Abstract
Because long-term potentiation (LTP) and long-term depression (LTD) are thought to be involved in learning and memory, it is important to delineate factors that modulate their induction and persistence, especially as studied in freely moving animals. Here, we investigated the effects of rat strain, circadian cycle, and high-frequency stimulation (HFS) pattern on LTP and concurrently induced LTD in the dentate gyrus (DG). Comparison of two commonly used rat strains revealed that medial perforant path field EPSP-population spike (E-S) coupling and LTP were greater in Long-Evans than Sprague-Dawley rats. Circadian cycle experiments conducted in Long-Evans rats revealed greater E-S coupling and enhanced LTP during the dark phase. Interestingly, concurrent LTD in the lateral perforant path did not significantly differ across strains or circadian cycle. Testing HFS protocols during the dark phase revealed that theta burst stimulation (100 Hz bursts at 5 Hz intervals) was ineffective in eliciting either LTP or concurrent LTD in DG, whereas 400 Hz bursts delivered at theta (5 Hz) or delta (1 Hz) frequencies produced substantial LTP and concurrent LTD. Thus, these natural and experimental factors regulate granule cell excitability, and differentially affect LTP and concurrent LTD in the DG of freely moving rats.
Keywords: in vivo electrophysiology, synaptic plasticity, Sprague-Dawley, Long-Evans, hippocampus
Introduction
The dentate gyrus (DG) is a hippocampal subregion in which long-term potentiation (LTP) and long-term depression (LTD) can be concurrently induced in neighboring synaptic pathways (LTP, Bliss and Gardner-Medwin, 1973; Douglas and Goddard, 1975; LTD, Levy and Steward, 1979; Abraham and Goddard, 1983; Doyère et al., 1997; Abraham et al., 2001). Concurrent LTD has been attributed to spike timing-dependent plasticity through the interaction of spontaneous and evoked synaptic potentials originating from the medial and lateral perforant path inputs from medial and lateral entorhinal cortex, respectively (White et al., 1988; Markram et al., 1997; Benuskova and Abraham, 2007). When studied in vivo, LTP and concurrent LTD can persist for many months (Abraham et al., 1994, 2002). Understanding how natural and experimental factors such as strain and circadian cycle influence LTP and concurrent LTD is important for establishing realistic models of their function in vivo (Manahan-Vaughan, 2000; Zhou and Poo, 2004; Artola et al., 2006; Manahan-Vaughan and Schwegler, 2011).
There has been considerable investigation of the properties of LTP and LTD in the DG of Long-Evans and Sprague-Dawley rats (Levy and Steward, 1979; Racine et al., 1991; Doyère et al., 1997; Abraham et al., 2002); nevertheless, there has been no direct comparison between these strains. Behaviorally, Long-Evans rats spend more time walking, rearing, and exploring an open field environment than Sprague-Dawley rats; these behaviors correlate with greater hippocampal EEG power at 9 to 10 Hz for Long-Evans rats (van Lier et al., 2003). Long-Evans rats also exhibit better spatial learning in the water maze task than Sprague-Dawley rats (Tonkiss et al., 1992). These strain differences in behavior and learning raise the question of whether there are corresponding differences in LTP and concurrent LTD, particularly when studied in freely-moving animals.
The phase of the circadian cycle is known to affect hippocampal function, including LTP and LTD (Harris and Teyler, 1983; Dana and Martinez, 1984; Chaudhury et al., 2005; Vyazovskiy et al., 2008). Disruption of the circadian cycle has an adverse effect on hippocampal-dependent memory, and significantly attenuates the induction of LTP (Campbell et al., 2002; Marks and Wayner, 2005; Craig and McDonald, 2008). Moreover, field excitatory postsynaptic potential (fEPSP) slopes and population spike (PS) amplitudes of granule cells fluctuate in synchrony with the circadian cycle. In diurnal animals, such as monkeys, fEPSP and PS peak during the light phase of the circadian cycle, while in nocturnal animals such as rats, fEPSP and PS peak during the dark phase (Barnes et al., 1977). When studied in vitro or in anesthetized rats and mice, dentate granule cells show greater LTP during dark phase than during light phase (Harris and Teyler, 1983; Dana and Martinez, 1984; Chaudhury et al., 2005); however, the effect of circadian cycle on either LTP or LTD has not been tested in awake animals. Here, we investigated whether circadian cycle influences LTP and concurrent LTD in freely moving rats.
Robust LTP occurs in the DG when perforant path inputs are activated with delta (1 Hz) spacing of 400 Hz pulse bursts (Douglas and Goddard, 1975; Winson and Dahl, 1986). Similarly, robust LTP occurs in area CA1 when Schaffer collaterals are activated by theta-burst stimulation (TBS), characterized by 5 Hz spacing of 100 Hz pulse bursts (Larson and Lynch, 1986; Staubli and Lynch, 1987; Abraham and Huggett, 1997;Raymond and Redman, 2006). TBS has also been shown to induce LTP in the DG of anesthetized rats (Christie et al., 1995a), but its efficacy in awake rats is largely unknown (Hargreaves et al., 1998). This is an important issue because theta is a naturally occurring frequency in the hippocampal EEG, and thus TBS is thought to be physiologically relevant (Larson and Lynch, 1986; Larson et al., 1986). Hence, we investigated whether TBS protocols could produce LTP and concurrent LTD in the DG in freely moving rats.
