Abstract
Extracellular ligand binding to G protein-coupled receptors (GPCRs) modulates G-protein and β-arrestin signaling by changing the conformational states of the cytoplasmic region of the receptor. Using site-specific 19F-NMR labels in the β2-adrenergic receptor (β2AR) in complexes with various ligands, we observed that the cytoplasmic ends of helices VI and VII adopt two major conformational states. Changes in the NMR signals reveal that agonist binding primarily shifts the equilibrium towards the G protein specific active state of helix VI. In contrast, β-arrestin-biased ligands predominantly impact the conformational states of helix VII. The selective effects of different ligands on the conformational equilibria involving helices VI and VII provide insights into the long-range structural plasticity of β2AR in partial and biased agonist signaling.
Signaling through G protein-coupled receptors (GPCRs) is a fundamental component of eukaryotic cellular communication. As a result, GPCRs comprise a large fraction of the “druggable proteome”, with more than 30% of available pharmaceuticals targeting GPCRs (1). In performing their functions, GPCRs such as the β2-adrenergic receptor (β2AR) recognize a diverse array of ligands and transmit signals through the cellular membrane to cytoplasmic G-proteins. In parallel to G-protein signaling, activated β2AR can be phosphorylated and binds to β-arrestin, initiating desensitization, endocytosis of β2AR and β-arrestin-dependent signaling (2). A number of β2AR ligands, including the FDA-approved β-blocker carvedilol (3) and the agonist isoetharine (4), have been shown to impart differing degrees of signaling in G-protein and arrestin pathways, a phenomenon called “functional selectivity” or “biased signaling” (5). Understanding the mechanism of biased signaling can provide leads for designing more specific and efficient drugs.
Crystal structures have been determined for β2AR with bound inverse agonists, antagonists, and agonists (6–10), including active-state β2AR complexes with a G-protein mimetic nanobody (8) and with a heterotrimeric G-protein (11). The β2AR–G-protein structure, which has also been analyzed by EM (12), reveals the location of the G-protein binding site, showing that the G-protein interacts with helices V and VI, and the intracellular loops ICL2 and ICL3, but makes no substantial contacts with helices VII and VIII (Fig. 1A,B). A comparison of inactive and active-state crystal structures of β2AR, rhodopsin (13) and the A2A adenosine receptor (14, 15) suggest a common pattern of structural differences between inactive and active states in GPCRs (Fig.1C), where a coupled motion of helices V and VI, and changes in helices III and VII are accompanied by distinct side chain rotamer switches on the cytoplasmic side of the protein (16, 17). Activation of β2AR has been investigated previously via fluorescence-based biophysical experiments (18–25) and NMR (26), and conformational changes during receptor activation were observed in helix VI and the cytoplasmic surface.
Fig. 1.
Locations of 19F-NMR labels in β2AR and activation-related changes in GPCR crystal structures. (A) Side view of β2AR in the active G protein-bound form (PDB ID 3SN6, shown in green). The trans-membrane helices I to VII and the C-terminal helix VIII are identified. The full agonist BI-167107 in the ligand-binding site is shown as a stick diagram. Green and yellow spheres highlight the three cysteine residues used for TET labeling, i.e., Cys2656.27 and Cys3277.54 at the cytoplasmic ends of helices VI and VII, respectively and Cys341 at the C-terminus. The bound G-protein heterotrimer is shown as red ribbons and surfaces. (B) Cytoplasmic view of the structure in (A), with the G-protein contact sites outlined by a broken red line. (C) Plot of distance root mean square deviations (RMSD) of individual residues between crystal structures of inactive and active-states of three GPCRs. Crystal structures used (from top to bottom): β2AR (PDB IDs 2RH1 vs. 3SN6), rhodopsin (PDB IDs 1GZM vs. 3DQB), A2A adenosine receptor (A2AAR; PDB IDs 3EML vs. 3QAK). The horizontal axes represent the amino acid sequences (β2AR residues 34–341, bovine rhodopsin residues 38–320, A2AAR residues 6–302). The vertical axis shows all-heavy-atom RMSDs per residue, while the color code defined in the upper right corner of the panel indicates corresponding Cα deviations. For each protein, selected residues are identified (see text). The locations of the helices I to VIII are indicated at the top. Periplasmic loop regions are highlighted in red, while cytoplasmic loops and helix VIII are highlighted in blue. The cytoplasmic ends of helices VI and VII, which contain Cys2656.27 and Cys3277.54, are “hot spots” with large conformational rearrangements between the crystal structures of inactive and active states; other transmembrane helices and intracellular helix VIII, which includes Cys341, show only small displacements.
