Abstract
Purpose of review
Agriculture represents a major industry worldwide, and despite protection against the development of IgE-mediated diseases, chronic exposure to agriculture-related organic dusts is associated with an increased risk of developing respiratory disease. This article will review the literature regarding new knowledge of important etiologic agents in the dusts and focus on the immunologic responses following acute and repetitive organic dust exposures.
Recent findings
Although endotoxin remains important, there is an emerging role for non-endotoxin components such as peptidoglycans from Gram-positive bacteria. Pattern recognition receptors including Toll-like receptor 4 (TLR4), TLR2 and intracellular nucleotide oligomerization domain-like receptors are partially responsible for mediating the inflammatory consequences. Repeated organic dust exposures modulate innate and adaptive immune function with a resultant adaptation-like response. However, repetitive exposures cause lung parenchymal inflammation, chronic disease, and lung function decline over time.
Summary
The immunological consequences of organic dust exposure in the farming industry are likely explained by the diversity of microbial motifs in dust that can elicit differing innate immune receptor signaling pathways. Whereas initial activation results in a robust inflammatory response, repetitive dust exposures modulate immunity. This can result in low-grade, chronic inflammation and/or protection against allergic disease.
Keywords: Farm, innate immunity, respiratory disease, adaptation, endotoxin, peptidoglycan, pattern recognition receptors
Introduction
Agricultural workers are at an increased risk of developing respiratory disorders including rhinosinusitis, asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD), and hypersensitivity pneumonitis. The resultant respiratory disease is predominately non-IgE mediated and marked by neutrophilic influx. As the agricultural industry has evolved, it is now recognized that livestock farmers as compared to crop farmers are at the highest risk of developing chronic bronchitis, COPD and lung function decline (1). This may be explained by the increase in large-scale, concentrated, closed, animal-feeding operations that can generate significant amounts of dust. Chronic inhalation of these complex organic dusts, rich with particulates and microbial-rich components, is implicated in disease development and severity (1). Although endotoxin remains an important inflammatory agent, peptidoglycans, Gram-positive bacteria cell wall components, (1→3)-β-D-glucans, and fungi are all emerging as potentially important players.
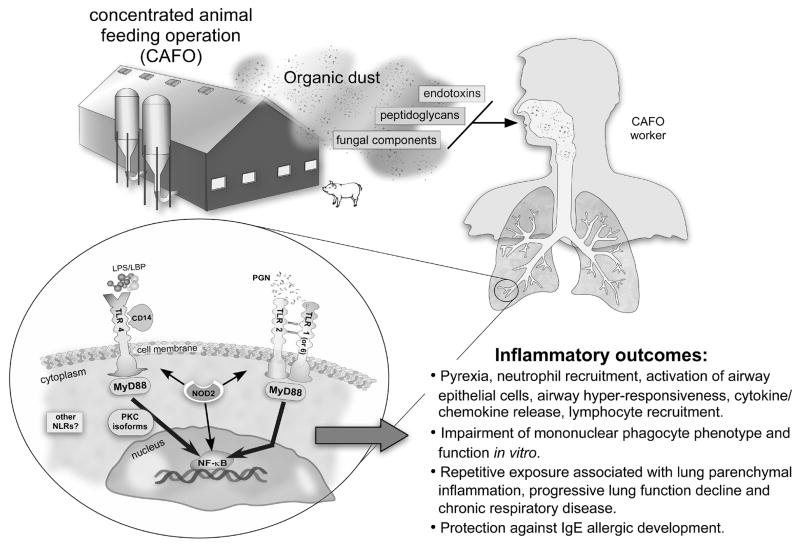
By focusing on the innate immune response to these various agents, there is new information on host genetic factors and potential novel targets. There has also been progress to better establish and understand the chronic inflammatory adaptation response, which describes the phenomenon whereby initial exposure to organic dust elicits an intense inflammatory response that subsequently wanes following repetitive exposures. Yet, repetitive exposure leads to chronic airway disease in agricultural workers. Finally, understanding the immunologic response to organic dust may also have implications in explaining the protective effect of agricultural exposures with allergy. This review will highlight these recent advances in organic dust-induced inflammatory consequences primarily focusing on industrial animal farming environments. This review will not discuss hypersensitivity pneumonitis, which was reviewed by Girard and Cormier in this journal last year (2). A schematic of the agents and immunologic consequences of organic dust exposure in the agriculture industry is shown (Figure 1).