Methods
Surgery
Adult (17–25 weeks old) male Long-Evans and Sprague-Dawley rats were anesthetized (ketamine, 75 mg/kg s.c.; domitor, 0.5 mg/kg s.c.), placed in a stereotaxic apparatus (Kopf, Tujunga, CA) and chronically implanted with two monopolar stimulating electrodes and one monopolar recording electrode (Abraham et al., 2001). Each electrode was fabricated from Teflon-insulated stainless steel wire (75 μm tip diameter) and had a resistance < 10 Ω. The stimulating electrodes were placed in the medial (from lambda: 4 mm lateral) and lateral (from lambda: 5 mm lateral) perforant path fibers as they run in the angular bundle; the recording electrode was placed in the dentate hilus (from bregma: 3.8 mm posterior, 2.5 mm lateral, Fig. 1A). Final electrode positions were adjusted to maximize the evoked field potentials for each pathway. Once positioning was finalized, electrodes were wired into a plastic socket that was secured to the skull with acrylic resin. Upon completing all surgical procedures, rats were administered antisedan (0.05 mg/kg s.c.) and closely monitored over a 2-week recovery period before being tested for stable electrophysiological recordings. All procedures were approved by the University of Otago Animal Ethics Committee, and complied with all NIH requirements for the humane care and use of laboratory rats.
FIGURE 1.
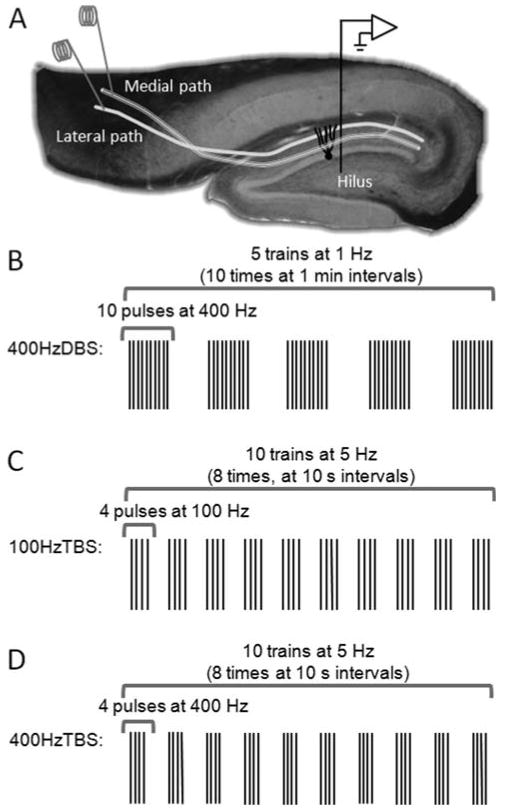
Electrophysiology methods. (A) Positions for stimulation electrodes in medial and lateral perforant paths, and the recording electrode in the hilus. (B–D) HFS protocols used to test for LTP on the medial path and concurrent LTD on the lateral path.
Recording Procedures
All experimental procedures were conducted while rats were in a quietly alert state (Abraham et al., 2002), and took place during either the light or dark phase of the circadian rhythm, depending on the experiment. Between procedures, rats were singly housed in standard rodent cages, and provided with ad libitum access to food and water. Stimulation was administered in the form of biphasic square-wave pulses: test pulses at 150 μs half-wave duration, high frequency stimulation (HFS) at 250 μs half-wave duration. Evoked potentials were amplified, band-pass filtered (0.3–3 kHz), recorded at a sample rate of 10 kHz, and stored on a computer for off-line analysis. Stimulation protocols and data acquisition applications were programmed with Labview software (National Instruments).
Electrophysiology
Medial and lateral path waveforms were evaluated with a standard set of electrophysiological tests. Paired-pulse testing was used to establish that stimulating electrodes were positioned in dissociable fiber pathways (McNaughton and Barnes, 1977). Convergence testing was used to verify that medial and lateral electrodes were activating a common set of postsynaptic granule cells (McNaughton and Barnes, 1977; Abraham and Goddard, 1983). Wave-shape dimensions were tested against previously defined criteria (Abraham and Goddard, 1983; Abraham et al., 2001). Rats with recordings that did not satisfy these criteria were excluded.
The initial slope of the fEPSP (mV/μs) and the amplitude of the PS (mV) were measured for each response. Input–output (I/O) testing was used to measure medial and lateral path waveform parameters (fEPSP and PS) across a range of stimulation intensities (20–200 μA) that were delivered as ascending and descending sequences (20 μA steps). The test-pulse stimulation intensity used for subsequent baseline testing was set to evoke medial path waveforms with fEPSP slopes ≥3.5 mV/μs in association with PS amplitudes ∼2 to 4 mV, at a stimulation current ≤500 μA. Lateral path waveforms had fEPSP amplitudes ≥4 mV at stimulation currents ≤500 μA. For baseline sessions, test pulses were administered alternately to medial and lateral paths every 15 s for a period of 30 min; these sessions were conducted once every 2 days and continued until medial and lateral fEPSP slope averages exhibited a variance of ≤5% for four consecutive sessions. The fact that responses drifted for some animals early during baseline testing before stabilization accounts for discrepancies between I/O curve data (e.g., Figs. 2 and 4) and the stabilized baseline values reported in Table 1.
FIGURE 2.
