus was grown in cell culture medium and incubated for 24 hours at 37°C with agitation. Prior to sterile filtration, the suspension was sampled, serial diluted, and plated to reveal a final bacterial cell density of 1.78×109 CFU/mL. The resulting PCM had a cell density slightly lower than that of the BCM. Therefore, for experimental use the volume of PCM was adjusted to equate to the CFU/mL of the BCM. Thus, HF cultures exposed to the BMC or PCM were all in contact with medium conditioned by approximately 2×109 CFU/mL. After filtration, the pH of the PCM was 7.36 and was not adjusted. The PCM was sterile-filtered once again and stored at −20°C until used.
Migration/Scratch Assay
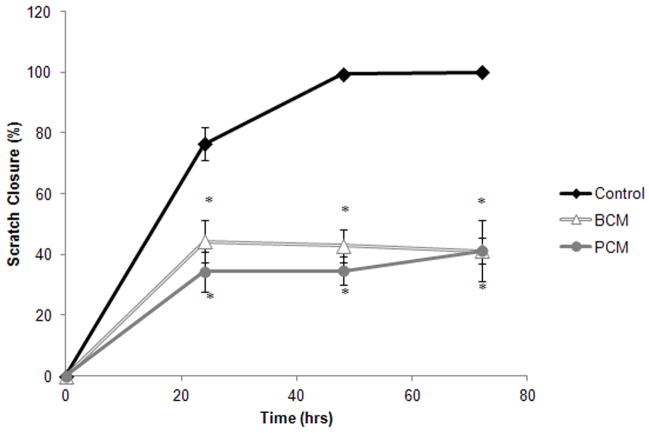
BCM and PCM-exposure produced similar HF scratch closure (Figure 2). After 24 hours, scratches in control cultures were 76.4±5.5% closed while BCM-exposed cultures were 44.4±7.1% closed and the PCM-exposed cells were 35.3±6.7%. At 48 hours, the control cultures were 99.4±1.0% closed, whereas the conditioned media groups demonstrated no further closure, 42.8±5.5 and 34.7 ±4.5% for the BCM and PCM, respectively. By 72 hours, the scratches in the control cultures had closed, while the scratches in the conditioned media cultures maintained their unclosed state. At all time points the scratched cultures receiving BCM and PCM had significantly less closure than the control group (p≤0.002). The BCM and PCM were statistically equivalent at all time points.
Figure 2.
Percent scratch area closed for the in vitro scratch assay. Results are shown for two experimental groups where HF were exposed to either MRSA BCM or PCM. The results for one control group are also shown. Results represented the mean±standard deviation, n=3. (*) Significantly different from control group at p≤0.002.
Keratinocyte Viability
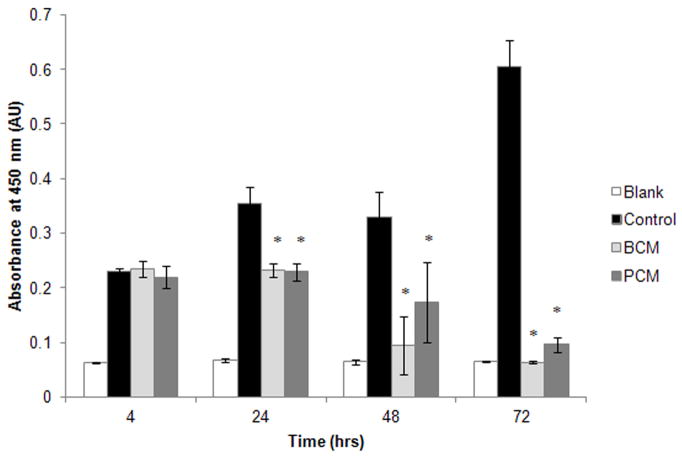
An XTT assay was employed to determine if there was a loss of cell viability post BCM and PCM exposure. By 24 hours, the viability of both BCM and PCM cultures were statistically lower than that of the control group (p≤0.0001, Figure 3), but were not statistically different from each other (p=0.999). At 48 hours, the viability of the conditioned media groups were not statistically different from the blank, no-cell control (p≥0.129). This trend continued through the 72-hour time point.
Figure 3.
Absorbance values for XTT in vitro cell viability assay. Results are shown for two experimental groups where HF were exposed to either MRSA BCM or PCM. The results for one control group and one blank are also shown. Results represented the mean±standard deviation, n=3. (*) Significantly different from control group at p≤0.007.