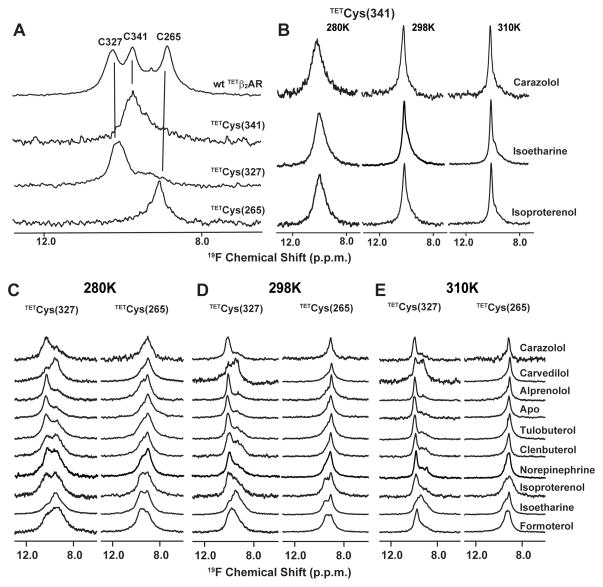
In order to probe for subtle conformational changes involved in receptor–ligand binding (27), 19F-NMR spectroscopy was employed. This highly sensitive method was used to provide information based on the observation of line shapes and chemical shifts of strategically located 19F-labels in the cytoplasmic region of the receptor (Fig. 1A, B) (28). Similar approaches have previously been successfully applied to studies of a number of membrane proteins (29–31). Three native cysteine residues (Cys2656.27, Cys3277.54, Cys341) (32) of β2AR (33) in the cytoplasmic region of the receptor are accessible for covalent labeling with 2,2,2 trifluoroethanethiol (TET) (31), and their NMR signals were unambiguously assigned through the use of site-specific mutagenesis of these residues and all other native cysteine residues that might be affected by TET (Fig. 2A) (34). To probe for structural changes, we primarily used singly-labeled receptors, which have reduced complexity of the 19F-NMR spectra when compared with wild type TETβ2AR (Fig. 2A). Cys2656.27 and Cys3277.54 are located at the cytoplasmic ends of helices VI and VII, respectively, both of which are known to undergo large conformational changes during receptor activation (Fig. 1C). In contrast, Cys341 is located in the non-transmembrane helix VIII at the C-terminus and β2AR(C265A, C327S, TETC341) was observed to be unresponsive to binding of different ligands (Fig. 2B). This C-terminal probe therefore served as an internal negative control relative to Cys2656.27 and Cys3277.54 in studies of the effects from the binding of different pharmacological ligands.
Fig. 2.
1D 19F-NMR spectra of different TET-labeled β2AR constructs under variable solution conditions. (A) 19F-NMR resonance assignments for carazolol-bound TETβ2AR at 298 K. Spectra of the following constructs were recorded: wt TETβ2AR; β2AR (TETC265, C327S, C341A), β2AR (C265A, TETC327, C341A) and β2AR (C265A, C327S, TETC341). The vertical lines connect peaks in the 19F-NMR spectrum of TETβ2AR with the corresponding peaks in the spectra of the single-residue TET-labeled mutants. At the top, the peak assignments are indicated by the one-letter amino acid code and the residue number. (B) β2AR (C265A, C327S, TETC341) in complex with an inverse agonist (carazolol), a biased agonist (isoetharine), and a full agonist (isoproterenol) at 280K, 298K and 310K. (C), (D) and (E) β2AR (TETC265, C327S, C341A) and β2AR (C265A, TETC327, C341A) free and bound to nine different ligands at 280K, 298K and 310K. In (B) to (E), the temperature and the ligands are indicated at the top and on the right, respectively. For all experiments, the following parameter settings were used to collect and process the spectra: data size 1024 complex points, acquisition time 51 ms, 24576 scans per increment. The data were multiplied with an exponential function with a line-broadening factor of 30 Hz, and zero-filled to 2048 points prior to Fourier transformation.