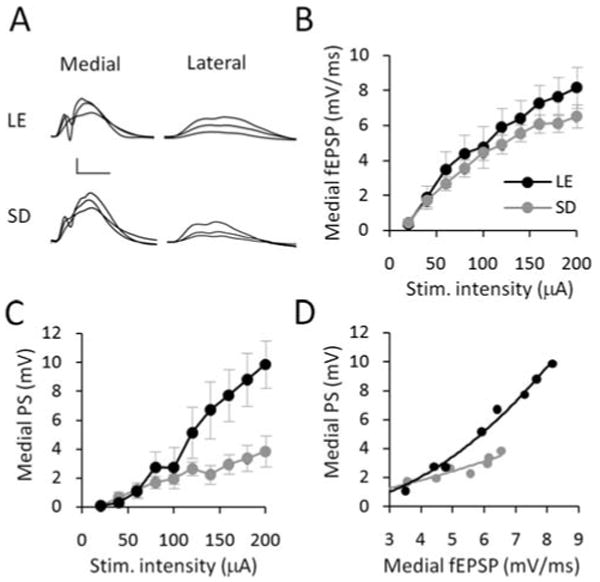
Effect of rat strain on I/O electrophysiology. (A) Medial and lateral path waveforms averaged across two sweeps, and displayed at stimulation intensities 20%, 40 and 100% of maximum (40 μA, 80 μA, and 200 μA stimulation intensity) for representative Long-Evans (LE) and Sprague-Dawley (SD) rats. Scale bars: 5 mV, 5 μs. (B) Strain differences between the medial path fEPSP slopes were small, but nevertheless significantly greater along the I/O curve for Long-Evans versus Sprague-Dawley rats. (C) Medial population spike amplitudes (PS) were markedly and significantly greater along the I/O curves for the Long-Evans rats. (D) Equivalent fEPSP slopes produced significantly larger PS amplitudes demonstrating greater E-S coupling in the Long-Evans rats.
FIGURE 4.
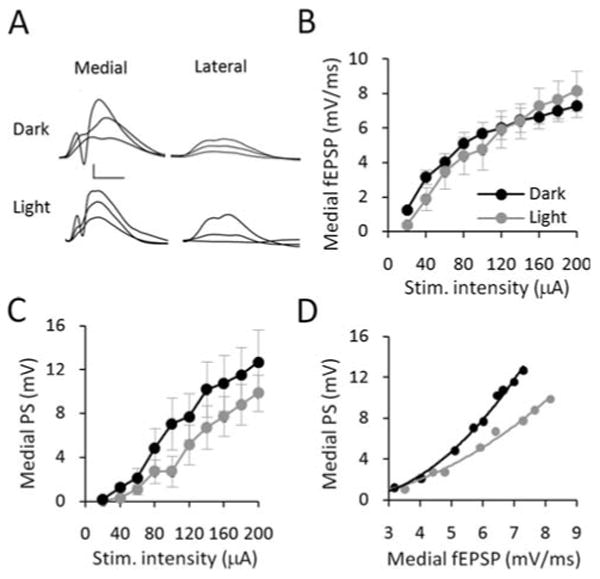
Effect of circadian cycle on I/O electrophysiology. (A) Example waveforms from I/O analysis. Medial and lateral path waveforms were averaged across two sweeps, and displayed at stimulation intensities 20%, 40 and 100% of maximum (40 μA, 80 μA, and 200 μA stimulation intensity) for representative dark phase and light phase in Long-Evans rats. Scale bars: 5 mV, 5 μs. (B) Medial fEPSP slopes across the I/O curve were equivalent between light phase and dark phase rats. (C) Dark phase and light phase PS amplitudes were not significantly different. (D) Equivalent fEPSP slopes produced significantly larger PS amplitudes demonstrating greater E-S coupling in the dark phase.
TABLE 1. Stabilized Baseline Electrophysiological Responses Were Comparable Across Conditions.
| n | Medial fEPSP (mV/ms) | Medial PS (mV) | Lateral fEPSP (mV/ms) | |
|---|---|---|---|---|
| Strain | ||||
| Long-Evans | 5 | 5.88 ± 0.68 | 1.91 ± 0.37 | 1.44 ± 0.06 |
| Sprague-Dawley | 5 | 7.30 ± 0.65 | 2.27 ± 0.52 | 1.47 ± 0.01 |
| Group difference | P = 0.17 | P = 0.58 | P = 0.89 | |
| Circadian (LE rats) | ||||
| Dark cycle | 5 | 5.65 ± 0.57 | 3.34 ± 0.61 | 1.65 ± 0.28 |
| Light cycle | 5 | 5.88 ± 0.68 | 1.91 ± 0.37 | 1.44 ± 0.06 |
| Group difference | P = 0.8 | P = 0.08 | P = 0.48 | |
| Stimulation protocol (LE rats) | ||||
| 50T | 5 | 5.65 ± 0.57 | 3.34 ± 0.61 | 1.65 ± 0.28 |
| 8TBS | 7 | 5.89 ± 0.50 | 2.01 ± 0.21 | 1.72 ± 0.09 |
| 8Hyb | 6 | 4.41 ± 0.98 | 2.23 ± 0.57 | 1.28 ± 0.09 |
| Group difference | P = 0.35 | P = 0.32 | P = 0.22 |
Values were averaged across the last 4 days of baseline stabilization prior to HFS. Data are mean ± S.E.M. and all group differences were nonsignificant by one-way ANOVA.
