Abstract
Objective
The physiology of menopausal hot flashes is not well understood. The autonomic nervous system may play a role in hot flashes, but the current understanding is limited. We previously demonstrated in the laboratory that decreases in high frequency heart rate variability, an index of cardiac vagal control, occur during hot flashes relative to preceding and following periods. In the present study, we tested whether we would observe a similar phenomenon in the ambulatory setting. We additionally considered respiratory rate in these associations.
Methods
21 peri- and postmenopausal women ages 40–60 reporting daily hot flashes were monitored both for physiologic and reported hot flashes and heart rate variability over a 24-hour period as they went about their daily lives. Heart rate variability estimates were derived using the band-limited variance method. The interval during the hot flash was compared to two non-flash periods prior to and following the hot flash via mixed effects models.
Results
Heart rate variability significantly decreased during hot flashes relative to periods preceding (b=0.31, SE=0.03 p<0.0001) and following (b=0.30, SE=0.03, p<0.0001) physiologic hot flashes (covariates: age, race, education, menopausal status, physical activity, body mass index, anxiety). Findings were comparable considering self-reported hot flashes. Findings persisted controlling for respiratory rate.
Conclusions
Significant decreases in cardiac vagal control occurred during hot flashes assessed during women’s daily lives. These findings extend our work in the laboratory to the ambulatory setting, further shedding light on the physiology of hot flashes and underscoring a potential role of parasympathetic function in hot flashes.
Keywords: hot flashes, hot flushes, vasomotor symptoms, heart rate variability, autonomic nervous system, menopause
Introduction
Hot flashes are a common midlife experience, reported by up to 70% of women during the menopausal transition.1 They are linked to a range of negative outcomes, such as impaired quality of life,2 sleep disturbance,3 cognitive decrements,4 and depressed mood.5 Hot flashes have even been linked to other poor physical health outcomes, such as cardiovascular risk6, 7 and low bone density.8 Hot flashes are a leading reported reason women seek medical care during midlife.9 However, despite their prevalence and importance to women’s lives, the understanding of the underlying physiology of hot flashes remains limited.
The leading model of hot flashes characterizes them as thermoregulatory events.10 However, hot flashes have also been linked to changes in the autonomic nervous system. In our prior work in the laboratory11 we found acute reductions in high frequency heart rate variability (HF-HRV), indicative of reductions in parasympathetic control of heart rate, during hot flashes. Other work has shown some indication of increases in power in lower frequency bands with reported12 and physiologic13 hot flashes during sleep. The latter findings were interpreted as indicating sympathetic activation during hot flashes.13 However, interpretation of these lower frequencies is limited by their multiple contributions, as a mixture of sympathetic and parasympathetic control contribute to low frequency HRV (LF-HRV) and a range of other physiologic stimuli to very low (VLF) and ultra low frequency (ULF) HRV.14 Notably, the parasympathetic branch of the autonomic nervous system is responsible for vegetative and restorative functions, whereas the sympathetic branch is associated with what is known as the “fight or flight” response. These two branches sometimes, but not always, act in a reciprocal fashion.14 Thus, initial evidence indicates autonomic nervous system involvement in hot flashes, and possibly parasympathetic withdrawal/sympathetic activation, but more research to better understand relations between hot flashes and autonomic function is clearly needed.
In this study, we examine whether hot flashes are associated with declines in HF-HRV in the ambulatory setting. Participants were monitored both for physiologic and subjective hot flashes and HRV over a 24-hour period as they went about their daily lives. We thereby extend our prior work in the laboratory to the ambulatory setting, a setting with greater generalizability to women’s daily lives. We hypothesize that reductions in cardiac vagal control will be observed during physiologically-measured hot flashes as compared to periods preceding and following hot flashes. We also examine cardiac vagal control during subjectively-reported hot flashes, and we examine relations between hot flashes and cardiac vagal control controlling for respiratory rate, a key contributor to HF-HRV and a possible explanatory factor for HF-HRV changes during hot flashes.14 Moreover, consistent with prior work11 we examine any differences in relations between HF-HRV and hot flashes by age, race, menopausal stage, and anxiety. Finally, we additionally examine changes in LF-HRV and the LF/HF ratio, a potential indicator of sympathovagal balance15 during hot flashes.
