Abstract
Objective
Severe burn injuries lead to a prolonged hyper-catabolic state resulting in dramatic loss of skeletal muscle mass. Post-burn muscle loss is well documented, but the molecular signaling cascade preceding atrophy is not. Our purpose was to determine the response to burn injury of signaling pathways driving muscle inflammation and protein metabolism.
Methods
Muscle biopsies were collected in the early flow phase after burn injury from the vastus lateralis of a non-injured leg in patients with 20–60% total body surface area burns and compared to uninjured, matched controls. Circulating levels of pro-inflammatory cytokines were also compared. Immunoblotting was performed to determine protein levels of key signaling components for translation initiation, proteolysis, and TNF/NFκB and IL-6/STAT3 signaling.
Results
Burn subjects had significantly higher levels of circulating pro-inflammatory cytokines, but no difference in muscle STAT3 activity and lower NFκB activity. No differences were found in any translational signaling components. Regarding proteolytic signaling in burn, calpain-2 was 47% higher, calpastatin tended to be lower, and total ubiquitination was substantially higher.
Conclusion
Surprisingly, a systemic pro-inflammatory response 3–10 d post-burn did not lead to elevated muscle STAT3 or NFκB signaling. Signaling molecules governing translation initiation were unaffected while indices of calcium-mediated proteolysis and ubiquitin-proteasome activity were up-regulated. These novel findings are the first in humans to suggest the net catabolic effect of burn injury in skeletal muscle (i.e. atrophy) may be mediated, at least during the early flow phase, almost entirely by increased proteolytic activity in the absence of suppressed protein synthesis signaling.
Keywords: Skeletal muscle atrophy, Burn injury, Inflammation, Cytokines
Introduction
Following a severe burn injury, the human body responds with a biphasic metabolic response. An initial ebb phase of 24 – 48 hours, characterized in part by a decreased metabolic rate, is followed by a subsequent flow phase characterized by a prolonged, elevated metabolic rate1. The hypermetabolic state that predominates during the flow phase persists for 9–12 months post-injury and continues well beyond complete wound healing 2. This is associated with a dramatic loss of skeletal muscle protein. Muscle proteolysis likely occurs in an attempt to provide amino acids to promote healing at wound sites 3; however, untreated patients with severe burn lose protein at a rate of 20–25 g/m2/day 2 which appears to exceed the amino acid requirements. The rapid loss of lean body mass increases the patient’s risk for infection and paradoxically impairs wound healing 4. This accelerated protein loss contributes to what is likely the most rapid rate of muscle degradation and subsequent muscle atrophy seen clinically, but the molecular events underlying this burn-induced skeletal muscle atrophy in humans have yet to be elucidated.
Maintaining net protein balance is necessary for maintenance of muscle mass. Therefore, based on the kinetics of muscle protein metabolism, a net negative balance leading to muscle atrophy results from decreased protein synthesis, increased protein degradation, or both. Diverse stimuli, such as inactivity/disuse, nerve injury, cancer, sepsis, and muscular dystrophies, lead to muscle atrophy through one or more of several upstream signaling pathways that ultimately regulate cell size. The net loss of muscle protein during disuse atrophy is normally due to a decrease in protein synthesis and this is reflected within the muscle in the downregulation of the PI3K/ Akt/ mTOR molecular signaling pathway5, while activation of mTOR signaling in response to mechanical load and growth factors (e.g., insulin like growth factor -1 (IGF-I)), can prevent atrophy 6. Studies on protein turnover after burn in humans have not observed a decrease in the protein synthesis rate 7, suggesting accelerated degradation, but the underlying cell signaling mechanisms have yet to be determined in humans.
Protein degradation-induced muscle atrophy occurs in response to the activation of signaling pathways within the muscle that increase the activity of the ubiquitin-proteasome, calcium-induced calpains, and/or apoptotic regulators such as caspase-3. The activation of these pathways occurs in response to a number of atrophy stimuli including decreased IGF-1/insulin signaling 8, glucocorticoids 9, and inflammatory cytokines and pro-inflammatory nuclear factor kappa B (NFκB) signaling 10. Animal models of burn induced muscle atrophy implicate several of these catabolic pathways as contributing to muscle loss. Indices of calcium-activated proteolysis (i.e. calpains and calpastatin) 11, 12, ubiquitin-proteasome activity (i.e. E3 ubiquitin ligases) 13, and apoptosis (i.e. caspase-3) 14, 15, have all been implicated in burn-induced muscle atrophy in rats. These same pathways are active in humans in other types of muscle atrophy such as denervation and unloading 16, cachexia 17, and sepsis-induced atrophy 18.