Figure 1.
An overview schematic is depicted of the agents and immunologic and inflammatory consequences of organic dust exposure in the agriculture industry. Large, concentrated animal feeding operations (CAFOs) can produce significant amounts of organic dust rich in microbial motifs (i.e. peptidoglycans, endotoxins, fungi) and particulates that can be easily respirable by exposed workers. These complex dusts are recognized by highly conserved innate immune receptor signaling pathways resulting in various immunologic inflammatory outcomes. Key: LPS: Lipopolysaccharide/endotoxin, LBP: LPS binding protein, PGN: peptidoglycan, TLR: Toll-like receptor, NLR: Nucleotide oligomerization domain-like receptors, NOD2: Nucleotide oligomerization domain 2, PKC: protein kinase C
Exposure Assessments and Disease Associations
To determine the etiology of airway disease manifestations in farmers, studies have investigated the relationships between quantifiable environmental agents with airway disease consequences. Generally, respirable particle counts, total dust, endotoxin, and ammonia levels are analyzed because of the relative ease in measuring these agents and their association with symptoms and disease. Whereas endotoxin (cell wall component of Gram-negative bacteria; lipopolysaccharide/LPS) is routinely measured and linked to airway inflammatory outcomes (3,4), there has been disagreement as to the association of endotoxin and disease in exposed workers. There can be high endotoxin exposure without symptoms (5,6) or low endotoxin exposure with a possible dose-response relationship (6). To date, endotoxin remains an important agent, but discrepancies in its association with disease continue to exist. Senthilselvan and colleagues reported that cross-shift changes in lung function among workers changed significantly in poultry layer operations (caged), which was associated with endotoxin concentration (7). However, endotoxin concentration was higher in the poultry broiler operations (floor), but no significant association was observed in lung function changes (7). A potential explanation may lie in respirable size fraction and its association with endotoxin. In floor-housed poultry operations, more endotoxin was found in larger size fraction (>9.8 μm) whereas in caged-housed operations, endotoxin was found to be greater at smaller size fractions (1.6–3.5 μm)(8).
To understand the real concentration and nature of airborne microorganisms, applied molecular approaches and gas chromatography-mass spectrometry (GC/MS) methods are being utilized (9). Phylogenetic analysis has revealed large number of sequences (>90%) related to Gram-positive anaerobic bacteria (10) and methanogenic archaea (11) in the bioaerosols from swine facilities. Mass spectrometry methods revealed high concentration of muramic acid, a marker of peptidoglycan (PGN) derived predominately from Gram-positive bacteria (i.e.~85% of cells wall), but also Gram-negative bacteria (i.e.~5% of cell wall), in settled dust samples from swine and dairy barns (12–14). Levels of muramic acid have been associated with inflammatory outcomes in humans following swine barn exposure in Europe (15,16). β-glucans have also been measured in agricultural environments and are recognized as potentially modulating inflammatory responses (17,18). These studies highlight the complexity of the exposure setting, and also underscore the importance of broadening environmental sampling approaches beyond endotoxin and dust particulate to understand the full-range of biological agents responsible for disease development. This information could be important for establishing and setting occupational guidelines for the industry.
Role of Innate Immune Pattern Recognition Receptors
Because organic dusts from agricultural settings contain a high quantity of microbial rich components, targeting pattern recognition receptors (PRRs) that recognize specific microbial components has been a strategy to determine the role of specific inflammatory agents within dusts and to potentially reduce dust-induced airway disease. One family of innate immune receptors responsible for recognizing highly conserved microbial motifs is the Toll-like receptors (TLRs). Of the human TLRs, TLR4 forms a complex with CD14 and LPS-binding protein (LBP) to recognize and respond to endotoxin. Earlier work established that corn dust-induced airway inflammation could be significantly and dramatically reduced in endotoxin-resistant mice (19,20). However, the same is not true for animal farming dusts. In the TLR4-deficient mouse, animals were significantly protected from neutrophil airway influx following a one-day exposure to swine barn air (21). However, there was no difference in swine barn air-induced cytokine release or airway hyper-responsiveness (AHR) in TLR4-deficient animals as compared to controls (21). There is a role for TLR4 variants in humans exposed to swine barns. Humans with variant in TLR4 (299/399) were found to have decreased cross-shift change in lung function (FEV1) following a high endotoxin swine barn exposure challenge, but no difference was observed after a low endotoxin swine barn exposure challenge (22). In a separate study, CD14 polymorphorisms (CD14/-159T and CD14/-1610G) were associated with increased prevalence of wheezing among farmers (23). These studies validate the role for endotoxin, but also highlight an important role for TLR4-independent pathways.