Methods
Study Sample
The study sample included 23 late perimenopausal and postmenopausal women between the ages of 40 and 60 who reported having daily hot flashes. Exclusion criteria included hysterectomy and/or oophorectomy; current smoking; reported heart disease, diabetes, or hypertension; pregnancy; use of oral or transdermal estrogen or progesterone, gabapentin, SSRI/SNRI antidepressants within the past three months; and currently undergoing chemotherapy for breast cancer. Two women were excluded; one due to missing skin conductance data and one due to total monitor failure. Therefore, 21 women (13 Caucasian, 8 African American women) were included in primary analytic models.
Design and Procedures
After a telephone and in-person screening to determine eligibility, height and weight were measured, questionnaires administered, and participants equipped with an ambulatory monitor that measured sternal skin conductance (hot flashes), electrocardiography (ECG, heart rate variability), and respiration. Women were also provided with an electronic diary to complete during waking hours when experiencing a hot flash. All women underwent monitoring for 24 hours as they went about their daily activities. Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written informed consent.
Measures
Hot flashes
Hot flash monitoring was conducted with an ambulatory sternal skin conductance monitor and an electronic diary. Sternal skin conductance was recorded via the Biolog ambulatory monitor (UFI, 3991/2-SCL; Morro Bay, CA), a portable device worn in a pouch around the waist. The Biolog measures sternal skin conductance sampled at 1 Hz from the sternum via a 0.5 Volt constant voltage circuit passed between two Ag/AgCl electrodes (UFI) filled with 0.05M KCL Velvachol/glycol paste.16 Participants were instructed to avoid heavy physical activity and showering while wearing the monitor.
Physiologic hot flashes were classified via standard methods, with skin conductance rise of 2 μmho in 30 seconds17 flagged automatically by UFI DPS software (v3.6; Morro Bay, CA) and edited for artifact.18 Given evidence that some women may show submaximal hot flashes failing to reach the 2 μmho criterion,19, 20 all potential hot flash events were also visually inspected and events showing the characteristic hot flash pattern yet <2 μmho/30 sec rise were also coded as hot flashes. This coding has been shown to be reliable (κ=.86).19, 20 A 20-minute lockout period was implemented after the start of the ash during which no hot ashes were coded. A 5-minute reporting window was adopted, in which physiologically defined hot flashes accompanied by a self-report within 5 minutes were considered reported. To report hot flashes, participants were instructed to 1) complete a portable electronic diary (Palm Z22, Palm, Inc.) (waking hours), and 2) press event mark buttons on the hot flash monitor (waking and sleeping hours) when experiencing a hot flash.
HRV and respiration
Heart rate was measured by ECG via three silver/silver chloride electrodes (Ultratrace 1690, Conmed; Utica, NY) in a standard 3-lead configuration sampled at 1 KHz via the Biolog ambulatory monitor (UFI, 3991/2-SCL; Morro Bay, CA). ECG processing was conducted online via UFI’s R-spike detection algorithm. The interbeat interval (IBI) event series was edited for artifact using custom software.21 Estimates of HF-HRV were derived using the band-limited variance method,22 with HF band cutoffs of 0.15–0.40 Hz and LF band cutoffs of 0.04–0.15. This method consisted of resampling the IBI event series excluding artifactual beats (10 Hz; linear interpolation), bandpass filtering the entire resampled series (241 pt Hamming filter), and taking the variance of the resulting filtered series across epochs. Minutes with ≥20% artifact were excluded (15% of minutes, 6% of physiologic hot flashes). HF and LF power values were natural log transformed for analysis. Respiratory rate was sampled at 10Hz via the Pneumotrace® respiration belt (UFI model 1132; Morro Bay, CA) worn around the chest. Respiration signals were amplified and filtered via the Biolog, and respiratory events were detected by UFI’s 3991× GPP v1.2 software.