Despite the involvement of inflammatory cytokines and NFκB signaling in muscle atrophy from causes other than burn, very little is known about their involvement in human burn-induced muscle atrophy. Additionally, the molecular pathways regulating human skeletal muscle synthesis and degradation post-burn have not been studied. Therefore, the purpose of this novel human study was to characterize burn-induced changes following severe burn injury in the cell signaling that regulates skeletal muscle mass and inflammation by analyzing key intermediates in the pro-inflammatory NFκB pathway and in the protein translational and degradative pathways.
Methods
Six patients admitted to the regional burn center with 20–60% total body surface area (TBSA) burns agreed to participate. The study was approved by the local Institutional Review Board and all subjects gave written informed consent prior to participation. Subjects had no known chronic disease prior to injury. During burn injury-related surgical procedures between three and 10 days post-burn (i.e. flow phase), a muscle sample was obtained from the vastus lateralis of a non-burned leg by percutaneous needle biopsy, as previously described 19. Blood samples were also collected at this time and serum isolated by centrifugation. Muscle samples were snap frozen in liquid nitrogen and all samples were stored at −80°C. Muscle and serum samples from six de-identified control subjects were used for comparison and matched to burn patients based on age, sex, height, and weight. All control subjects were screened via health history questionnaire and deemed healthy at the time of sample collection.
Serum Cytokine Analysis
Serum samples were analyzed for the inflammatory cytokines interleukins (IL) IL-1β, IL-6, IL-8, and tumor necrosis factor – alpha (TNF-α) using the MSD Human Proinflammatory 4-plex II kit according to manufacturer’s instructions (Mesoscale Discovery, Gaithersburg, MD) as described previously 20. Analysis was performed by an experienced technician in blinded fashion in the Integrated Molecular Analysis Core Facility.
Immunoblotting
Our laboratory has previously published detailed methods for muscle protein isolation and immunoblotting 20–22. Briefly, muscle samples (~30 mg) were thoroughly homogenized after a 15 min pre-incubation in 6 µl/mg muscle of ice cold lysis buffer with protease and phosphatase inhibitors and then centrifuged at 15,000g for 40 min at 4°C. Supernatant was stored at −80°C until assayed for mixed muscle protein lysate concentration using the bicinchoninic acid (BCA) technique with BSA as a standard. Twenty-five µg of this mixed muscle protein were diluted in loading buffer and resolved on 4–12% SDS-PAGE gels and transferred to PVDF membranes. Immunoprobing was performed using primary antibodies (1:1000) from Cell Signaling Technologies (Danvers, MA) unless noted. Translation initiation signaling was analyzed using antibodies against phosphorylated (T421/S424) and total p70S6k, phosphorylated (T37/46)- 4EBP1, and total eIF2Bε. Protein degradation was measured with antibodies against caspase 3, calpain 2, calpastatin, as well as total ubiquitination levels. Inflammation signaling through the NFκB pathway (e.g., via IL-1β or TNF-α) was determined with antibodies against NFκB p105/p50, phosphorylated (S180) and total inhibitor of kappa B kinase alpha/beta (IKKαβ), and phosphorylated (S32) and total (inhibitor of kappa B (IκBα), while IL-6 signaling was assessed using antibodies against phosphorylated (S727 and T705) and total signal transducer and activator of transcription 3 (STAT3). Horseradish peroxidase (HRP)-conjugated secondary antibody (Pierce – ThermoScientific, Waltham, MA) was used at 1:50,000 (w/v) followed by chemiluminescent detection (SuperSignal West Femto Maximum Sensitivity Substrate: ThermoScientific, Waltham, MA) in a ChemiDoc imaging system with band densitometry performed using Quantity One (Bio-Rad, software package 4.5.1, Hercules, CA) using established protocols 21.
Statistics
All values are reported as means ± SEM. Two-tailed, unpaired, student’s T tests were performed to compare burn patients and matched controls. Significance was defined as p < 0.05.
Results
The six burn injured patients, who suffered on average 32.4% ± 3.5% TBSA burn, were 34.8 ± 3.4 years old at the time of injury. Serum and muscle samples were collected 5.7 ± 1.4 days post injury. The six healthy controls (aged 36.3 ± 2.3 years) were well matched, with no significant differences between groups for age, height, or weight. All patients survived the burn injury. One patient was treated with oxandrolone and insulin and another only insulin. All had been receiving general standard of care for burn injuries. Every patient was fed enterally, either a p.o. diet or a high-protein/high calorie tube feed. We had calorie counts on the patients and each patient consumed ~80% of calories needed. We consistently achieved this 80% prior to biopsy and typically within the first 48–72 h after burn injury.