Apoptosis
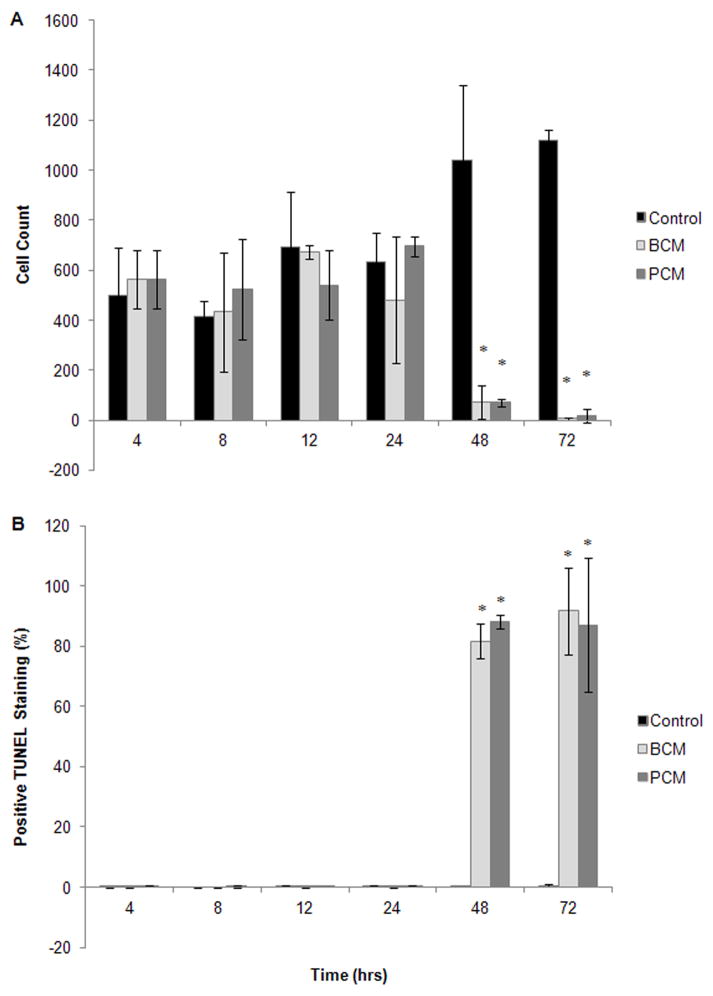
The dual staining technique of the APO-BrdU TUNEL Assay Kit allowed all cells to be stained by propidium iodide, while only those undergoing apoptosis also stained positive for TUNEL. Using this technique, cell counts could be obtained, and the percentage undergoing late-state apoptosis could be calculated. Differences between groups were not detected until the 48 hour time point (Figure 4A–B). After 48 hours of exposure, there were significantly fewer cells remaining attached to the plate in the BCM and PCM groups compared to the controls (p≤0.006, Figure 4A), and of those remaining cells, the majority of them stained positive for TUNEL, 81.7±5.8 and 88.2±2.3% for BCM and PCM, respectively (Figure 4B). By 72 hours, the percentage of TUNEL positive cells increased to 91.7±14.4 and 87.1±22.4% for the BCM and PCM groups, respectively. Only 0.5±0.4% of the control group population stained positive for TUNEL at 72 hours (p≤0.008 compared to BCM and PCM). The BCM and PCM were statistically equivalent at all time points for both cell counts and TUNEL staining.
Figure 4.
Results from in vitro TUNEL assay: (A) Number of cells in culture and stained with PI, and (B) percentage of cells in culture staining positive for TUNEL. Results are shown for two experimental groups where HF were exposed to either MRSA BCM or PCM. The results for one control group are also shown. Results represented the mean±standard deviation, n=3. (*) Significantly different from control group at p≤0.004.