Covariates
Height and weight were measured via a fixed stadiometer and a calibrated balance beam scale, respectively. Demographics, menstrual history, and health behaviors were assessed by demographic and medical history questionnaires. Sleep/wake times were determined from a sleep diary completed before the woman went to bed at night and upon waking the following morning. Race/ethnicity was determined in response to “How would you describe your primary racial or ethnic group?” Menopausal status was obtained from reported bleeding patterns, categorized as perimenopausal (bleeding in previous three months with decrease in cycle predictability in past year or >3-<12 months amenorrhea), or postmenopausal (≥12 months amenorrhea). Physical activity was assessed via the International Physical Activity Questionnaire (IPAQ) which yields estimates of metabolic equivalent-minutes/week.23 Depressive symptoms were assessed via the Center for Epidemiologic Studies Depression (CESD) Survey,24 and state/trait anxiety via the Spielberger State Trait Anxiety Inventory.25 Only state anxiety was included due to its high correlation with the CESD and trait anxiety, due to its prior documented robust associations with hot flashes1, 19 and for consistency with prior work.11
Statistical analysis
Relations between average HF-HRV values and participant characteristics were estimated using Pearson and point biserial correlations. Pre-flash, flash, and post-flash time periods were identified by comparing HF-HRV during the minute at which the physiological onset of the hot flash occurred (i.e., minute zero) to each of the preceding and following 20 minutes within a single mixed effects regression. Using p=0.10 for the purposes of defining relevant time periods, this model yielded three distinct periods: 1) a pre-flash period, ranging from 20 to 2 min prior to flash onset; 2) a flash period, ranging from 1 min prior to 7 min following onset; and 3) a post-flash period, ranging from 8 min to 20 min following onset. Nearly identical results were obtained using k-means clustering procedures. These pre- and post-flash periods were next entered into subsequent models as within-woman factors, with the during-flash time segment considered the referent.
Relations between flash intervals and HF-HRV values were estimated with linear mixed effects models, with random intercepts for flashes nested within participants and maximum likelihood estimation. The within-group correlation structure was modeled as a first order continuous autoregressive, nested within flashes and participants. Covariates included age, race/ethnicity, menopausal status, education, physical activity, and state anxiety, consistent with our prior work.11 Physiologic flashes (regardless of whether they were reported) and onset times were used for all primary models; subjective flashes (regardless of whether they were met by a physiologic flash) and times were considered in secondary models. Additional models considered HF-HRV changes during reported hot flashes that lacked a corresponding physiologic hot flash, as well as during physiologic hot flashes lacking a corresponding self-reported flash. In secondary analyses, respiratory rate was added to covariate-adjusted models to examine whether it accounted for any relations between (physiologic or self-reported) hot flashes and HF-HRV. Interactions between hot flashes and state anxiety, menopausal status, ethnicity and sleep-wake status were tested via the likelihood ratio test consistent with our prior work. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria). All models were 2-sided, alpha=0.05.
Results
Participants were on average 52 years old, postmenopausal and overweight (Table 1). Across participants, 245 physiologic hot flashes (7612 minutes) and 156 reported flashes (5031 minutes) were included in the analysis, for an average of 12 physiologically detected hot flashes and 7 reported hot flashes per woman/24-hour monitoring session. The only participant characteristic related to mean 24-hour HF-HRV was state anxiety, with higher anxiety associated with lower HF-HRV (r=−0.47, p=0.03).
Table 1.