Following baseline stabilization, HFS was applied to the medial path (beginning at time 0) to test for LTP on that pathway and concurrent LTD on the lateral path. The 400 Hz-DBS protocol consisted of five trains of 10 pulses delivered at 400 Hz at a delta (1 Hz) interburst frequency repeated 10 times at 1 min intervals (Fig. 1B, Abraham et al., 2002). The 100 Hz-TBS had 10 trains of 4 pulses delivered at 100 Hz and a theta (5 Hz) interburst frequency repeated eight times at 10 s intervals (Fig. 1C, Christie and Abraham, 1994; Christie et al., 1995b). The 400 Hz-TBS was a novel hybrid of the two protocols involving 10 trains at TBS (5 Hz) but using 400 Hz intraburst frequency, repeated eight times at 10 s intervals (Fig. 1D). To assess induction of LTP and concurrent LTD, test-pulse stimulation was continued for an hour. To evaluate persistence of the plasticity, recording sessions identical in format to the baseline sessions were conducted on days 1, 3, 5, 7, and twice a week thereafter, up to 35 days post-HFS.
Data Analysis and Statistics
For I/O testing, the two responses were averaged for each of the stimulus intensities, and then compared across experimental conditions using a 2-way analysis of variance (ANOVA) with repeated measures. For analysis of fEPSP-spike (E-S) coupling, the medial path PS amplitudes were plotted against the medial path fEPSP slopes. Because the relationship between these variables was fit by an exponential function, the data were log-transformed (base 10) and a linear regression was applied. The slopes of this function were compared using analysis of covariance (ANCOVA), with fEPSP slope as the covariate.
LTP and concurrent LTD values were expressed as a percentage change in fEPSP slope relative to the average value taken from the last 4 days of baseline stimulation. Data taken on the day of HFS were analyzed by dividing the 60-min period following the onset of HFS into six, 10 min bins; values were then compared across experimental conditions with a two-way ANOVA with repeated measures. Data recorded on the days after HFS were compared across conditions using a two-way ANOVA with repeated measures. The LTP and concurrent LTD group data were expressed as mean ± S.E.M. The post-HFS data were fit by a single exponential function (Abraham et al., 2002). For all analyses, an alpha level of P ≤ 0.05 was defined as statistically significant, and Tukey's post hoc testing was performed where appropriate.
Results
Effect of Rat Strain on Dentate Granule Cell Excitability
For this study, Sprague-Dawley (n = 5) and Long-Evans (n = 5) rats were maintained on a conventional 12:12 h light/dark circadian cycle, and all experimentation took place 7 to 9 h after lights-on. Analysis by two-way ANOVA with repeated measures revealed that differences in the slope of the medial path fEPSPs were small across the I/O, but significantly greater for Long-Evans rats (strain main effect F(1,54) = 4.942, P = 0.041; Figs. 2A,B). There was no significant difference in the lateral path fEPSP slope (F(1,54) = 0.009, P = 0.926; Fig. 2A). Interestingly, the same range of stimulation intensities evoked much greater population spike amplitudes in Long-Evans than in Sprague-Dawley rats (strain × intensity, F(9,54) = 5.86, P < 0.001; Figs. 2A,C). The curve-fitting procedures described above demonstrated a strong correlation between medial fEPSP slope and PS amplitude that approximated a single exponential function (Long-Evans R2 = 0.95, Sprague-Dawley, R2 = 0.83; Fig. 2D). Following log transformation, ANCOVA of the E-S slope revealed that comparable levels of fEPSP activation were associated with significantly greater population spike amplitudes in Long-Evans rats (strain main effect, F(1,75) = 6.2,414, P = 0.015). These findings suggest that the excitability of dentate granule cells, as measured by E-S coupling, was greater in Long-Evans rats.
Effect of Rat Strain on LTP but not Concurrent LTD
The 400 Hz-DBS protocol (termed 50T in previous publications) has been used extensively to study the properties of LTP and concurrent LTD in freely moving Sprague-Dawley rats, but the efficacy of this protocol has not been characterized in the Long-Evans strain. To address this issue, we first adjusted the stimulation intensities to obtain baseline responses that met the criteria described in the Methods. These baseline responses did not differ significantly between the strains (Table 1), although a trend towards a difference in the E-S coupling was still apparent. Note that this lack of effect is not inconsistent with the above I/O data, as the baseline analysis was for only a single stimulus intensity set relatively low on the E-S curve. During HFS, a stronger stimulus intensity was utilized (Methods). In both strains, robust LTP (Long-Evans, 35.6 ± 3.3%; Sprague-Dawley, 15.7 ± 3.4%) and LTD (Long-Evans, −28.2 ± 8.2%; Sprague-Dawley, −39.4 ± 4.1%; Fig. 3A) were generated, as measured 50 to 60 min post-HFS. However, Long-Evans rats demonstrated significantly greater LTP of the medial fEPSPs than Sprague-Dawley rats (F(1,40) = 12.74, P = 0.007; Figs. 3A,B). Analysis of LTP persistence revealed that this strain difference in LTP was sustained for at least one week following HFS (F(1,39) = 8.194, P = 0.021; Fig. 3C). No significant strain effect was observed in the induction or persistence of concurrent LTD (Fig. 3).