Enzyme-linked Immunosorbent Assays
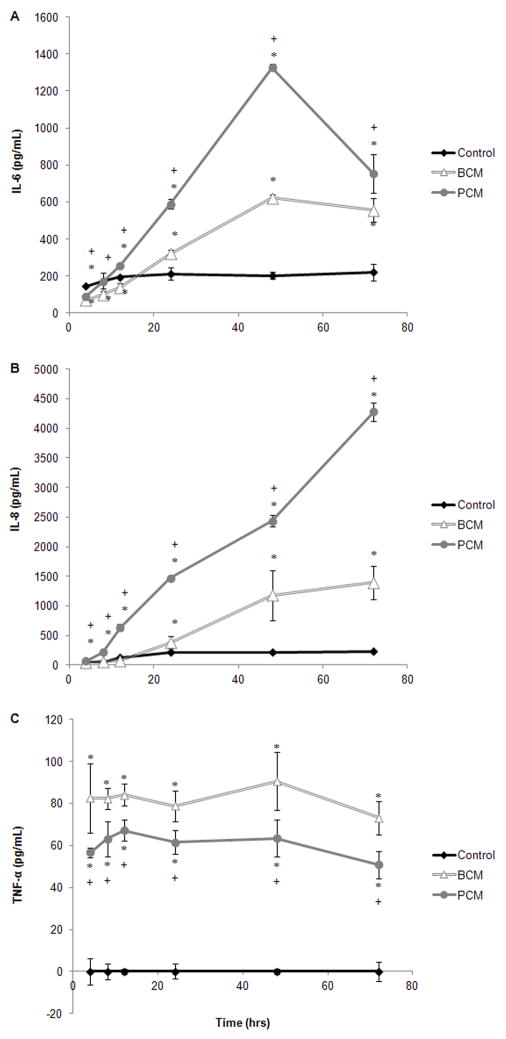
ELISAs were used to detect cytokine, growth factor, and protease production by HF in response to soluble factors produced by planktonic MRSA and MRSA biofilms. HF cytokine production was characterized by probing for inflammatory cytokines, IL-6, IL-8, and TNF-α (Figure 5A–C). IL-6 production was initially reduced for both conditioned media; however by 12 hours for the PCM group and 24 hours for the BCM group, IL-6 production had increased and was significantly higher than the scratched controls (Figure 5A, p≤0.018). IL-6 production for the PCM group was significantly higher than that of the BCM group for all time points (p≤0.003) except at 72 hours (p=0.134).
Figure 5.
Results from in vitro ELISA assays for a selection of cytokines: (A) IL-6, (B) IL-8, and (C) TNF-α. Results are shown for two experimental groups where HF were exposed to either MRSA BCM or PCM. The results for one control group are also shown. Results represented the mean±standard deviation, n=3. (*) Significantly different from control group at p≤0.05, and (+) significantly different from BCM at p≤0.04.
PCM-exposed cells produced significantly more IL-8 than the BCM-exposed cells and the control cells (p≤0.018, Figure 5B). This trend began at the 4 hour time point and extended through the 72 hour time point. The production of IL-8 from BCM-exposed cells was not significantly higher than the controls until the 48 hour time point (p=0.008, Figure 5C). While the IL-6 and IL-8 production by the conditioned media groups generally increased with time, TNF-α production remained constant with respect to time (Figure 5C); however, at all time points TNF-α production by the BCM-exposed cells was significantly higher than the PCM-exposed cells (p≤0.038), and both conditioned-media groups produced significantly more TNF-α than the controls (p≤0.001).
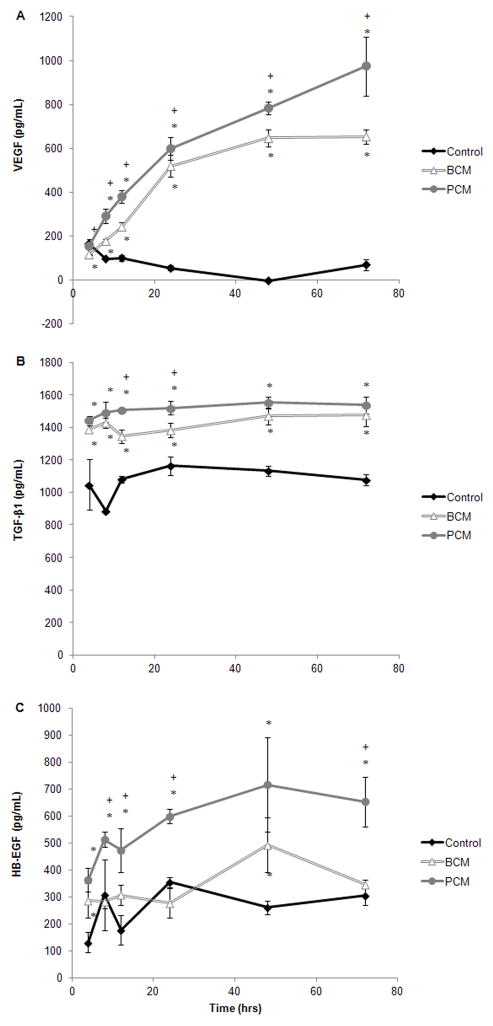
Numerous growth factors are involved in the wound healing process. HF production of representative growth factors (VEGF, TGF-β1, and HB-EGF) in response to conditioned media was examined (Figure 6A-C). VEGF production was significantly higher in the conditioned media groups compared to the controls starting at the 8 hour time point and the differences increased with time (p≤0.004, Figure 6A). Furthermore, the VEGF concentration in the PCM cultures were higher than those in the BCM cultures (significance p≤0.007 except for the 12 hour time point p=0.127). TGF-β1 levels were significantly higher in the conditioned media groups compared to the controls at all time points (Figure 6B, p≤0.008). Differences between conditioned media groups were only significant at the 12 and 24 time points, p=0.001 and p=0.032, respectively. HB-EGF levels were also significantly elevated (p≤0.005) in the PCM group compared to the control group at all time points (Figure 6C). HB-EGF production by the control cells and the BCM-exposed cells fluctuated; however, at the 4 and 48 hour time points, HB-EGF in the BCM group was significantly higher than the controls (p≤0.02).