Participant characteristics (N=21)
| Age, years (M, SD) | 51.9 (3.9) |
| Race/ethnicity (n, %) | |
| Caucasian | 13 (61.9) |
| African American | 8 (38.1) |
| Menopausal status (n, %) | |
| Perimenopausal | 4 (19.1) |
| Postmenopausal | 17 (81.0) |
| Time since last menstrual period (M, SD), years‡ | 4.3 (3.4) |
| BMI (M, SD) | 27.2 (5.8) |
| Education (n, %) | |
| High school or less, vocational | 11 (52) |
| Some college or higher | 9 (48) |
| SBP, mmHg (M, SD) | 124.5 (13.7) |
| DBP, mmHg (M, SD) | 78.9 (11.1) |
| IPAQ Physical Activity Score, MET-min/wk (Median, IQR)† | 2628 (3481) |
| State anxiety (M, SD) | 34.7 (10.9) |
| Self-reported hot flashes, 24 hours (M, SD) | 7.4 (4.5) |
| Physiologically-detected hot flashes, 24 hours (M, SD) | 11.7 (7.2) |
Natural log transformed for analysis
Ranged from 0.4–16.0 years
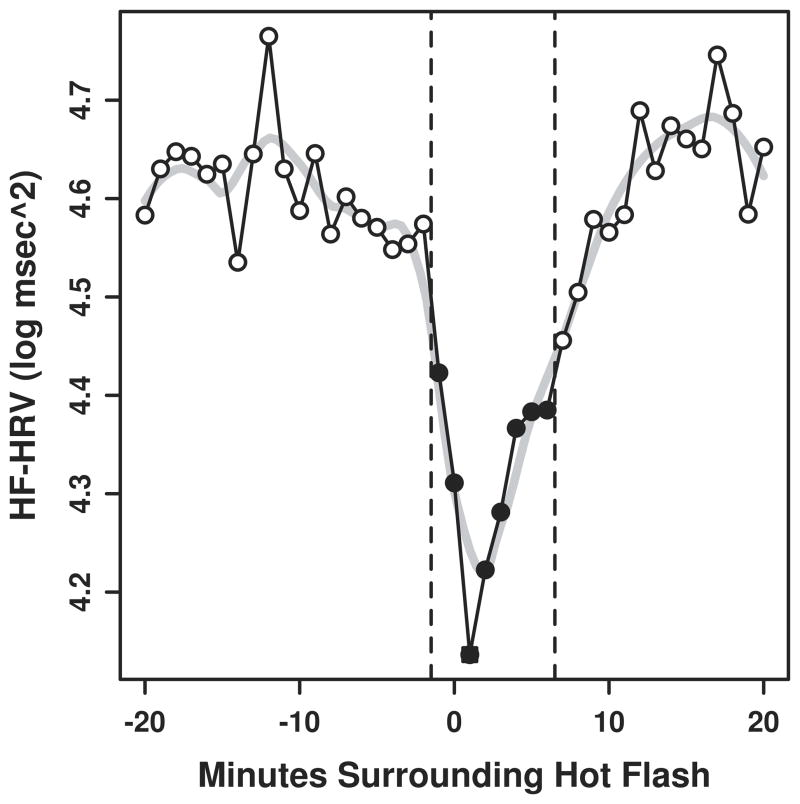
Significant reductions in HF-HRV were observed during physiologically monitored hot flashes as compared to the periods preceding the following the hot flash (Figure, Table 2). These findings were evident in minimally adjusted as well as fully adjusted models. When self-reported hot flashes were examined, findings were similar, but with slightly attenuated effect sizes relative to models considering physiologic hot flashes (Table 3).
Figure.
Minute by minute changes in HF-HRV during hot flash
Note: Closed circles represent minutes significantly different from open circles at p<0.05, N=21
Table 2.
HF-HRV changes associated with physiologically-assessed hot flashes
| HF-HRV
|
||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| B (SE) | p | B (SE) | p | B (SE) | p | |
| Flash interval | ||||||
| Pre-flash | 0.28 (0.03) | <0.0001 | 0.31 (0.03) | <0.0001 | 0.33 (0.03) | <0.0001 |
| Post-flash | 0.30 (0.03) | <0.0001 | 0.30 (0.03) | <0.0001 | 0.29 (0.03) | <0.0001 |
| Flash (referent) | -- | -- | -- | |||
Model 1: Univariate
Model 2: Covariates age, race, menopausal stage, education, physical activity, state anxiety, BMI
Model 3: Covariates age, race, menopausal stage, education, physical activity, state anxiety, BMI, respiratory rate
Note: Models 1 and 2: N=21; Model 3: N=19
Table 3.