FIGURE 3.
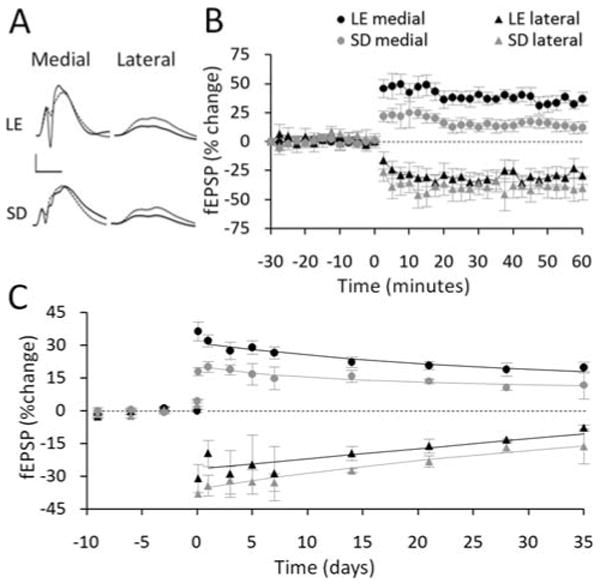
Effect of rat strain on LTP but not concurrent LTD. (A) Pre-HFS waveforms (dashed lines) are averages of 30 sweeps sampled immediately before 400 Hz-DBS; post-HFS waveforms (solid lines) are averages of the final 30 sweeps obtained at 45 to 60 min. Scale bars: 5 mV, 5 μs. (B) Following 400 Hz-DBS, Long-Evans rats (LE) exhibited more LTP of medial path fEPSPs than Sprague-Dawley (SD) rats. No strain difference was observed in the induction of concurrent LTD in the lateral path. (C) LTP persisted at a higher level for 7 days post-HFS in Long-Evans rats than Sprague-Dawley rats. No significant strain effect occurred in the persistence of concurrent LTD in the lateral path. Solid lines are exponential functions fitted to the averaged group data.
Effect of Circadian Cycle on Dentate Granule Cell Excitability
To test for circadian modulation of synaptic plasticity, a second group of Long-Evans rats (n = 5) was studied at the same time as the above animals, but maintained on a reversed 12:12 h dark/light cycle. All experimentation for these rats took place 7 to 9 h after lights-off. The results from this group were compared with the data obtained from the Long-Evans rats studied during the light phase. Two-way ANOVA of I/O data revealed no significant effect of circadian cycle on either medial or lateral path fEPSPs before HFS (Figs. 4A,B). Similarly, there was no significant effect on population spike amplitudes, although there was a trend for larger spikes during the dark phase (Figs. 4A,C). Subsequent E-S analysis revealed that the relationship between PS amplitude and fEPSP slope was fit by an exponential function (light R2 = 0.95, dark R2 = 0.97, Fig. 4D). The difference in the slope of the log-transformed E-S data between the light and dark phases was highly significant (strain, F(1,66) = 18.11, P < 0.001, Fig. 4D). This result indicates that E-S coupling was enhanced during the dark phase.
Effect of Circadian Cycle on LTP but not Concurrent LTD
In both circadian groups, 400 Hz-DBS induced LTP in the medial path (light phase, 35.6 ± 3.3%; dark phase, 42.5 ± 5.3%) and concurrent LTD in the lateral path (light phase, −28.2 ± 8.1%; dark phase, −25.2 ± 4.6%, Figs. 5A,B). There was no significant difference between dark and light phases in LTP measured at 50 to 60 min post-HFS (Fig. 5B). LTP was slightly but significantly greater during the dark phase than the light phase over the next 14 days post-HFS (F(1,32) = 6.03, P < 0.05) with the strongest differences at 7 and 14 days (P < 0.05), but not beyond (Fig. 5C). Although there was an apparent trend for concurrent LTD to be greater in the light phase, this effect did not reach statistical significance (F(1,20) = 0.708, P = 0.439; Figs. 5A–C).
FIGURE 5.
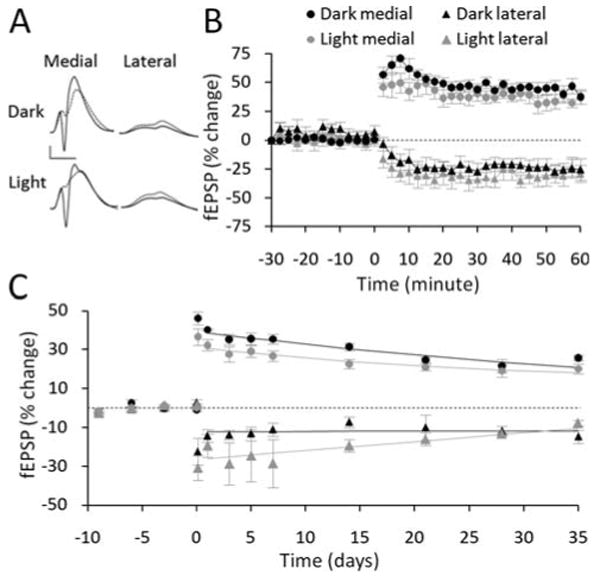
Effect of circadian cycle on LTP but not concurrent LTD. (A) Pre-HFS waveforms (dashed lines) are averages of 30 sweeps sampled immediately before 400 Hz-DBS; post-HFS waveforms (solid lines) are averages of the final 30 sweeps obtained at 45 to 60 min. Scale bar: 5 mV, 5 μs. (B) No significant differences in the induction of LTP and concurrent LTD compared across the dark and light phases. (C) Comparison across the subsequent 35 days revealed slight but significantly greater LTP at days 7 and 14 during the dark phase. No significant circadian differences were observed in the persistence of lateral path LTD.