Figure 6.
Results from in vitro ELISA assays for a selection of growth factors: (A) VEGF, (B) TGF-β1, and (C) HB-EGF. Results are shown for two experimental groups where HF were exposed to either MRSA BCM or PCM. The results for one control group are also shown. Results represented the mean±standard deviation, n=3. (*) Significantly different from control group at p≤0.02, and (+) significantly different from BCM at p≤0.03.
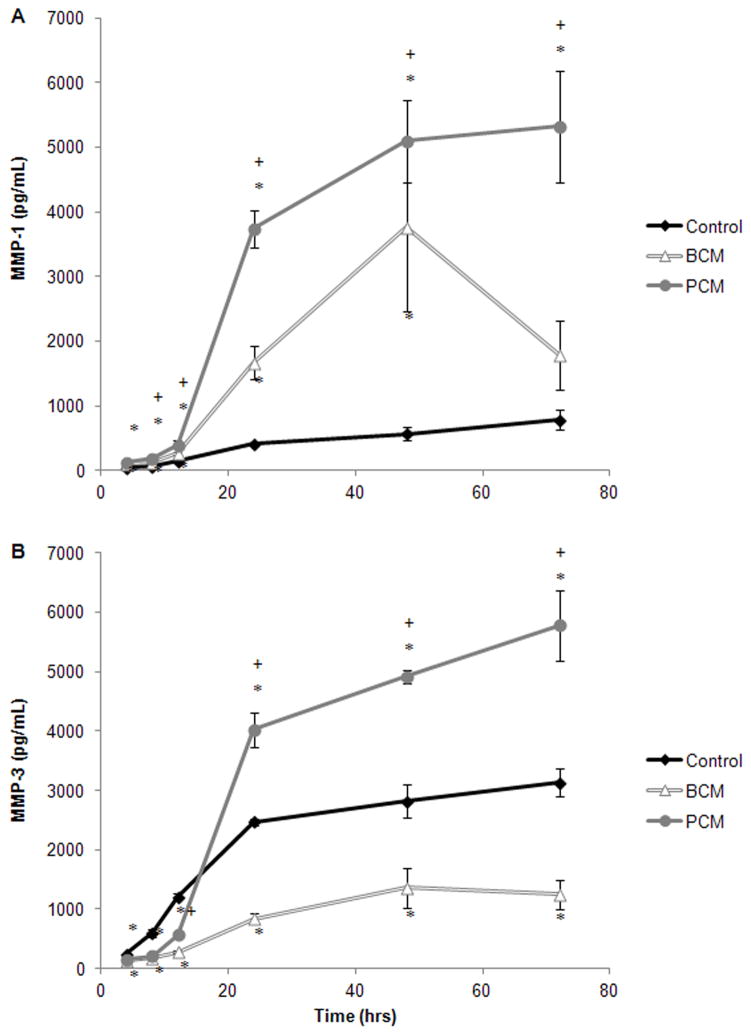
MMPs also play a pivotal role in wound healing and the production of MMP-1 and MMP-3 by HF in response to conditioned media was also characterized (Figure 7A-B). The MMP-1 concentration in the PCM group increased with time. Starting at the 8 hour time point, these concentrations were significantly higher than the BCM-group (p≤0.042), which was also elevated compared to the controls (Figure 7A). For all groups, the MMP-3 concentration increased with time (Figure 7B). Beginning at the 24 hour time point the MMP-3 concentration in the PCM group exceeded the controls (p<0.001), and it continued to increase with time. Conversely, the MMP-3 concentrations in the BCM-group never exceeded those found for the controls (p≤0.001).