In further support of non-endotoxin components in swine facility organic dust playing important biological roles, dust extracts reduced of endotoxin retain significant ability to elicit inflammatory and biological responses in a variety of cell types (13,24–28). Given the abundance of Gram-positive bacteria in dusts, recent studies have focused on the TLR2 pathway because TLR2 recognizes lipoteichoic acid, lipoproteins, and peptidoglycans from Gram-positive bacteria. Swine facility organic dust exposure upregulated airway epithelial cell TLR2 (29), and blocking epithelial cell TLR2 resulted in a dampening of pro-inflammatory cytokine release after organic dust exposure in vitro (30). In addition, neutrophil influx, cytokine release, and lung parenchymal inflammation was significantly less in TLR2-deficient mice following single and repetitive organic dust intranasal inhalation challenges as compared to control animals (31). However, AHR and NO release were not reduced (32). Although a role for TLR2 gene variants in agriculture workers is not known, children of farmers with polymorphism in TLR2/-16934 were less likely to have asthma and allergic symptoms compared to children of non-farmers with same polymorphism (33). Thus, there is a critical role for the TLR2 signaling pathway, but it is noted that inflammatory outcomes were not completely eliminated, suggesting that TLR2-independent pathways are also important.
Another innate immune PRR group that senses microbial motifs is the NACHT-LRR protein family. Of this large family of intracellular proteins, there is a role for nucleotide oligomerization domain 2 (NOD2), which senses muramyl dipeptide, a component of virtually all types of bacterial-derived peptidoglycan. Organic dust extracts, as well as endotoxin and peptidoglycan, upregulated NOD2 expression in mononuclear phagocytes, which was dependent on dust-induced NF-κB activity as opposed to a TNF-α autocrine/paracrine mechanism (34). In comparison, endotoxin and peptidoglycan products alone upregulate NOD2 expression via TNF-α in epithelial cells (35–37). Loss of NOD2 resulted in an enhancement of select mediator production from isolated lung macrophages following organic dust stimulation, and in vivo, there was small, but significant increases in airway inflammation in the NOD2-deficient mice following organic dust challenges (34). Although other groups have also reported that NOD2 may play a negative regulatory role following TLR2 signaling (38) and TLR4-signaling (39), there are also reports that in the absence of NOD2, pro-inflammatory cytokine production is reduced after stimulation with various TLR agonists (40,41), suggesting a positive regulatory role. Although not described in agriculture workers, NOD2 polymorphisms have been implicated in atopic diseases (42). Interestingly, NOD1, which senses muropeptides commonly found in Gram-negative bacteria, has been associated in several studies with asthma susceptibility and development (42–44).
Chronic Inflammatory Adaptation Response
Organic dust exposures in agricultural environments result in an intense inflammatory response that attenuates over time, but repetitive exposures can result in chronic respiratory disease. This chronic inflammatory adaptation response (45) is well recognized, but the mechanisms to explain it are not clear. Recent advances have improved our insight into this phenomenon. Sundblad and colleagues (46) assessed whether inflammatory responses to organic dust and endotoxin challenges were similar in pig farmers and smokers (both groups routinely exposed to organic material) compared to healthy nonsmokers (naïve group) with the hypothesis that tolerance/adaptation developed in the regularly organic dust exposed groups and that cross reactivity between different types of organic dust exposures existed. They confirmed that as compared to controls and consistent with the adaptation observation, pig farmers demonstrated an attenuation of symptoms, lung function, bronchial responsiveness, and markers of airway inflammation, but yet had markers of low-grade, on-going inflammation. However, the results were mixed with smokers because in some parameters smokers responded like controls, but in other parameters responded like the farmer group, suggesting that an indoor swine farming environment and tobacco smoke exposure do not activate identical adaptive mechanisms. Additionally, they observed that exposure in the pig barn was a much stronger pro-inflammatory stimulus than the inhalation of pure endotoxin (LPS), even though the doses of the LPS challenge were 200-fold higher than the doses inhaled in the pig barns. This latter observation is consistent with the concept that non-endotoxin components are playing an important role in the response to animal farming agricultural dusts.