Effect of Stimulation Pattern on LTP and Concurrent LTD
Experiments investigating the influence of stimulation pattern on LTP and concurrent LTD were conducted during the dark phase (Fig. 6A). Two additional groups of Long-Evans rats, receiving either the 100 Hz-TBS (n = 7) or 400 Hz-TBS (n = 6) protocols, were interleaved and compared with the 400 Hz-DBS group described above. ANOVA revealed a highly significant difference in the efficacy of HFS protocols to induce medial path LTP (F(2,75) = 16.493, P < 0.001) and concurrent lateral path LTD (F(2,50) = 9.18, P = 0.005). The 400 Hz-DBS protocol resulted in robust LTP (42.5 ± 5.4%), whereas 100 Hz-TBS did not induce LTP (9.8 ± 4.9%; P = 0.15, Figs. 6B,C). Increasing the pulse frequency of the TBS protocol to 400 Hz produced LTP comparable to that produced by the 400 Hz-DBS protocol (35.0 ± 4.5% Fig. 6C). A similar pattern of results was observed for LTD (Figs. 6B,C). Notably, both the 100 Hz-TBS and 400 Hz-TBS protocols had identical pulse number (320) and train spacing (5 Hz); thus it was the switch in frequency from 100 Hz to 400 Hz that was critical for generating the LTP and LTD. The difference apparent early in the temporal profile of LTP induced by 400 Hz-DBS and 400 Hz-TBS (Fig. 6B) is largely attributable to the difference in spacing of the HFS episodes (Figs. 1B–D). Thus, the 1 min intervals between the 400 Hz-DBS episodes permitted measurement of potentiated evoked responses over the initial 10 min period from HFS onset, but spacing of the TBS episodes (10 s intervals) was not sufficiently long to permit recording of responses and assessment of between episodes for this protocol (Fig. 6B).
FIGURE 6.
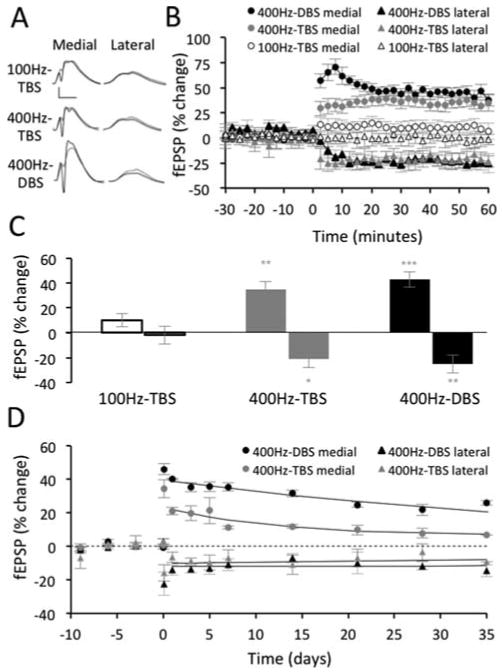
Effect of stimulation pattern on LTP and concurrent LTD. (A) Pre-HFS waveforms (dashed lines) are averages of 30 sweeps sampled immediately before HFS; post-HFS waveforms (solid lines) are averages of the final 30 sweeps obtained at 45 to 60 min. Scale bars: 5 mV, 5 μs. (B) Summary of the LTP and concurrent LTD measured from the final 10 min post-HFS for the three induction protocols. (C) The 400 Hz protocols produced greater LTP and concurrent LTD than 100 Hz-TBS (*P < 0.05, **P < 0.01, ***P < 0.001 displayed relative to 100 Hz-TBS). There was no significant difference between 400 Hz-TBS and 400 Hz-DBS. (D) LTP evoked by 400 Hz-DBS exhibited greater persistence than the LTP evoked by 400 Hz-TBS. The persistence of concurrent LTD did not differ between the two groups. LTP and concurrent LTD induced by 100 Hz-TBS was not sufficiently strong to warrant further analysis.
Although LTP was comparable at 60 min post-HFS for the two 400 Hz protocols, the LTP induced by 400 Hz-DBS persisted for longer and at higher levels than LTP induced by 400 Hz-TBS (F(1,36) = 41.092, P < 0.001, Fig. 6D). Since these protocols had the same stimulation frequency (400 Hz), the greater efficacy of the DBS protocol are likely attributable to differences in either train spacing (1 vs. 5 Hz) or total pulse number (500 vs. 320, Figs. 1B,D).
Discussion
These experiments served to identify several conditions that modulate LTP and concurrent LTD in the DG of freely moving rats. Regarding strain differences, Long-Evans rats had greater baseline E-S coupling suggesting that they have more excitable dentate granule cells than Sprague-Dawley rats. In addition, LTP was significantly greater in Long-Evans rats. Comparison of circadian effects in Long-Evans rats revealed greater baseline E-S coupling and more LTP during the dark phase. Interestingly, concurrent LTD was not significantly affected by strain or circadian cycle. In the testing of HFS protocols, conventional 100 Hz-TBS did not elicit LTP or concurrent LTD; however, when the same number of stimuli were delivered at 400 Hz, robust LTP and concurrent LTD were produced. It is unlikely that any of these effects were due to behavioral differences at the time of recording, because all rats were well habituated to the recording environment, and recordings only commenced after rats were in a quietly alert state (Abraham et al., 2002).