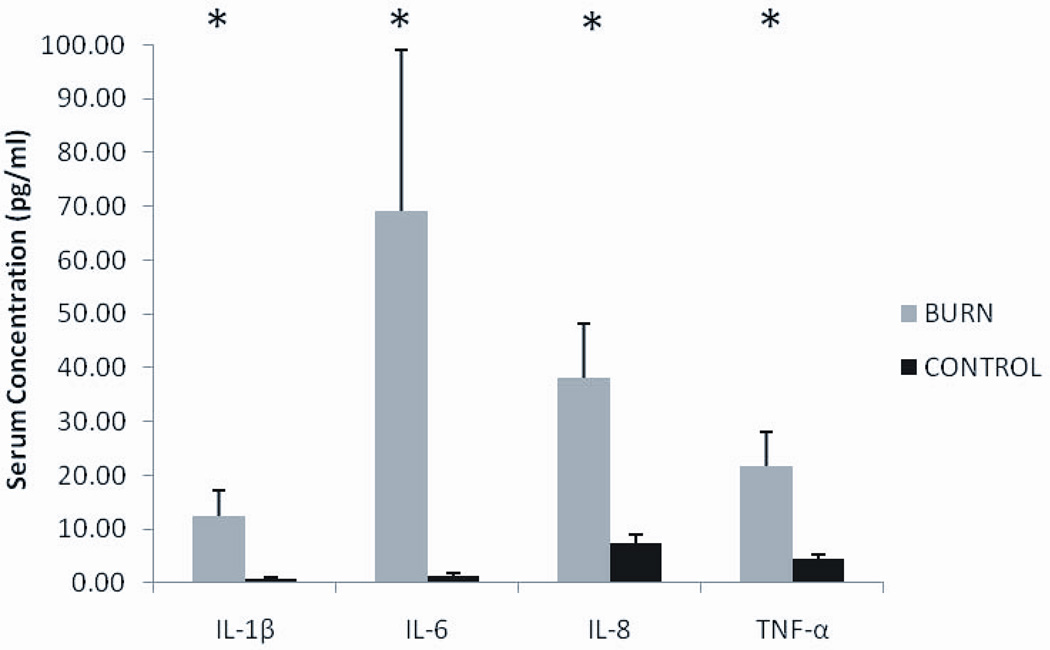
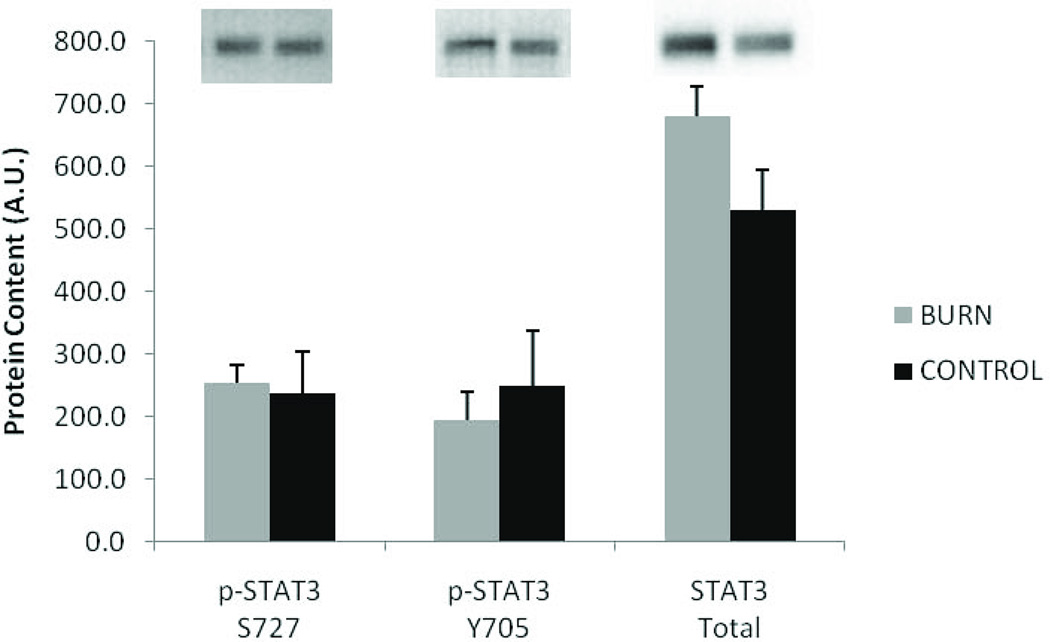
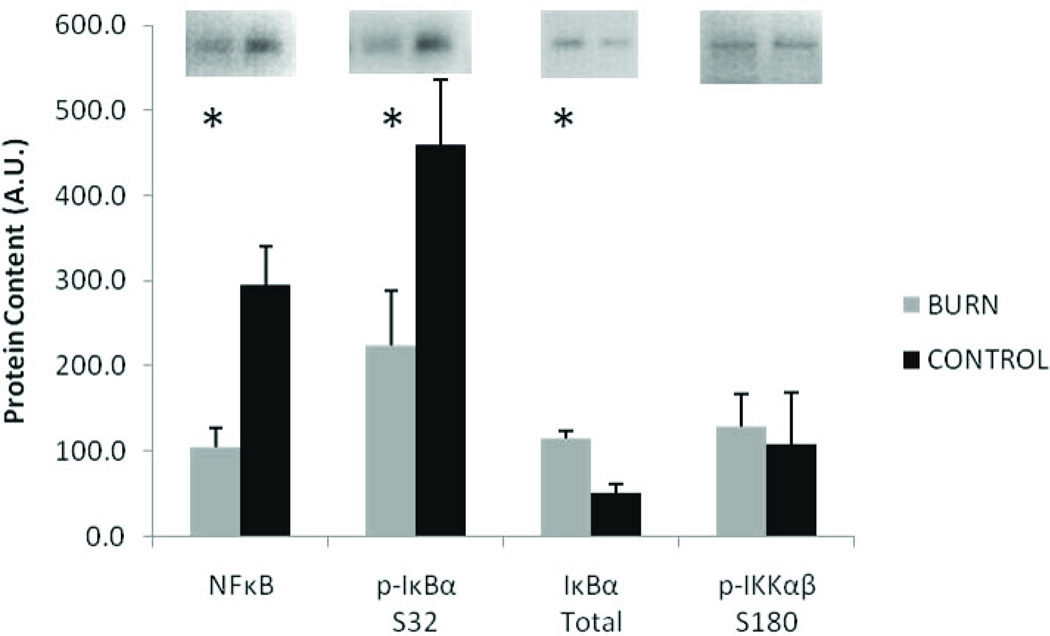
Circulating inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α were all substantially elevated (5–70 fold) compared to matched controls (P<0.05). (Figure 1). Despite the remarkably high levels of serum IL-6, muscle levels of phosphorylated and total STAT3 were not significantly different between burn and control (Figure 2). Similarly, the high serum TNF-α did not lead to elevated NFκB signaling in skeletal muscle (Figure 3). Muscle levels of the active p50 fragment of transcription factor NFκB were actually lower in burn compared to control (p < 0.05), as was the phosphorylation state of IκBα (p < 0.05). Total levels of IκBα were elevated in burn (p < 0.05), while no group differences were observed in the phosphorylation state of IKKαβ.
Figure 1.
Serum cytokine levels in burn and control subjects. Data presented as mean ± SEM. *Significantly different from control (p < 0.05).
Figure 2.
Muscle levels of phosphorylated and total STAT3 protein in burn and control subjects. Data presented as mean ± SEM. No significant differences observed.
Figure 3.
Muscle protein levels of NFκB signaling pathway. Data presented as mean ± SEM. *Significantly different from control (p < 0.05).