Earlier work demonstrated that swine workers have increased levels of soluble L-selectin, which may be important in the adaptation response because soluble L-selectin decreases inflammatory cell migration (47). Recent studies have demonstrated that pig farmers as compared to controls demonstrate an increase in circulating neutrophils and IL-13- and IL-4-producing Th2 cells as well as diminished TLR2 expression on peripheral blood monocytes (48). Moreover, a relative monocytosis and a monocyte TNF-α hyper-responsiveness occurred after swine barn exposure (49). These findings suggested alteration in systemic innate and adaptive immune responses following organic dust challenges.
In cell culture models, repeat exposure of healthy human peripheral blood monocytes to organic dust and its components (endotoxin, peptidoglycan and endotoxin-reduced dust) resulted in a tolerant response with regards to TNF-α and IL-6, but not CXCL8/IL-8 and IL-10, which was partially mediated by protein kinase C (PKC) activity (24). Notably, in bronchial epithelial cells, organic dust-stimulated IL-6 and CXCL8/IL-8 release are strongly mediated by sequential activation of PKCα and PKCε following an autocrine/paracrine dust-induced TNF-α response (50). Repetitive exposure to organic dust modulates innate immune host defense in macrophages and dendritic cells (DCs). Specifically, when monocytes are differentiated into macrophages in the presence of dust extracts, cell-surface marker expression (HLA-DR, CD80, CD86), phagocytosis, intracellular bacterial killing, and cytokine responsiveness are significantly impaired as compared to control macrophages differentiated without dust extracts (13). In addition, organic dust exposure altered monocyte differentiation to immature DCs and prevented maturation of immature DCs to mature DCs (25).
Animal models to understand the chronic adaptation inflammatory response have been developed. One model whereby rodents are placed in hanging cages within the swine barn demonstrated increased AHR, cellular influx, and cytokine release after one day of exposure and resolution of AHR and dampening of cellular and cytokine releases with repetitive exposures (51). Although chronic lung parenchymal changes were observed, changes were subtle (51). Other animal models utilizing intranasal or nebulization administration of dust extracts also demonstrated similar adaptation responses, but produce an overall exaggerated airway inflammatory response (20,32). Specifically, robust increases in airway inflammatory mediators and AHR following single exposure were significantly dampened following repetitive exposures (32). Importantly, there were semi-quantifiable increases in peribronchiolar and alveolar compartment inflammation with the development of discrete mononuclear cellular aggregates composed of T-cells, B cells, and mononuclear phagocytes (32). Together these studies suggest that the adaptation response observed in humans can be applied to animal models. Future directions should investigate manipulating these models to understand the cellular and molecular mechanisms responsible for the chronic inflammatory adaptation response.
Farming Exposures and Protective Effects
For over a decade, it has been recognized that children who grow up on the farm and farmers have less allergic (IgE-mediated) disease as compared to those who are not from farming communities (52). This observation is consistent with the hygiene hypothesis that suggests that lack of microbial exposure might be a risk factor for allergic disorders. The role for microbial agents modulating adaptive/T cell responses away from the classic allergic, Th2 phenotype, has been recently reviewed (53). As this current review highlights, organic dust exposure(s) modulate innate immunity, and it could be speculated that having an adapted/tolerant innate immune response might protect one from responding to allergens. There is evidence that exposure to farm environments can alter innate immunity in children and associated atopic disease. Maternal contact with farm animals and cats during pregnancy significantly protected children in the first 2 years of life from developing atopic dermatitis (54). Furthermore, elevated gene expression of TLR5 and TLR9 (trends were observed for other TLRs and CD14) in cord blood was associated with decreased atopic dermatitis (54). A separate study found that although allergic children had an exaggerated innate immune response at birth, by age 5 years these children had significant attenuations in their TLR2-9 ligand responsiveness compared to nonallergic subjects (55).