No previous study has directly compared DG electrophysiology between Long-Evans and Sprague-Dawley rats, even though each strain has been used extensively in previous studies of DG LTP induction and persistence (Douglas and Goddard, 1975; McNaughton et al., 1978; Levy and Steward, 1979; Abraham and Goddard, 1983; Doyère et al., 1997). We observed that E-S coupling, a measure dependent on the intrinsic excitability of the dentate granule cells, feed-forward inhibition and the activity of neuromodulatory inputs (Kairiss et al., 1987; Kitchigina et al., 1997; Marder and Buonomano, 2003) was higher in Long-Evans rats than Sprague-Dawley rats. It is not possible to determine from our field potential studies which of these variables accounts for the strain difference in granule cell excitability. It is notable, however, that Long-Evans rats also exhibited greater LTP induction and persistence, a finding that may relate to the increased cell excitability (present data) and spatial memory performance (Tonkiss et al., 1992) of these animals.
The greater E-S coupling we found during baseline recordings in the dark phase of Long-Evans rats is consistent with previous findings (Barnes et al., 1977). Here we also report for the first time that LTP induction in freely moving rats is significantly enhanced in the dark phase as well. As above, we hypothesize that enhanced LTP in the dark cycle is in part due to the elevated excitability of the granule cells, although variations in other factors including the level of neuromodulatory transmitters, such as noradrenaline, could also play a role in both the excitability change and the LTP enhancement (Stanton and Sarvey, 1987; Wagner et al., 1993; Kitchigina et al., 1997).
Although the first studies of LTP used 15 Hz stimulation (Bliss and Lømo, 1973; Bliss and Gardner-Medwin, 1973), it was soon realized that higher frequencies produced more robust and longer lasting LTP at perforant path synapses (Douglas and Goddard, 1975; Douglas, 1978). Contemporary studies typically use frequencies in the 250 to 400 Hz range in vivo (Winson and Dahl, 1986; Doyère et al., 1997; Abraham et al., 2002). In the DG, it was shown that 400 Hz-DBS is capable of producing LTP lasting up to 1 year (Abraham et al., 2002), whereas in area CA1, 100 Hz-TBS can produce LTP lasting weeks (Staubli and Lynch, 1987), making these benchmark protocols for comparison.
To our knowledge, no published studies have used 100 Hz TBS protocols in the DG of freely moving animals. However, we have reported previously that this protocol elicits LTP and concurrent LTD in the DG of anesthetized rats (Christie and Abraham, 1992, 1994; Christie et al., 1995b), leading to the prediction that it would also be effective in awake animals. To our surprise, 100 Hz-TBS failed to elicit either LTP or concurrent LTD in the DG of freely moving rats. However, simply increasing the intratrain frequency to 400 Hz produced robust LTP and concurrent LTD, similar in magnitude and persistence to that generated by the 400 Hz-DBS that has been our standard protocol in prior studies. The reason for the standard 100 Hz-TBS being effective only in anesthetized animals is not clear. We speculate, however, that the 400 Hz protocols are generally more effective because they generate a more rapidly rising depolarization that overcomes strong inhibitory influences and the relatively high threshold for LTP of mature dentate granule cells (McNaughton and Barnes, 1977; Douglas et al., 1983; Hanse and Gustafsson, 1992).
In this investigation, we have demonstrated that rat strain and the circadian state have an impact on the excitability of dentate granule cells, and correspondingly affect LTP, although not concurrent LTD. Furthermore, we found that synaptic plasticity in the DG is preferentially responsive to 400 Hz as opposed to 100 Hz stimulation bursts. This frequency effect is unique to recordings from animals in the awake state, and thus may reflect a categorical difference in the function of the DG when rats are awake and freely moving. Overall, these findings extend our understanding of how key natural and experimental factors affect the induction and persistence LTP and concurrent LTD in freely moving animals.
Acknowledgments
The authors thank Mrs. Sara E. Mason-Parker for her assistance with the surgery and recording procedures.
Grant sponsor: National Institutes of Health; Grant numbers: NS021184 and EB2170 (to K.M.H.); Grant sponsor: University of Otago Postgraduate Scholarship (to J.B.B.)