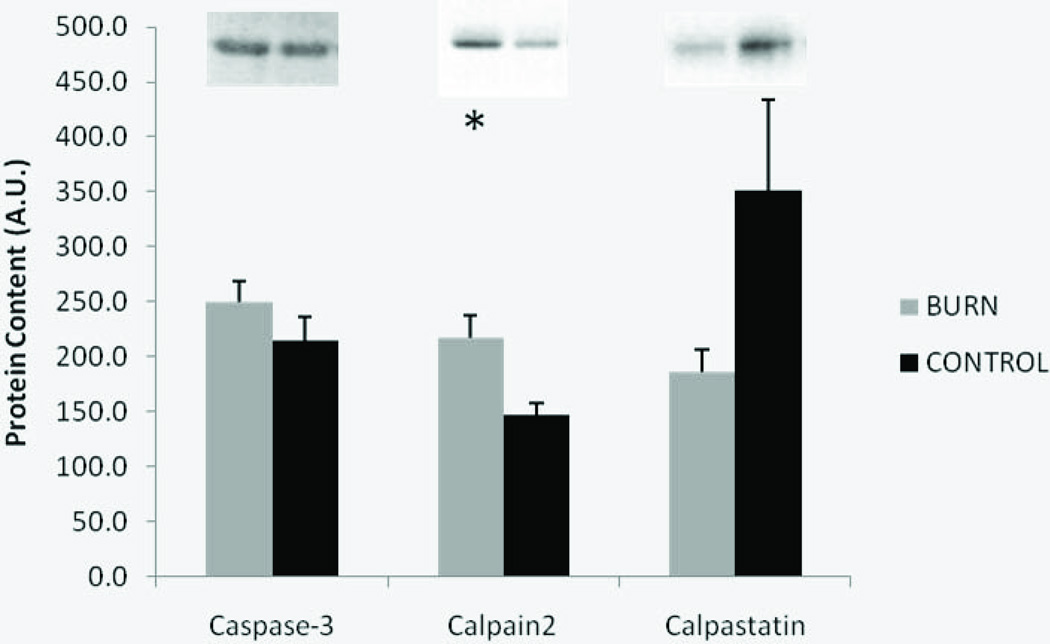
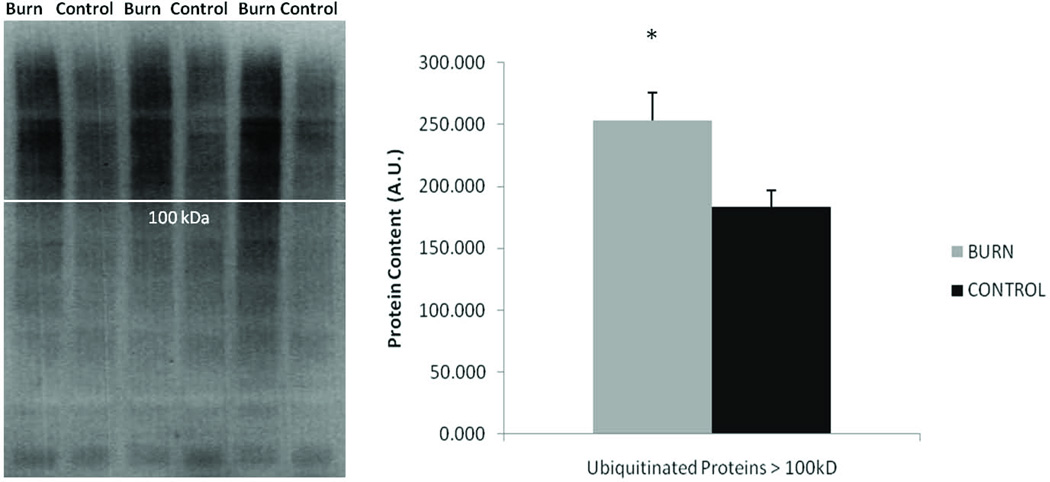
Several group differences were observed in muscle levels of protein degradation signaling. Calpain2 levels were significantly higher in burn compared to control (p < 0.05) and calpastatin, the specific calpain endogenous inhibitor, tended to be lower in burn relative to control (p = 0.08), but no difference was observed in the protein levels of the active fragment of caspase 3 (Figure 4). In burn subjects, total ubiquitination levels of proteins within the muscle were 13% higher (p < 0.05), while analysis of only ubiquitinated proteins over 100 kilodaltons in size revealed a 38% elevation post-burn (Figure 5).
Figure 4.
Protein degradation signaling molecules in burn and control subjects. Data presented as mean ± SEM. *Significantly different from control (p < 0.05).
Figure 5.
Blots of total ubiquitination of burn and control samples. White line indicates approximately 100 kDa protein size. Graph of total ubiquitnated proteins larger than 100 kDa. Data presented as mean ± SEM. *Significantly different from control (p < 0.05).