HF-HRV changes associated with self-reported hot flashes
| HF-HRV
|
||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| B (SE) | p | B (SE) | p | B (SE) | p | |
| Flash interval | ||||||
| Pre-flash | 0.13 (0.03) | <0.0001 | 0.14 (0.03) | <0.0001 | 0.17(0.03) | <0.0001 |
| Post-flash | 0.24 (0.03) | <0.0001 | 0.23 (0.03) | <0.0001 | 0.22(0.03) | <0.0001 |
| Flash (referent) | -- | -- | -- | |||
Model 1: Univariate
Model 2: Covariates age, race, menopausal stage, education, physical activity, BMI, state anxiety
Model 3: Covariates age, race, menopausal stage, education, physical activity, BMI, state anxiety, respiratory rate
Note: Models 1 and 2: N=21; Model 3: N=19
We next considered associations between hot flashes and HF-HRV controlling for respiratory rate. Due to missing respiration data on two women, these secondary models included 219 physiologic flashes (6567 minutes) and 143 subjective flashes (4371 minutes) for models with physiologic and self-reported hot flashes, respectively. The average respiratory rate across intervals was 17.7 breaths/min (0.295 Hz). Hot flashes were associated with small (mean difference <0.1 breath/min) but statistically significant increases in respiratory rate (vs. flash interval, pre-flash: b(SE)=−0.002(0.0009), p=0.007; post-flash: b(SE)= −0.003(0.001), p=0.001), and higher respiratory rate was associated with lower HF-HRV (b(SE) = −1.40(0.36), p<0.0001). When respiratory rate was included in multivariable models examining changes in HF-HRV during hot flashes, associations between hot flashes and HF-HRV remained significant in the case of both physiologic hot flashes and self-reported hot flashes (Tables 2, 3).
We conducted several additional analyses. First, consistent with our prior work, we examined any interactions by age, race/ethnicity, menopausal status, and anxiety in the physiologic hot flash models. We found significant interactions for menopausal status (pre-flash: p=0.03, post-flash: p=0.02) and anxiety (pre-flash: p=0.0001, post-flash: p=0.04), and to a lesser extent age (pre-flash: p=0.01, post-flash: p=0.15), indicating that the declines in HF-HRV associated with HF-HRV were greatest for those who were postmenopausal (compared to perimenopausal), older, and more anxious. Next, given prior work examining HRV during hot flashes during sleep,12, 13 we examined whether the observed associations differed by sleep/wake. First, HF-HRV was on average higher during sleep than during waking hours (b(SE)= −0.79 (0.11), p<0.0001). Second, significant interactions were found for both pre-flash (multivariable models, physiologic flashes, b(SE)= −0.19(0.06), p=0.001) and post-flash (b(SE)= −0.14(0.06), p=0.02) intervals, indicating that the drops in HF-HRV seen with hot flashes were somewhat more pronounced at night. Further, examination of changes in HF-HRV during physiologic hot flashes that were not reported revealed a similar pattern as primary results (multivariable models, vs. flash interval; pre-flash: b(SE)=0.36 (0.04), p<0.0001; post-flash: b(SE)=0.30(0.05), p<0.0001).
Finally, given prior evidence of increased LF-HRV during hot flashes,13 we examined LF-HRV changes during hot flashes. We found some evidence of decreases in LF-HRV (pre-flash: B(SE)=0.08 (0.03), p=0.006; post-flash: B(SE)=0.13 (0.03), p<0.0001; vs. flash interval, multivariable models) during physiologic hot flashes, but slight increases in LF-HRV (pre-flash: B(SE)= −0.06 (0.03), p=0.04; post-flash: B(SE)= −0.004 (0.03), p=0.92; vs. flash interval, multivariable models) during reported hot flashes. Given the both parasympathetic and sympathetic contributions to LF-HRV that make these findings difficult to interpret, we also examined the LF/HF HRV ratio during hot flashes. The LF/HF ratio showed significant increases during physiologic flashes (pre-flash: B(SE)= −0.63(0.10), p<0.0001; post-flash: B(SE) −0.44(0.11), p=0.0001; vs. flash interval, multivariable models) and self-reported flashes (pre-flash: B(SE)= −0.57(0.12), p<0.0001; post-flash: B(SE) −0.75(0.13), p<0.0001; vs. flash interval, multivariable models), suggesting a greater decline in HF relative to LF-HRV, or sympathetic predominance/vagal withdrawal during the physiologic or self-reported hot flash.