Figure 7.
Results from in vitro ELISA assays for a selection of MMPs: (A) MMP-1 and (B) MMP-3. Results are shown for two experimental groups where HF were exposed to either MRSA BCM or PCM. The results for one control group are also shown. Results represented the mean±standard deviation, n=3. (*) Significantly different from control group at p≤0.02, and (+) significantly different from BCM at p≤0.04.
DISCUSSION
Dermal fibroblasts play a pivotal role in the wound healing process (12). Shortly after wounding, fibroblasts migrate into the provisional matrix and proliferate, increasing the cellularity of the wound and initiating the repair phase of wound healing. They synthesize growth factors and extracellular matrix (ECM) molecules, mainly collagen, fibronectin, and hyaluronan, which provide structural integrity to the wound and form wound granulation tissue (12). During the remodeling phase of wound healing, dermal fibroblasts synthesize ECM proteins such as, collagen, elastin, and proteoglycans, but also MMPs, which degrade the matrix (13). Specialized dermal fibroblasts, myofibroblasts, also contribute to wound contraction.
The chronic wound biofilm is likely also colonizing dermal tissue, and it is important to investigate the effects of bacterial biofilms on the dermal fibroblast. The approach taken herein involved conditioning cell culture medium with similar bacterial densities of either biofilm or planktonic MRSA, exposing HF to the conditioned media, and evaluating HF in a number of ways. The soluble products produced by both the planktonic and biofilm MRSA, produce similar deleterious effects on HF migration and viability by inducing apoptosis (Figures 2–4). No significant differences between the experimental groups were revealed. However, HF response to the conditioned media was also monitored by investigating the production of wound healing cytokines, growth factors, and proteases. Several distinctions between BCM and PCM were observed.
Given appropriate stimulation, HF are strong producers of a variety of cytokines. IL-6 is a pleiotropic cytokine produced by normal the constituents of the skin, including fibroblasts. IL-6 has numerous biological activities, including stimulating growth factor production in fibroblasts (14) and increasing neutrophil adhesion to dermal fibroblasts (15). IL-8 is a neutrophil-attractant chemokine, and is produced by resident skin cells and infiltrating leukocytes. IL-8 also exerts proliferative and migratory effects on keratinocytes, reduces fibroblast-associated contraction (16), and has also been found to be a potent angiogenic factor (17). TNF-α is also produced by the normal constituents of the skin and activated immune cells, and plays a profound role in wound healing depending upon concentration and length of exposure (18). Chronic wound fluid has demonstrated excessive levels of IL-8 (19), IL-6 (19, 20), and TNF-α (20, 21).
In this chronic wound model, HF exposed to the soluble products of both planktonic and biofilm MRSA produced significantly more IL-6, IL-8, and TNF-α compared to control HF (Figure 5). However, PCM induced the significantly more IL-6 and IL-8 production than BCM, whereas BCM induced significantly more TNF-α production. The difference between the two groups was not as dramatic as that seen in IL-6 and IL-8 production. Although the specific induction factors in this investigation were not isolated, it has been that demonstrated that dermal fibroblasts produce IL-6 and IL-8 in response to S. aureus protein A and lipoteichoic acid (22), and synovial fibroblasts from rheumatoid arthritis and osteoarthritis patients were found to increase IL-6 and IL-8 production in response to S. aureus peptidoglycans (23) and staphylococcal enterotoxin A (24). Thus, planktonic S. aureus may be producing more soluble toxins than biofilm S. aureus.
HF production of growth factors in response to BCM or PCM exposure was also examined by assaying for representative growth factors, TGF-β1, HB-EGF, and VEGF. TGF-β family of growth factors includes TGF-β1–3, bone morphogenic proteins (BMP), and activins; however, TGF-β1 is predominant in cutaneous wound healing (18). It is produced by platelets, macrophages, keratinocytes, and fibroblasts, and it is important in all phases of the wound healing process (18). The EGF superfamily of growth factors is also well described, and the members most associated with wound healing include, EGF, transforming growth factor-α (TGF-α), and HB-EGF (18). All three bind to the same receptor, but HB-EGF is also a heparin binding protein. HB-EGF is a mitotic and migration factor for keratinocytes and fibroblasts (25). Finally, VEGF is produced by a number of cell types, including fibroblasts. VEGF promotes early angiogenic events such as vasodilation, degradation of basement membrane, endothelial cell migration and proliferation (26). Stimuli for VEGF production include hypoxia and a number of other growth factors such as, TGF-β1 and EGF (26).