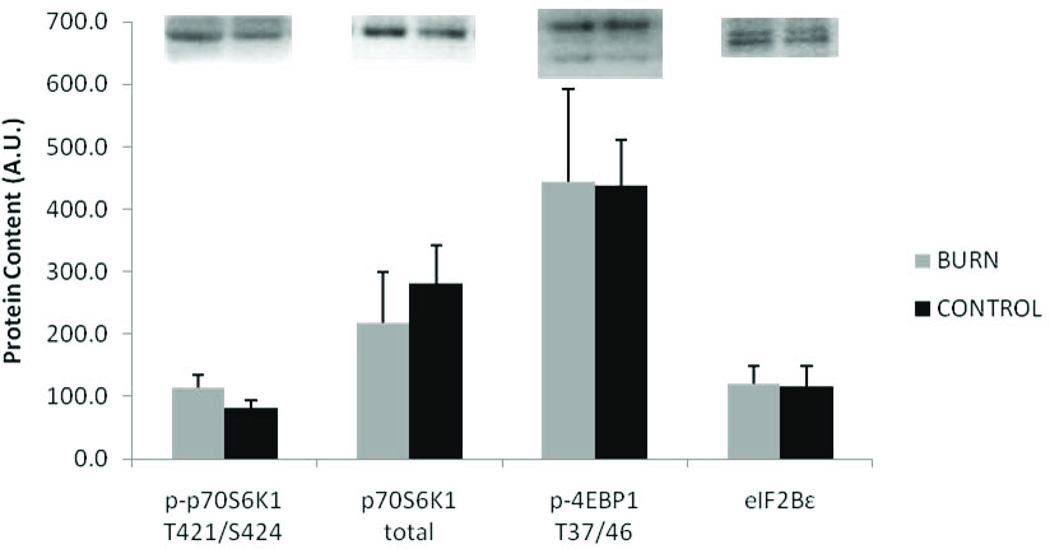
Markers indicative of the initiation of protein translation showed no differences in burn vs. control. No significant differences were seen in eIF2Bε total protein content, 4EBP1 phosphorylation (T37/46), or p70S6K1 total or phosphorylation (T421/S424) (Figure 6).
Figure 6.
Protein synthesis markers. Data presented as mean ± SEM. No signifcant differences between burn and control.
Discussion
As evidenced by serum pro-inflammatory cytokine levels, our findings demonstrate that severe burn injury initiates a dramatic systemic inflammatory response in humans. These results are consistent with the increase observed by other groups in response to burn 23, 24 and in humans and animal models of chronic disorders involving severe muscle atrophy 25–27. Surprisingly, the high inflammatory state did not translate to higher levels of inflammatory signaling within the muscle based on STAT3 activation and activation of the transcription factor NFκB. In fact, the p50 subunit of NFκB, which is the key protein translocated to the nucleus on NFκB signaling activation, was paradoxically lower in burn subjects. In skeletal muscle STAT3 and NFκB pro-inflammatory signaling pathways are initiated by IL-6 and TNF-α respectively and ultimately result in atrophy 28, 29. IL-6 leads to phosphorylation of STAT3 at tyrosine 705 and its subsequent activation and nuclear translocation via serine 727 phosphorylation. TNF-α is a potent activator of pro-inflammatory NFκB signaling by phosphorylating and activating IKK and Iκβα allowing NFκB to translocate to the nucleus where it activates atrophy-inducing genes 10. In agreement with lower levels of the active p50 fragment of NFκB in burn subjects, phosphorylation of Iκβα was also lower. It is not immediately apparent why these pathways were not upregulated in muscle post-burn in the face of the marked systemic pro-inflammatory state or how these novel findings may influence skeletal muscle mass. With the rapid atrophy experienced by burn victims, the possibilty exists that the downregulation of NFκB signaling in the muscle is a compensatory mechanism in order to prevent even more rapid losses of muscle mass. This speculation, however, requires further study.
Consistent with observations that severe burn results in muscle atrophy, calpain-2, a marker of protein degradation in the calcium-activated proteolysis pathway, was significantly higher after burn. Additionally, the endogenous inhibitor of the calpains, calpastatin, tended to be lower in burn. This is similar to observations from animal studies of burn 11, 12, but these are the first such data in humans. It should be pointed out, however, that upregulation of calcium-activated proteolysis cannot completely explain burn-induced muscle atrophy because calpains only partially break down proteins in the muscle. The proteins must be further broken down, and this is normally accomplished by the ubiquitin-proteasome pathway, which is responsible for over 80% of intracellular muscle protein turnover 30. Total ubiquitination (indicative of up-regulated proteasome activity) was substantially higher after burn compared to controls, particularly among the higher molecular weight proteins. The putative regulatory enzymatic step of proteasomal degradation in muscle is the E3 ligase. Attempts were made to determine protein levels of the muscle specific E3 ubiquitin ligases, MuRF1 and atrogin-1, which are upregulated at the mRNA level in animal models of burn-induced muscle atrophy 13, 31 and thought to be necessary for human muscle atrophy 32, 33. Unfortunately, no suitable, consistent, commercial antibodies were found as confirmed by preliminary immunoblotting experiments using muscle samples from MuRF1 or atrogin-1 k/o mice (a kind gift from S. Bodine, UC-Davis). To determine if increased apoptotic activity may have contributed to increased muscle protein breakdown, activated caspase-3 protein levels were also determined, but no significant difference was found between burn and control. Caspase-3 is a key mediator of apoptosis and was found to be significantly upregulated in skeletal muscles of burned rats 14. A number of factors may explain lack of agreement between our human data and the observations in rodents, with the timing of our muscle biopsy being a plausible candidate. Unlike some of the animal models, we collected human muscle specimens well into the flow phase.