Discussion
This study replicated and extended our prior laboratory research, showing acute decreases in HF-HRV during hot flashes as compared to the periods preceding and following hot flashes in the ambulatory setting. This study included longer monitoring periods (24 hours), a larger sample of hot flashes per woman, and given its ambulatory design, greater generalizability to women’s everyday lives. This study also incorporated respiratory measures, showing that changes in HF-HRV during hot flashes were not due to changes in respiratory rate. Further, we found more pronounced findings for physiologically-measured hot flashes, as well as persistent associations for physiologic hot flashes that were not reported. Thus, results showed that women experience declines in cardiac vagal control during hot flashes throughout their daily lives, an effect not explained by respiration, and not due solely to the experience of hot flashes.
Hot flashes are generally believed to represent thermoregulatory phenomena, or dramatic heat dissipation events occurring in the context of the narrowed thermoneutral zone of symptomatic menopausal women.10 However, the understanding of the physiology of hot flashes is far from complete, and there have been urgent calls to better understand the underlying physiology of hot flashes.26 The autonomic nervous system has been implicated in hot flashes. Some earlier work has suggested changes in central noradrenergic function,27 or peripheral catecholamines28 with hot flashes. More recently, investigators have adopted measures of HRV to understand the role of the autonomic nervous system in hot flashes. We previously showed acute decreases in HF-HRV during physiologically-measured hot flashes during the daytime in the laboratory.11 In the present study, we replicated laboratory work in the ambulatory setting in a separate sample of women, showing a pattern of autonomic changes indicating vagal withdrawal during physiologically-assessed or self-reported hot flashes.
When considering these findings in the context of the existing literature,11–13 they collectively suggest vagal withdrawal and/or sympathetic activation during hot flashes. However, there are important differences between studies. Freedman et al.,13 monitored women overnight during sleep in the laboratory, and Hoikkala et al.12 examined acute changes in HRV during reported hot flashes during sleep. Freedman et al.13 found acute increases in frequency powers 0–0.15 Hz during sleeping physiologic hot flashes, a band representing LF, VLF, and ULF-HRV, but did not find changes in HF-HRV. Hoikkala et al.12 found somewhat deceased HF-HRV and increases in both LF- and ULF/VLF-HRV during overnight hot flashes that were reported. We found significant decreases in HF-HRV with hot flashes here and in the laboratory,11 and significant, but less pronounced, decreases in LF-HRV and an increase in the LF/HF ratio during physiologic hot flashes (suggesting vagal withdrawal). For self-reported hot flashes, we found an even clearer pattern of sympathetic predominance/vagal withdrawal during hot flashes. The reasons for the different findings between the various studies are not immediately apparent, although there were important methodologic differences. First, we focus on HF-HRV, because it is a clearer index of parasympathetic control of the heart14 and has been linked to a range of clinical health outcomes, including cardiovascular risk.15, 29, 30 LF-HRV or VLF-HRV changes during hot flashes are difficult to interpret given the multiple contributions to these frequency bands, as a mixture of sympathetic and parasympathetic control contributes to LF-HRV, and a range of other physiologic stimuli not fully understood, including thermoregulation and plasma renin activity, to ULF/VLF-HRV.14 In fact, the decreases in LF-HRV during physiologic hot flashes observed here, when considered in the context of the changes in HF-HRV and the LF/HF ratio, is likely driven by withdrawal of the vagal contribution to LF-HRV. Moreover, we consider physiologic and self-reported hot flashes over the day and night. Freedman et al. and Hoikkala et al. considered only overnight hot flashes, and in the case of Hoikkala et al., only those hot flashes reported during the night (events for which women were awake to report). Reported hot flashes have the additional contribution of the often negative experience of hot flashes, which could further contribute to the elevations in LF and declines in HF-HRV. Interestingly, we found somewhat more pronounced reductions in HF-HRV with hot flashes observed at night relative to the day, for which a contributing factor may have been the higher mean HF-HRV at night. Another important methodological difference is that we consider 1 minute epochs, whereas Freedman et al. considered 5 minute epochs and Hoikkala et al. an average of 5 minute windows with 50% overlap. Finally, in the one study with null findings for HF-HRV,13 the definition of the HF band appeared to differ somewhat than what is standard and was used here. Thus, any of these methodological differences could have contributed to the variation in findings between studies. However, in general, these studies support an overall sympathovagal balance suggesting vagal withdrawal and/or sympathetic predominance during hot flashes.