Agricultural exposures might also be protective against the development of cancer because a meta-analysis reported that occupational exposure to endotoxin in agriculture is protective against lung cancer (56). However, there might be important differences in gender because it was reported that between 1984 and 1998, after adjustment for smoking, there was significant excess proportionate lung cancer mortality among women in the United States working in agriculture (57). To further highlight a potential difference in gender in respiratory outcomes in agriculture work, upper, but not lower respiratory tract symptoms were found to be more frequent in women than in men employed in a variety of organic dust-producing industries (58).
CONCLUSION
Organic dust exposure in the farming industry, particularly large animal farming, is associated with the development of chronic respiratory diseases. The exact etiology of disease remains unclear, but emerging evidence shows that it cannot be ascribed to one or two agents alone. Targeting host defense response to pathogen-associated molecular patterns and/or common downstream proteins is one potential future strategy to dissect out the key components mediating disease in this complex environment as well as to prevent and reduce disease manifestations. Genetic factors in innate immune host defense are important, and future studies to understand gene-environment interactions are warranted. Utilization of newly developed cell culture and animal models to expand our understanding of the complex inflammatory adaptation response is necessary. The goal remains to identify key environmental factors and host defense responses that can ultimately be targeted to alleviate disease in exposed agriculture workers. Information from cohort studies, such as the young Danish farmer longitudinal study, should provide new information over the forthcoming years (59). Importantly, the creation of AGRICOH, a consortium of agricultural cohorts in nine countries, should provide a venue for affirming key observations with larger numbers of persons from diverse areas regarding immunologic and inflammatory responses to agricultural exposures (60).
KEY POINTS.
Agriculture work is associated with increased risk of developing chronic respiratory disease despite a protection against the development of IgE-mediated diseases.
The inflammatory consequences from organic dust exposure in the agriculture industry, particularly the animal industry, are likely explained by the diversity and synergy of microbial motifs with endotoxins and peptidoglycans as key components.
Pattern recognition receptor signaling pathways involving TLR2, TLR4, and NOD2 are partially responsible for mediating inflammatory consequences to organic dust environments.
Whereas the initial exposure to organic dust elicits a robust inflammatory response, there is a dampening, but not complete resolution, of airway inflammatory consequences, which is consistent with the so-called chronic inflammatory adaptation response.
The creation of agricultural cohorts like the AGRICOH should provide a venue for affirming gene-environment observations with larger numbers of persons from diverse areas in regards to the immunologic and inflammatory responses to agricultural exposures.
Acknowledgments
The authors wish to thank Art Heires for his artistic figure development and Lisa Chudomelka for manuscript preparation assistance.
Footnotes
Conflicts of interest: The authors acknowledge their respective funding sources: National Institute of Environmental Health Sciences (K08 ES015522-01, ES015522-03S1[ARRA], R01 ES019325; JAP), National Institute of Occupational Safety Health (R01 OH008539-01; DJR and 1 U54 OH010162-01; JAP, DJR).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1**.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136:716–725. doi: 10.1378/chest.08-2192. This study demonstrates in humans the importance of various exposures agents in large animal farming environmental dusts. [DOI] [PubMed] [Google Scholar]
- 2*.Girard M, Cormier Y. Hypersensitivity pneumonitis. Curr Opin Allergy Clin Immunol. 2010;10:99–103. doi: 10.1097/ACI.0b013e3283373bb8. This is an important review article of hypersensitivity pneumonitis with relevance to agricultural exposures. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DA, Landas SK, Lassise DL, Burmeister LF, Hunninghake GW, Merchant JA. Airway injury in swine confinement workers. Ann Intern Med. 1992;116:630–635. doi: 10.7326/0003-4819-116-8-630. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, et al. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am J Respir Crit Care Med. 1995;151:47–53. doi: 10.1164/ajrccm.151.1.7812571. [DOI] [PubMed] [Google Scholar]