Since burn injury is known to increase whole body protein metabolism 2 and net loss of muscle protein, and our findings indicated heightened proteolytic activity, we also measured key regulatory steps in muscle protein synthesis (i.e. translation initiation signaling). Cap-dependent translation initiation (i.e. protein synthesis) is regulated through the putative phosphatide inositol 3-kinase (PI3K)/ Akt/ mammalian target of rapamycin (mTOR)pathway as well as through eukaryotic initiation factor 2B (eIF2B) signaling. Useful biomarkers of mTOR signaling are the phosphorylation states of 4E binding protein-1 (4EBP1) and p70S6 kinase-1 (p70S6K1) 34, while total protein levels of eIF2Bε are indicative of eIF2 activity. No differences in any of these signaling molecules were found between the burn and control groups. Under the assumption that the increase in markers for muscle protein breakdown ultimately leads to muscle protein loss, the lack of a concomitant increase in markers of muscle protein synthesis strongly suggest that the burn subjects were in negative muscle protein balance and likely to experience atrophy. Overall these novel observations suggest calcium-mediated proteolysis may explain the changes in directly measured human muscle protein metabolism following burn injury observed by others 35.
Conclusions
To our knowledge, these are the first results of muscle protein metabolic signaling in human burn victims. Despite the small sample size and variability of treatments received by subjects (oxandrolone and insulin given to one and only insulin to another), we found important differences in muscle protein catabolic signaling and inflammation following burn injury. As expected in persons with severe burns, molecular signaling leading to muscle protein breakdown was increased and this did not appear counterbalanced by signaling events that direct protein synthesis. It is noteworthy that protein synthesis signaling was not up-regulated in the two patients who received insulin, even though insulin is a putative stimulus of protein synthesis via the PI3K/Akt/mTOR pathway. On an individual basis, ubiquitination was elevated in five of six burn patients, with the only exception being the patient who received insulin + oxandrolone therapy. Oxandrolone is a well-characterized androgenic/anabolic steroid capable of increasing muscle mass. It is currently unclear whether oxandrolone directly influences ubiquitin-proteasome activity in skeletal muscle; however, it would be of value to pursue this in future studies since oxandrolone therapy is fairly common in acute burn care.
A potential limitation of this work is that the degree of hypermetabolism was not determined, via indirect calorimetry or other means. Such assessments would be extremely valuable in future studies. On the other hand, elevated proteolytic signaling is certainly suggestive of hypercatabolism. The unexpected mismatch of serum inflammatory cytokines and muscle inflammatory signaling was surprising and certainly deserves further study. The underlying cause behind this paradox could hold important insights into the regulation of muscle mass post-burn. As one of the first studies to approach the causes of human burn-induced muscle atrophy from a protein-level cell signaling perspective, the results presented here demonstrate the need for better understanding of the process in order to explore targeted therapeutic approaches to prevent muscle loss and speed recovery. The results also demonstrate that molecular changes affecting skeletal muscle protein metabolism occur soon after injury and likely favor muscle atrophy if continued for a prolonged period of time 35. This underscores the importance of starting effective muscle-loss countermeasures in the earliest phases of treatment after severe burn injury.
Acknowledgements
Supported by NIH 5R01 AG017896 (MMB), UAB Burn Center, and VA RRD Merit Review (MMB)
Appendix
Akt ( Protein kinase B): serine/threonine protein kinase that plays a key role in transcription
eIF2: eukaryotic initiation factor 2 – required for the initiation of translation
IGF-1: insulin-like growth factor 1
mTOR: mammalian target of rapamycin – serine/threonine protein kinase that regulates protein synthesis
NFκB: nuclear factor kappa-light-chain-enhancer of B cells - a protein complex that controls the transcription of DNA
p70S6K1: p70S6 kinase-1 – phosphorylates ribosomal protein S6; is involved in the induction of protein synthesis
PI3K: phosphatidylinositol 3 kinase – signal transducer enzyme involved in protein synthesis
STAT3: signal transducer and activator of transcription-3 – transcription factor associated with cytokines
4EBP-1: eukaryotic initiation factor 4E binding protein 1 - translation repressor protein that when phosphorylated disassociates from eIF4E and allows activation of mRNA translation
Caspase-3: cysteine-dependent aspartate-directed protease – molecule that partakes in the regulation of apoptosis, programmed cell death, signaling.