The 19F-NMR spectra ofβ2AR(TETC265, C327S, C341A) and β2AR(C265A, TETC327, C341A) (Fig. 2C–E) show prominent changes upon addition of saturating concentrations of a collection of ligands (Table S1) which represent a full spectrum of ligand efficacy (35). In general, the signals at 280K of both β2AR(TETC265, C327S, C341A) and β2AR(C265A, TETC341A, C327) consist of two components, suggesting that the β2AR structure contains two independent equilibria between locally different conformations manifested at these two amino acid positions. While ligand-free and inverse agonist-bound TETβ2ARs have almost identical spectra, in which the populations of C327I and C265I are much more prominent than those of C327A and C265A, spectra of TETβ2AR bound to the full agonist isoproterenol exhibit approximately equal populations at both residues (Fig. 3A). Shifts of this equilibrium are observed with different agonists bound to TETβ2AR, suggesting that the peaks A and I are likely to represent the active and inactive state of both helices VI and VII of β2AR, respectively. The temperature dependence of the 19F-signals with regard to line width and peak intensity (Figs. 2C–E, S4) suggests that the active state of both helices VI and VII of β2AR exhibits a larger degree of conformational plasticity than the inactive state, i.e., the active state samples a wider range of conformers with slightly different chemical shifts, either in a static ensemble or in a manifold of conformers that also includes conformational exchange.
Fig. 3.
Relative populations of active (A, red) and inactive (I, blue) states of β2AR derived from the 1D 19F-NMR spectra at 280K. (A) Peak volumes for the individual components in the 1D 19F-NMR signals of β2AR (TETC265, C327S, C341A) and β2AR(C265A, TETC327, C341A) obtained by a non-linear least-squares fit to a double-Lorentzian function. The experimental data and the double-Lorentzian are indicated by thin and thick black lines, respectively. The fit used the chemical shift positions of peaks A and I indicated by the red and blue vertical lines. (B) Plot of the relative peak volumes for C265A versus the relative peak volumes for C327A. The relative peak volumes are the ratios of the volume of peak A and the sum of the volumes of peaks A and I. Agonists (tulobuterol, clenbuterol, norepinephrine (NE), isoproterenol, formoterol) are shown as black circles highlighted by a yellow background, biased ligands (carvedilol, isoetharine) as red triangles highlighted by a green background, a neutral antagonist (alprenolol) as a black square, an inverse agonist (carazolol) as a black diamond, and the apo-protein as an open square.
The 19F-NMR data were analyzed with a double-Lorentzian function to obtain quantitative information on the conformational equilibria (Fig. 3A). This revealed that positions and widths of the NMR peaks remain practically unchanged for all ligands studied, and confirmed that the major effects of agonist binding are population shifts from inactive state peaks (I) to those of active state (A). A plot of relative peak volumes for Cys265A versus those for Cys327A, which represent the populations of the active state in helix VI and helix VII, respectively, reveals varying degrees of agonist-mediated shifts towards the active state manifested by Cys2656.27 and Cys3277.54 (Fig. 3B). For most agonists the shifts along the two axes follow the same overall trend as their reported pharmacological efficacy on G-protein activation (36). For instance, the partial agonists tulobuterol, clenbuterol and norepinephrine have a less pronounced effect on Cys2656.27 than the full agonist isoproterenol, while the full agonist formoterol elicits a greater shift in conformational equilibrium on Cys2656.27. These results suggest that the degree to which agonists shift the equilibrium towards the active state of helix VI results in differing G-protein signaling capacity. The antagonist/weak partial agonist alprenolol can induce a small shift on Cys2656.27, but has a minimal effect on Cys3277.54, in agreement with previous findings that it is a weak partial agonist (25, 37). The inverse agonist carazolol, on the other hand, induces no apparent shifts as compared to the apo form. In contrast, the two β-arrestin biased ligands, isoetharine and carvedilol, cause large shifts of the equilibria in Cys3277.54 towards the active state. Interestingly, carvedilol, like other antagonists and inverse agonists, has little influence on the equilibrium of Cys2656.27, while isoetharine, like other agonists, also produces a prominent shift of Cys2656.27 towards the active state.