- 5.Rask-Andersen A, Malmberg P, Lundholm M. Endotoxin levels in farming: absence of symptoms despite high exposure levels. Br J Ind Med. 1989;46:412–416. doi: 10.1136/oem.46.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kateman E, Heederik D, Pal TM, Smeets M, Smid T, Spitteler M. Relationship of airborne microorganisms with the lung function and leucocyte levels of workers with a history of humidifier fever. Scand J Work Environ Health. 1990;16:428–433. doi: 10.5271/sjweh.1764. [DOI] [PubMed] [Google Scholar]
- 7**.Senthilselvan A, Beach J, Feddes J, Cherry N, Wenger I. A prospective evaluation of air quality and workers' health in broiler and layer operations. Occup Environ Med. 2011;68:102–107. doi: 10.1136/oem.2008.045021. This is an important recent exposure assessment study focusing on poultry workers and respiratory health outcomes. [DOI] [PubMed] [Google Scholar]
- 8*.Kirychuk SP, Reynolds SJ, Koehncke NK, Lawson J, Willson P, Senthilselvan A, et al. Endotoxin and dust at respirable and nonrespirable particle sizes are not consistent between cage- and floor-housed poultry operations. Ann Occup Hyg. 2010;54:824–832. doi: 10.1093/annhyg/meq047. This study describes that the biological relevance of constituents of dust are impacted by more than one characteristic such as endotoxin plus size of particle. [DOI] [PubMed] [Google Scholar]
- 9.Sebastian A, Larsson L. Characterization of the microbial community in indoor environments: a chemical-analytical approach. Appl Environ Microbiol. 2003;69:3103–109. doi: 10.1128/AEM.69.6.3103-3109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol. 2008;10:665–675. doi: 10.1111/j.1462-2920.2007.01489.x. [DOI] [PubMed] [Google Scholar]
- 11.Nehme B, Gilbert Y, Letourneau V, Forster RJ, Veillette M, Villemur R, et al. Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl Environ Microbiol. 2009;75:5445–5450. doi: 10.1128/AEM.00726-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A. 2010;73:684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole JA, Alexis NE, Parks C, MacInnes AK, Gentry-Nielsen MJ, Fey PD, et al. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol. 2008;122:375–382. 382, e1–4. doi: 10.1016/j.jaci.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szponar B, Larsson L. Use of mass spectrometry for characterising microbial communities in bioaerosols. Ann Agric Environ Med. 2001;8:111–117. [PubMed] [Google Scholar]
- 15.Zhiping W, Malmberg P, Larsson BM, Larsson K, Larsson L, Saraf A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med. 1996;154:1261–1266. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]
- 16.Larsson BM, Larsson K, Malmberg P, Palmberg L. Gram positive bacteria induce IL-6 and IL-8 production in human alveolar macrophages and epithelial cells. Inflammation. 1999;23:217–230. doi: 10.1023/a:1020269802315. [DOI] [PubMed] [Google Scholar]
- 17.Halstensen AS, Nordby KC, Wouters IM, Eduard W. Determinants of microbial exposure in grain farming. Ann Occup Hyg. 2007;51:581–592. doi: 10.1093/annhyg/mem038. [DOI] [PubMed] [Google Scholar]
- 18.Samadi S, Wouters IM, Houben R, Jamshidifard AR, Van Eerdenburg F, Heederik DJ. Exposure to inhalable dust, endotoxins, beta(1->3)-glucans, and airborne microorganisms in horse stables. Ann Occup Hyg. 2009;53:595–603. doi: 10.1093/annhyg/mep040. [DOI] [PubMed] [Google Scholar]
- 19.George CL, Jin H, Wohlford-Lenane CL, O'Neill ME, Phipps JC, O'Shaughnessy P, et al. Endotoxin responsiveness and subchronic grain dust-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2001;280:L203–213. doi: 10.1152/ajplung.2001.280.2.L203. [DOI] [PubMed] [Google Scholar]
- 20.Jagielo PJ, Thorne PS, Watt JL, Frees KL, Quinn TJ, Schwartz DA. Grain dust and endotoxin inhalation challenges produce similar inflammatory responses in normal subjects. Chest. 1996;110:263–270. doi: 10.1378/chest.110.1.263. [DOI] [PubMed] [Google Scholar]
- 21.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res. 2008;34:19–35. doi: 10.1080/01902140701807779. [DOI] [PubMed] [Google Scholar]