The present findings may also contribute to emerging work on hot flashes and cardiovascular risk. Some emerging work, with notable exceptions, has shown links between hot flashes and cardiovascular risk, including measures of subclinical cardiovascular disease,6, 7 adverse risk factors,31, 32 and possibly clinical events in the setting of hormone use.33, 34 Notably, reduced HF-HRV has been prospectively linked to greater cardiovascular morbidity and mortality.15, 29 In fact, an autonomic nervous system pattern characterized by reduced parasympathetic and increased sympathetic tone has been implicated in cardiovascular risk.15, 29 The clinical significance of phasic changes in HF-HRV, such as the acute decreases in HF-HRV observed here, is not as well established in contrast to mean HF-HRV differences between individuals. However, it does suggest considering the role of the autonomic nervous system in understanding associations between hot flashes and cardiovascular risk.
Reductions in HF-HRV during hot flashes were observed across women, but were most pronounced among women who were postmenopausal, more anxious, and to a lesser extent older. The reasons for these interactions are not entirely clear, and these between-woman comparisons should be interpreted with caution given the relatively small sample, particularly of perimenopausal women. Notably, postmenopausal status, anxiety, and older age are all factors that have been associated with lower absolute HF-HRV.11, 15, 35, 36 Although speculative, it is possible that a hot flash experienced in the setting of compromised vagal tone may have a particularly important autonomic impact.
Results must be interpreted in light of several limitations. The sample was small, and findings should be replicated with a larger sample. However, it is worth noting that this analysis was a within-flash, not a between-woman analysis, and the large number of hot flashes considered here afforded a well-powered test of the primary hypothesis. Conversely, power to test interactions by between-woman characteristics was likely limited. Further, the women in this study were selected to be at relatively low cardiovascular risk, which may limit conclusions to a broader range of women. However, findings from our laboratory study showing similar findings included women with several comorbidities.11 Additionally, physiologic measures of hot flashes have known limitations,19, 37 and while our coding of hot flashes addressed some of these limitations, interpretative questions remain. However, confidence in our findings is bolstered by the fact that significant findings persisted when considering subjectively reported hot flashes alone.
This study had several strengths. First, it is the first study to examine acute changes in HF-HRV during hot flashes over 24-hours. We prospectively and simultaneously recorded hot flashes physiologically and via self-report, as well as ECG and respiratory rate for the monitoring period. The physiologic measurement of hot flashes, with interpretative caveats mentioned above, allows a more precise estimation of the exact timing of hot flash occurrence and does not reply upon compliance to diaries. We measured hot flashes during wake and sleep, allowing examination of both intervals. Women were free of reported cardiovascular disease, diabetes, and hypertension and related confounding medications. Improving upon our prior work, we measured respiratory rate, confirming that decreases in HF-HRV during hot flashes were not due to this factor. Finally, we replicated our prior findings from the laboratory, extending them to a separate sample of women monitored in the ambulatory setting.