Using mastectomy wound fluid as a model for acute wounds, researchers found that chronic wound fluid to have significantly more TGF-β and EGF than the mastectomy wound fluid (20). However, Trengrove et al found no difference in the concentration of TGF-β or EGF in the wound fluid of healing and non-healing pressure ulcers (21) and Cooper et al found variable levels of EGF in chronic pressure ulcers (27). Elevated VEGF levels have been detected in chronic wound fluid compared to acute wound fluid (28). In this investigation, both soluble products from both planktonic and biofilm MRSA-exposed HF produced significantly more TGF-β1 and VEGF compared to control HF (Figure 6), and PCM induced significantly more TGF-β1, HB-EGF, and VEGF, than BCM. These results were similar to IL-6 and IL-8.
MMPs also play a pivotal role in the wound healing process. While they are primarily associated with the remodeling phase of wound healing, they can also influence other processes including inflammation and re-epithelialization (13). There are 24 mammalian MMPs, and their functions include the release of growth factors from the cell membrane or the ECM, cleavage of growth factor receptors from the cell surface, shedding of cell adhesion molecules, the activation of other MMPs, and cleavage of ECM molecules (13). MMP-1 (interstitial collagenase, fibroblast collagenase, or collagenase-1) cleaves fibrillar collagens types I, II, and III. It also cleaves multiple chemokines and aids in the establishment of a chemotactic gradient, which is a critical step of the inflammatory phase of wound healing (13). During wound repair, MMP-1 also guides and orients keratinocyte migration (29). MMP-3 (stromelysin-1), degrades several collagen types, fibronectin, elastin, laminins, gelatin and proteoglycan core proteins, and also participates in the inflammatory and repair phase of wound healing. In the remodeling phase, MMP-3 is also required for wound contraction (13). The elevated level of protease activity in chronic wounds is well documented (19, 20). Specifically, MMP-1 levels were elevated in diabetic foot ulcers (30), chronic leg ulcers (31). MMP-3 levels were also elevated in chronic leg ulcers (31).
HF exposed to the soluble products of both planktonic and biofilm MRSA produced significantly more MMP-1 compared to controls (Figure 7A). However, more interesting results were obtained when assaying for MMP-3. The PCM group produced significantly more MMP-3 than the control, while the BCM groups produced significantly less MMP-3 than the control (Figure 7B). These results are partially corroborated by previous investigations. Kanangat et al., found that HF exposed to culture supernatant and whole cell lysates of planktonic S. aureus had significantly enhanced expression of MMP-1 and MMP-3, as well as MMP-2, -7, -10, and -11, compared to control cultures; however the effects of biofilm-derived soluble product were not investigated (32). The BCM suppression of MMP-3 production is a unique result and warrants further investigation.
The scratch, viability, and apoptosis assays suggest that HF experience the same fate when exposed to either planktonic or biofilm MRSA, limited migration followed by death; however, the ELISA data demonstrate that HF may contribute differentially to the overall wound environment depending on the presence of either planktonic or biofilm MRSA. HF cytokine, growth factor, and protease production are all affected by PCM or BCM, suggesting that all phases of wound healing could be disrupted by the presence of bacteria. Specifically, the ELISA data demonstrated that soluble products from planktonic MRSA generally induced a stronger response from the HF than those of biofilm MRSA, with the exception of TNF-α and MMP3. This may be due to the fact that S. aureus may be more virulent in the planktonic state (33). Comparative proteome and trascriptome analysis of S. aureus biofilm and planktonic cells demonstrated that biofilm cells express more factors for binding and sessile growth, while planktonic cells are more virulent (33). Factors associated with acute infections, immunodominate antigen A (IsaA), staphylococcal secretor antigen (SsaA), nucleases, proteases, and toxins were expressed in lower amounts in biofilm cells (33). In the chronic wound environment, where S. aureus-containing biofilms are commonly found (7), reduced virulence may ensure a moderate host response, a more hospitable environment, and a possible bacterial pathogenesis strategy (10, 34).