- 22**.Senthilselvan A, Dosman JA, Chenard L, Burch LH, Predicala BZ, Sorowski R, et al. Toll-like receptor 4 variants reduce airway response in human subjects at high endotoxin levels in a swine facility. J Allergy Clin Immunol. 2009;123:1034–1040. 1040.e1–2. doi: 10.1016/j.jaci.2009.02.019. This study highlights the importance of genetic variation in human and differing responses to specific environmental exposure challenges. [DOI] [PubMed] [Google Scholar]
- 23.LeVan TD, Von Essen S, Romberger DJ, Lambert GP, Martinez FD, Vasquez MM, et al. Polymorphisms in the CD14 gene associated with pulmonary function in farmers. Am J Respir Crit Care Med. 2005;171:773–779. doi: 10.1164/rccm.200404-530OC. [DOI] [PubMed] [Google Scholar]
- 24.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, et al. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol. 2007;120:366–373. doi: 10.1016/j.jaci.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Poole JA, Thiele GM, Alexis NE, Burrell AM, Parks C, Romberger DJ. Organic Dust Exposure Alters Monocyte-Derived Dendritic Cell Differentiation and Maturation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L767–776. doi: 10.1152/ajplung.00107.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol. 2002;93:289–296. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Suur C, Larsson K, Grunewald J. Organic dust-induced interleukin-12 production activates T- and natural killer cells. Eur Respir J. 2002;20:686–690. doi: 10.1183/09031936.02.02002002. [DOI] [PubMed] [Google Scholar]
- 28.Demanche A, Bonlokke J, Beaulieu MJ, Assayag E, Cormier Y. Swine confinement buildings: effects of airborne particles and settled dust on airway smooth muscles. Ann Agric Environ Med. 2009;16:233–238. [PubMed] [Google Scholar]
- 29.Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1049–1054. doi: 10.1152/ajplung.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Scheele I, Larsson K, Palmberg L. Budesonide enhances Toll-like receptor 2 expression in activated bronchial epithelial cells. Inhal Toxicol. 2010;22:493–499. doi: 10.3109/08958370903521216. [DOI] [PubMed] [Google Scholar]
- 31.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, et al. Toll-like receptor 2 (TLR2) Regulates Organic Dust-Induced Airway Inflammation. Am J Respir Cell Mol Biol. 2011;45:711–719. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, et al. Intranasal Organic Dust Exposure-Induced Airway Adaptation Response Marked By Persistent Lung Inflammation and Pathology in Mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1085–1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 34*.Poole JA, Kielian T, Wyatt TA, Gleason AM, Stone J, Palm K, et al. Organic Dust Augments Nucleotide-Binding Oligomerization Domain (NOD2) Expression via an NF-{kappa}B Pathway to Negatively Regulate Inflammatory Responses. Am J Physiol Lung Cell Mol Physiol. 2011;301:L296–306. doi: 10.1152/ajplung.00086.2011. This study highlights a potential role for intracellular sensors with mechanistic focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farkas L, Stoelcker B, Jentsch N, Heitzer S, Pfeifer M, Schulz C. Muramyldipeptide modulates CXCL-8 release of BEAS-2B cells via NOD2. Scand J Immunol. 2008;68:315–322. doi: 10.1111/j.1365-3083.2008.02145.x. [DOI] [PubMed] [Google Scholar]
- 36.Koslowski MJ, Beisner J, Stange EF, Wehkamp J. Innate antimicrobial host defense in small intestinal Crohn's disease. Int J Med Microbiol. 2010;300:34–40. doi: 10.1016/j.ijmm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 39.Tsai WH, Huang DY, Yu YH, Chen CY, Lin WW. Dual roles of NOD2 in TLR4-mediated signal transduction and -induced inflammatory gene expression in macrophages. Cell Microbiol. 2010;13:717–730. doi: 10.1111/j.1462-5822.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim YG, Park JH, Daignault S, Fukase K, Nunez G. Cross-tolerization between Nod1 and Nod2 signaling results in reduced refractoriness to bacterial infection in Nod2-deficient macrophages. J Immunol. 2008;181:4340–4346. doi: 10.4049/jimmunol.181.6.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Reijmerink NE, Bottema RW, Kerkhof M, Gerritsen J, Stelma FF, Thijs C, et al. TLR-related pathway analysis: novel gene-gene interactions in the development of asthma and atopy. Allergy. 2010;65:199–207. doi: 10.1111/j.1398-9995.