Calpain: Calcium activated non-lysosomal cysteine protease
Calpastatin: inhibitor of calpains
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe RR. Review: acute versus chronic response to burn injury. Circ Shock. 1981;8(1):105–115. [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000 Oct;232(4):455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006 Jul-Aug;30(4):331–338. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 4.McClave SA, Mitoraj TE, Thielmeier KA, Greenburg RA. Differentiating subtypes (hypoalbuminemic vs marasmic) of protein-calorie malnutrition: incidence and clinical significance in a university hospital setting. JPEN J Parenter Enteral Nutr. 1992 Jul-Aug;16(4):337–342. doi: 10.1177/0148607192016004337. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Chen X, Fan M. Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses. 2007;69(2):310–321. doi: 10.1016/j.mehy.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 6.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004 Dec 29;119(7):907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002 Jul;87(7):3378–3384. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 8.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004 Oct;287(4):E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 9.Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999 May;2(3):201–205. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med. 2008 Oct;86(10):1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang CH, Li BG, Wray CJ, Hasselgren PO. Insulin-like growth factor-I inhibits lysosomal and proteasome-dependent proteolysis in skeletal muscle after burn injury. J Burn Care Rehabil. 2002 Sep-Oct;23(5):318–325. doi: 10.1097/00004630-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Fang CH, Tiao G, James H, Ogle C, Fischer JE, Hasselgren PO. Burn injury stimulates multiple proteolytic pathways in skeletal muscle, including the ubiquitin-energy-dependent pathway. J Am Coll Surg. 1995 Feb;180(2):161–170. [PubMed] [Google Scholar]
- 13.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R328–R336. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- 14.Duan H, Chai J, Sheng Z, et al. Effect of burn injury on apoptosis and expression of apoptosis-related genes/proteins in skeletal muscles of rats. Apoptosis. 2009 Jan;14(1):52–65. doi: 10.1007/s10495-008-0277-7. [DOI] [PubMed] [Google Scholar]
- 15.Yasuhara S, Perez ME, Kanakubo E, et al. Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocrinol Metab. 2000 Nov;279(5):E1114–E1121. doi: 10.1152/ajpendo.2000.279.5.E1114. [DOI] [PubMed] [Google Scholar]
- 16.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol. 2009 Sep;107(3):645–654. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- 17.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009 Apr;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 18.Klaude M, Fredriksson K, Tjader I, et al. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin Sci (Lond) 2007 Jul;112(9):499–506. doi: 10.1042/CS20060265. [DOI] [PubMed] [Google Scholar]
- 19.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14(1):101–102. [PubMed] [Google Scholar]
- 20.Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics. Feb 4;40(3):141–149. doi: 10.1152/physiolgenomics.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamman MM, Ragan RC, Kim JS, et al. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol. 2004 Oct;97(4):1329–1337. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- 22.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006 Aug;101(2):531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 23.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006 Jul;26(1):13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 24.Finnerty CC, Jeschke MG, Herndon DN, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008 Sep-Oct;14(9–10):553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debigare R, Cote CH, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med. 2001 Nov 1;164(9):1712–1717. doi: 10.1164/ajrccm.164.9.2104035. [DOI] [PubMed] [Google Scholar]
- 26.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. Faseb J. 2005 Apr;19(6):668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 27.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003 Oct 15;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005 Mar;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 29.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. Faseb J. 1998 Jul;12(10):871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 30.Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci. 2008 Apr;86(14 Suppl):E19–E35. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramaniam A, Joshi R, Su C, et al. Ghrelin inhibits skeletal muscle protein breakdown in rats with thermal injury through normalizing elevated expression of E3 ubiquitin ligases MuRF1 and MAFbx. Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R893–R901. doi: 10.1152/ajpregu.00015.2008. [DOI] [PubMed] [Google Scholar]
- 32.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001 Nov 23;294(5547):1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 33.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001 Dec 4;98(25):14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational Signaling Responses Preceding Resistance Training-Mediated Myofiber Hypertrophy in Young and Old Humans. J Appl Physiol. 2009 Jul 9; doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000 Aug;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]