The protein composition of biofilm and planktonic-conditioned media used in this experiment has yet to be elucidated; however the PCM and BCM produced using HK culture medium was found to have different compositions (10). BCM contained a more complex protein composition, including proteins related to translation, while PCM was found to contain several enzymes involved in glycolysis.
Finally, the results presented herein differ from those found previously for HK (9, 10). BCM made with HK culture medium was found to strongly induce apoptosis in HK, while PCM did not (9, 10). Furthermore, cytokine production in HK was significantly higher at the 4 hour time point for cells exposed to BCM compared to PCM; however, at the 24-hour time point cytokine production was higher for the PCM-exposed HK. In this investigation, both BCM and PCM induced HF apoptosis after prolonged exposure, and at most time points HF protein (cytokine, growth factor, and protease) production was higher in PCM-exposed cells compared those exposed to BCM. One notable exception was TNF-α, which was higher for BCM-exposed HF and HK at all time points. TNF-α is capable of inducing apoptosis in many cell types, and may be responsible for apoptotic response observed in HF and HK (10). Further investigation into the TNF-α pathway is currently ongoing.
Different responses from HF and HK to bacteria have been previously reported. For example, supernatants from Peptostreptococcus spp, were profoundly inhibited keratinocyte scratch closure and endothelial tubule formation, but not fibroblast scratch closure (35). LPS derived from P. aeruginosa or E.coli inhibited keratinocyte migration, but not fibroblast migration (36). Bacterial cytolethal distending toxins (CDTs) (produced by Gram-negative bacteria including Salmonella enterica serovar Typhi, Escherichia coli, Shigella dysenteriae, and Campylobacter species) induced cell-cycle arrest differently depending on the eukaryotic cell type (37). Interesting results were also obtained with keratinocytes/fibroblast co-cultures. When exposed to Haemophilus ducreyi, co-cultures of fibroblasts and HaCaT keratinocytes produced cytokine profiles that were similar to those found with an in vitro skin system, but were different than those obtained when the when the cell types were cultured separately (38). This last observation suggests that in order to more accurately mimic the in vivo chronic wound, the next iteration of a chronic wound biofilm model should contain a HK/HF co-culture or three-dimensional skin model (39).
CONCLUSION
Chronic wounds affect approximately 6.5 million patients in the United States and represent $25 billion in annual health care costs. This burden is rapidly increasing due to escalating health care costs, an aging population, and the rising incidence of diabetes and obesity (40). Several studies indicate that chronic wounds are often contaminated with bacterial biofilms, and understanding how biofilms contribute to wound chronicity may have significant impact on chronic wound care. Unfortunately, most studies use planktonic bacteria when studying host/pathogen interactions. The results presented herein demonstrate that planktonic and biofilm bacteria have differential effects on host cells. Furthermore, the results illustrate that to best characterize how bacteria participate in chronic wound pathogenesis, bacterial biofilms should be investigated; effects found using planktonic bacteria, may not accurately represent the state of a chronic wound.
Acknowledgments
The project described was supported by grant number 1P20GM078445-01 from the National Institute of General Medical Sciences (NIGMS). The contents of this project are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS.