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 43.Melen E, Kho AT, Sharma S, Gaedigk R, Leeder JS, Mariani TJ, et al. Expression analysis of asthma candidate genes during human and murine lung development. Respir Res. 2011;12:86. doi: 10.1186/1465-9921-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidinger S, Klopp N, Rummler L, Wagenpfeil S, Novak N, Baurecht HJ, et al. Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J Allergy Clin Immunol. 2005;116:177–184. doi: 10.1016/j.jaci.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health. 2003;9:185–196. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 46**.Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J. 2009;34:80–88. doi: 10.1183/09031936.00105308. This is an important recent study describing the chronic inflammatory adaptation response in humans highlighting the differing role of organic material exposures and outcomes. [DOI] [PubMed] [Google Scholar]
- 47.Israel-Assayag E, Cormier Y. Adaptation to organic dust exposure: a potential role of L-selectin shedding? Eur Respir J. 2002;19:833–837. doi: 10.1183/09031936.02.02182001. [DOI] [PubMed] [Google Scholar]
- 48.Sahlander K, Larsson K, Palmberg L. Altered innate immune response in farmers and smokers. Innate Immun. 2010;16:27–38. doi: 10.1177/1753425909106317. [DOI] [PubMed] [Google Scholar]
- 49.Willson PJ, Khozani TT, Juurlink BH, Senthilselvan A, Rennie DC, Gerdts V, et al. In vitro production of tumor necrosis factor-alpha by human monocytes stimulated with lipopolysaccharide is positively correlated with increased blood monocytes after exposure to a swine barn. J Toxicol Environ Health A. 2008;71:1401–1406. doi: 10.1080/15287390802241015. [DOI] [PubMed] [Google Scholar]
- 50.Wyatt TA, Slager RE, Heires AJ, Devasure JM, Vonessen SG, Poole JA, et al. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am J Respir Cell Mol Biol. 2010;42:706–715. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res. 2005;6:50. doi: 10.1186/1465-9921-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Mutius E, Radon K. Living on a farm: impact on asthma induction and clinical course. Immunol Allergy Clin North Am. 2008;28:631–647. ix–x. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Thorburn AN, Hansbro PM. Harnessing regulatory T cells to suppress asthma: from potential to therapy. Am J Respir Cell Mol Biol. 2010;43:511–519. doi: 10.1165/rcmb.2009-0342TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Roduit C, Wohlgensinger J, Frei R, Bitter S, Bieli C, Loeliger S, et al. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol. 2011;127:179–185. 185.e1. doi: 10.1016/j.jaci.2010.10.010. An interesting study drawing attention to the role of farming exposure impact on innate immunity in early life. [DOI] [PubMed] [Google Scholar]
- 55.Tulic MK, Hodder M, Forsberg A, McCarthy S, Richman T, D'Vaz N, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol. 2011;127:470–478.e1. doi: 10.1016/j.jaci.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Lenters V, Basinas I, Beane-Freeman L, Boffetta P, Checkoway H, Coggon D, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control. 2010;21:523–555. doi: 10.1007/s10552-009-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson CF, Sullivan PA, Li J, Walker JT. Occupational lung cancer in US women, 1984–1998. Am J Ind Med. 2011;54:102–117. doi: 10.1002/ajim.20905. [DOI] [PubMed] [Google Scholar]
- 58*.Schachter EN, Zuskin E, Moshier EL, Godbold J, Mustajbegovic J, Pucarin-Cvetkovic J, et al. Gender and respiratory findings in workers occupationally exposed to organic aerosols: a meta analysis of 12 cross-sectional studies. Environ Health. 2009;8:1. doi: 10.1186/1476-069X-8-1. This work is important because it demonstrates differences in farming exposures related to gender. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Elholm G, Omland O, Schlunssen V, Hjort C, Basinas I, Sigsgaard T. The cohort of young Danish farmers - A longitudinal study of the health effects of farming exposure. Clin Epidemiol. 2010;2:45–50. doi: 10.2147/clep.s9255. This study is cited because it highlights the importance of longitudinal studies in this area of research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leon ME, Beane Freeman LE, Douwes J, Hoppin JA, Kromhout H, Lebailly P, et al. AGRICOH: a consortium of agricultural cohorts. Int J Environ Res Public Health. 2011;8:1341–1357. doi: 10.3390/ijerph8051341. [DOI] [PMC free article] [PubMed] [Google Scholar]