References
- Abraham WC, Christie BR, Logan B, Lawlor P, Dragunow M. Immediate early gene expression associated with the persistence of heterosynaptic long-term depression in the hippocampus. Proc Natl Acad Sci USA. 1994;91:10049–10053. doi: 10.1073/pnas.91.21.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Goddard GV. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-erm depression. Nature. 1983;305:717–719. doi: 10.1038/305717a0. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: A BCM-like modifiable threshold for LTP. Proc Natl Acad Sci USA. 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD, LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23:261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Goddard GV, Douglas RM, Adamec R. Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science. 1977;197:91–92. doi: 10.1126/science.194313. [DOI] [PubMed] [Google Scholar]
- Benuskova L, Abraham WC. STDP rule endowed with the BCM sliding threshold accounts for hippocampal heterosynaptic plasticity. J Comput Neurosci. 2007;22:129–133. doi: 10.1007/s10827-006-0002-x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. NMDA-dependent heterosynaptic long-term depression in the dentate gyrus of anaesthetized rats. Synapse. 1992;10:1–6. doi: 10.1002/syn.890100102. [DOI] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. Differential regulation of paired-pulse plasticity following LTP in the dentate gyrus. Neuroreport. 1994;5:385–388. doi: 10.1097/00001756-199401120-00003. [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Evidence for common expression mechanisms underlying heterosynaptic and associative long-term depression in the dentate gyrus. J Neurophysiol. 1995a;74:1244–1247. doi: 10.1152/jn.1995.74.3.1244. [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Reduction of the threshold for long-term potentiation by prior theta-frequency synaptic activity. Hippocampus. 1995b;5:52–59. doi: 10.1002/hipo.450050107. [DOI] [PubMed] [Google Scholar]
- Craig LA, McDonald RJ. Chronic disruption of circadian rhythms impairs hippocampal memory in the rat. Brain Res Bull. 2008;76:141–151. doi: 10.1016/j.brainresbull.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Dana RC, Martinez JL. Effect of adrenalectomy on the circadian rhythm of LTP. Brain Res. 1984;308:392–395. doi: 10.1016/0006-8993(84)91086-2. [DOI] [PubMed] [Google Scholar]
- Douglas RM. Unpublished PhD Thesis. Dalhousie University; 1978. The conditional nature of synaptic modification in the fascia dentata of the rat. [Google Scholar]
- Douglas RM, Goddard GV. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975;86:205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Douglas RM, McNaughton BL, Goddard GV. Commissural inhibition and facilitation of granule cell discharge in fascia dentata. J Comp Neurol. 1983;219:285–294. doi: 10.1002/cne.902190304. [DOI] [PubMed] [Google Scholar]
- Doyère V, Srebro B, Laroche S. Heterosynaptic LTD, depotentiation in the medial perforant path of the dentate gyrus in the freely moving rat. J Neurophysiol. 1997;77:571–578. doi: 10.1152/jn.1997.77.2.571. [DOI] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Long-term potentiation and field EPSPs in the lateral and medial perforant paths in the dentate gyrus in vitro: A comparison. Eur J Neurosci. 1992;4:1191–1201. doi: 10.1111/j.1460-9568.1992.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Peters M, Mason-Parker SE, Abraham WC. Double dissociation of 400 Hz and theta-burst stimulation protocols on LTP persistence in the dentate gyrus and CA1 of the rat hippocampus. Soc Neurosci Abstr View Itinerary Planner. 1998;24:1321. [Google Scholar]
- Harris KM, Teyler TJ. Age differences in a circadian influence on hippocampal LTP. Brain Res. 1983;261:69–73. doi: 10.1016/0006-8993(83)91284-2. [DOI] [PubMed] [Google Scholar]
- Kairiss EW, Abraham WC, Bilkey DK, Goddard GV. Field potential evidence for long-term potentiation of feed-forward inhibition in the rat dentate gyrus. Brain Res. 1987;401:87–94. doi: 10.1016/0006-8993(87)91167-x. [DOI] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979;175:233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb Cortex. 2000;10:482–487. doi: 10.1093/cercor/10.5.482. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Schwegler H. Strain-dependent variations in spatial learning and in hippocampal synaptic plasticity in the dentate gyrus of freely behaving rats. Front Behav Neurosci. 2011;5:7. doi: 10.3389/fnbeh.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder CP, Buonomano DV. Differential effects of short- and long-term potentiation on cell firing in the CA1 region of the hippocampus. J Neurosci. 2003;23:112–121. doi: 10.1523/JNEUROSCI.23-01-00112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Marks CA, Wayner MJ. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull. 2005;66:114–119. doi: 10.1016/j.brainresbull.2005.03.018. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977;175:439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. Synaptic enhancement in fascia dentata: Cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Moore KA, Wicks S. Activation of the NMDA receptor: A correlate in the dentate gyrus field potential and its relationship to long-term potentiation and kindling. Brain Res. 1991;556:226–239. doi: 10.1016/0006-8993(91)90310-r. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ. Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J Physiol. 2006;570:97–111. doi: 10.1113/jphysiol.2005.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull. 1987;18:115–119. doi: 10.1016/0361-9230(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Res. 1987;435:227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Shultz P, Galler JR. Long-Evans and Sprague-Dawley rats differ in their spatial navigation performance during ontogeny and at maturity. Dev Psychobiol. 1992;25:567–579. doi: 10.1002/dev.420250804. [DOI] [PubMed] [Google Scholar]
- van Lier H, Drinkenburg WH, Coenen AM. Strain differences in hippocampal EEG are related to strain differences in behaviour in rats. Physiol Behav. 2003;78:91–97. doi: 10.1016/s0031-9384(02)00893-4. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G, Levy WB, Steward O. Evidence that associative interactions between synapses during the induction of long-term potentiation occur within local dendritic domains. Proc Natl Acad Sci USA. 1988;85:2368–2372. doi: 10.1073/pnas.85.7.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J, Dahl D. Long-term potentiation in dentate gyrus: Induction by asynchronous volleys in separate afferents. Science. 1986;234:985–988. doi: 10.1126/science.3775372. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]