Conclusion
This is the first study to examine cardiac vagal control during physiologically-assessed as well as self-reported hot flashes in the ambulatory setting over the day and night. Findings indicated acute reductions in HF-HRV during hot flashes. These associations could not be explained by confounding factors, including respiratory rate. These findings further shed light on the physiology of hot flashes, suggesting a role for the autonomic nervous system, and particularly parasympathetic function, in hot flashes.
Summary Sentence.
Among women with hot flashes, acute decreases in high frequency heart rate variability were observed during physiologically-monitored and self-reported hot flashes in the ambulatory setting. These findings indicate acute decreases in cardiac vagal control during hot flashes, supporting a role for the autonomic nervous system in hot flashes.
Acknowledgments
Funding Source: This publication was supported by the National Institutes of Health via the National Institute on Aging (K23AG029216, PI: Thurston) the University of Pittsburgh Institute on Aging (PI: Thurston).
This work was supported by the National Institutes of Health via the National Institute on Aging (K23AG029216, PI: Thurston) the University of Pittsburgh Institute on Aging (PI: Thurston).
Footnotes
The authors have no conflicts of interest.
References
- 1.Gold E, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16(5):860–9. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15(5):848–56. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103(1–3):267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurston RC, Sutton-Tyrrell K, Everson-Rose S, Hess R, Powell L, Matthews K. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18(4):352–38. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: Findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall CJ, Zheng Y, Crawford SL, et al. Presence of vasomotor symptoms is associated with lower bone mineral density: a longitudinal analysis. Menopause. 2009;16(2):239–46. doi: 10.1097/gme.0b013e3181857964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58(4):348–58. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Freedman RR. Physiology of hot flashes. Am J Human Biol. 2001;13(4):453–64. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 11.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control: A link to cardiovascular risk? Menopause. 2010;17(3):456–61. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoikkala H, Haapalahti P, Viitasalo M, et al. Association between vasomotor hot flashes and heart rate variability in recently postmenopausal women. Menopause. 2010;17(2):315–20. doi: 10.1097/gme.0b013e3181c2bb6d. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Kruger ML, Wasson SL. Heart rate variability in menopausal hot flashes during sleep. Menopause. 2011 doi: 10.1097/gme.0b013e31820ac941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 15.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 16.Dormire SL, Carpenter JS. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology. 2002;39(4):423–6. doi: 10.1017.S0048577201393149. [DOI] [PubMed] [Google Scholar]
- 17.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–9. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–15. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 19.Thurston R, Matthews K, Hernandez J, De La Torre F. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009;46(2):285–92. doi: 10.1111/j.1469-8986.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston R, Hernandez J, Del Rio J, De la Torre F. Support vector machines to improve physiologic hot flash measures: Application to the ambulatory setting. Psychophysiology. 2011;48(7):1015–21. doi: 10.1111/j.1469-8986.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christie IC. A Matlab algorithm for the automated and manual detection of IBI artifacts. Psychophysiology. 2004;41(Suppl 1):S39. [Google Scholar]
- 22.Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol Psychol. 2007;74(2):243–62. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall A, Sjostrom M, et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 25.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 26.Sturdee DW. The menopausal hot flush--anything new? Maturitas. 2008;60(1):42–9. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70(2):332–7. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 28.Kronenberg F, Cote LJ, Linkie DM, Dyrenfurth I, Downey JA. Menopausal hot flashes: thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas. 1984;6(1):31–43. doi: 10.1016/0378-5122(84)90063-x. [DOI] [PubMed] [Google Scholar]
- 29.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74(2):224–42. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Liao D, Cai J, Rosamond WD, et al. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 31.Gast GC, Grobbee DE, Pop VJ, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51 (6):1492–8. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 32.Thurston R, El Khoudary S, Sutton-Tyrrell K, et al. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women’s Health Across the Nation. Menopause. doi: 10.1097/gme.0b013e31821f5d39. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 34.Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause. 2009;16 (4):639–43. doi: 10.1097/gme.0b013e31819c11e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74(2):185–99. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol. 2003;285(5):H2188–93. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- 37.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79(6):777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]