References
- 1.Resch A, Rosenstein R, Nerz C, Gotz F. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol. 2005;71(5):2663–76. doi: 10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 3.Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis. 2004;32(1):88–94. doi: 10.1016/j.bcmd.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 4.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008;16(1):23–9. doi: 10.1111/j.1524-475X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen. 2010;18(5):467–77. doi: 10.1111/j.1524-475X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA, Wolcott RD. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008;8:43. doi: 10.1186/1471-2180-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirker KR, Secor PR, James GA, Fleckman P, Olerud JE. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009;17:690–9. doi: 10.1111/j.1524-475X.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. Staphylococcus aureus Biofilm and Planktonic Cultures Differentially Impact Gene Expression, MAPK Phosphorylation, and Cytokine Production in Human Keratinocytes. BMC Microbiol. 2011;11(1):143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukano Y, Knowles NG, Usui ML, Underwood RA, Hauch KD, Marshall AJ, Ratner BD, Giachelli C, Carter WG, Fleckman P, Olerud JE. Characterization of an in vitro model for evaluating the interface between skin and percutaneous biomaterials. Wound Repair Regen. 2006;14(4):484–91. doi: 10.1111/j.1743-6109.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 12.Clark RA. Wound repair. Curr Opin Cell Biol. 1989;1(5):1000–8. doi: 10.1016/0955-0674(89)90072-0. [DOI] [PubMed] [Google Scholar]
- 13.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–47. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9(11):3199–204. [PubMed] [Google Scholar]
- 15.Giuliani AL, Spisani S, Cavalletti T, Reali E, Melchiorri L, Ferrari L, Lanza F, Traniello S. Fibroblasts increase adhesion to neutrophils after stimulation with phorbol ester and cytokines. Cell Immunol. 1993;149(1):208–22. doi: 10.1006/cimm.1993.1148. [DOI] [PubMed] [Google Scholar]
- 16.Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93(1):41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- 17.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4(3):155–60. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiegand C, Schonfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res. 2010;302(6):419–28. doi: 10.1007/s00403-009-1011-1. [DOI] [PubMed] [Google Scholar]
- 20.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321–5. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- 21.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8(1):13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 22.Perfetto B, Donnarumma G, Criscuolo D, Paoletti I, Grimaldi E, Tufano MA, Baroni A. Bacterial components induce cytokine and intercellular adhesion molecules-1 and activate transcription factors in dermal fibroblasts. Res Microbiol. 2003;154(5):337–44. doi: 10.1016/S0923-2508(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 23.Kyburz D, Rethage J, Seibl R, Lauener R, Gay RE, Carson DA, Gay S. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum. 2003;48(3):642–50. doi: 10.1002/art.10848. [DOI] [PubMed] [Google Scholar]
- 24.Mourad W, Mehindate K, Schall TJ, McColl SR. Engagement of major histocompatibility complex class II molecules by superantigen induces inflammatory cytokine gene expression in human rheumatoid fibroblast-like synoviocytes. J Exp Med. 1992;175(2):613–6. doi: 10.1084/jem.175.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333(3):F179–99. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 26.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219(6):688–91. doi: 10.1097/00000658-199406000-00012. discussion 91–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauer G, Sollberg S, Cole M, Flamme I, Sturzebecher J, Mann K, Krieg T, Eming SA. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115(1):12–8. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 29.Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7(6):423–32. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 30.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45(7):1011–6. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 31.Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res. 1998;290 (Suppl):S47–54. doi: 10.1007/pl00007453. [DOI] [PubMed] [Google Scholar]
- 32.Kanangat S, Postlethwaite A, Hasty K, Kang A, Smeltzer M, Appling W, Schaberg D. Induction of multiple matrix metalloproteinases in human dermal and synovial fibroblasts by Staphylococcus aureus: implications in the pathogenesis of septic arthritis and other soft tissue infections. Arthritis Res Ther. 2006;8(6):R176. doi: 10.1186/ar2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resch A, Leicht S, Saric M, Pasztor L, Jakob A, Gotz F, Nordheim A. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics. 2006;6(6):1867–77. doi: 10.1002/pmic.200500531. [DOI] [PubMed] [Google Scholar]
- 34.Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson KL, Dunman PM, Joyce S, Skaar EP. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe. 2007;1(2):109–19. doi: 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens P, Wall IB, Wilson MJ, Hill KE, Davies CE, Hill CM, Harding KG, Thomas DW. Anaerobic cocci populating the deep tissues of chronic wounds impair cellular wound healing responses in vitro. Br J Dermatol. 2003;148(3):456–66. doi: 10.1046/j.1365-2133.2003.05232.x. [DOI] [PubMed] [Google Scholar]
- 36.Loryman C, Mansbridge J. Inhibition of keratinocyte migration by lipopolysaccharide. Wound Repair Regen. 2008;16(1):45–51. doi: 10.1111/j.1524-475X.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 37.Smith JL, Bayles DO. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol. 2006;32(4):227–48. doi: 10.1080/10408410601023557. [DOI] [PubMed] [Google Scholar]
- 38.Zaretzky FR, Kawula TH. Examination of early interactions between Haemophilus ducreyi and host cells by using cocultured HaCaT keratinocytes and foreskin fibroblasts. Infect Immun. 1999;67(10):5352–60. doi: 10.1128/iai.67.10.5352-5360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charles CA, Ricotti CA, Davis SC, Mertz PM, Kirsner RS. Use of tissue-engineered skin to study in vitro biofilm development. Dermatol Surg. 2009;35(9):1334–41. doi: 10.1111/j.1524-4725.2009.01238.x. [DOI] [PubMed] [Google Scholar]
- 40.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]