The large activation changes in Cys3277.54 caused by carvedilol and isoetharine suggest that helix VII primarily impacts β-arrestin signaling pathways. This is consistent with the result of a previous study that β2AR-phosphorylation by GPCR kinases, which is a prerequisite for β-arrestin binding, primarily targets a region directly adjacent to helix VII on helix VIII (38). Furthermore, recent crystallographic (11) and EM studies (12) of aβ2AR–Gαβγ heterotrimer complex show that the Gα subunit makes major contact with helices V and VI of β2AR, resulting in a 14 Å outward movement of the cytoplasmic ends of these helices, whereas there is no contact between Gαβγ and helix VII (Fig. 1A,B), suggesting that the conformational changes in helix VII are not directly involved in G-protein binding and signaling.
To further characterize the environment of Cys3277.54 in the active state, we evaluated the effect of the water-soluble paramagnetic relaxation agent gadopentetic dimeglumine (magnevist®) (39) on individual resonances of β2AR(C265A, TETC327, C341A) bound to isoproterenol. A comparison of the line widths of peaks A and I in the 1D 19F-NMR spectra at various magnevist concentrations revealed that peak I is more susceptible to the line broadening by magnevist than peak A (Fig. S1A), indicating that Cys3277.54 in the inactive state is more readily solvent-accessible than in the active state. This interpretation is consistent with crystal structure data, where Cys3277.54 was found to be solvent-exposed in the carazolol-bound structure (6), but buried in the trans-membrane helix bundle of the receptor in its complexes with a nanobody (8) or the G-protein (11) (Fig. S1B).
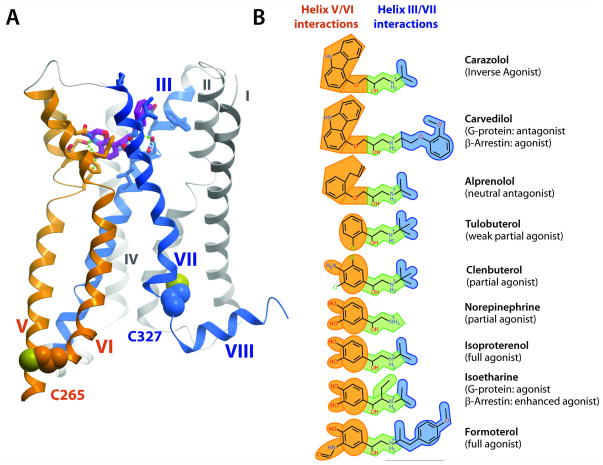
Additional insight from analysis of the 19F-NMR results can be obtained by examining the chemical structures of the agonists and their interactions with the individual helices in the β2AR binding pocket (Fig. 4). The majority of known high-affinity adrenergic ligands have a common structural motif consisting of an aromatic “head group” and an ethanolamine “tail group”. The head group (e.g., catechol) of agonists is directly connected to the ethanolamine tail, while antagonists and inverse agonists, both of which act as competitive inhibitors to agonists and either have no impact or a negative impact on the receptor basal activity, have an additional two-atom linker between the head and tail groups. Crystal structures of the β2AR complexes with various ligands (6–11), as well as biochemical (40–42) and modeling studies (43, 44) have established that the aromatic head groups interact with helices V and VI, while the ethanolamine tails are anchored by ionic and polar interactions at helices III and VII (Fig. 4A and Fig. S2). For agonists, polar interactions of their head groups with helix V side chains thereby mediate an inward shift of Pro2115.50, and subsequent conformational changes involving Ile1213.40 and Phe2826.44 (8) result in the activation move of the cytoplasmic tip of helix VI.
Fig. 4.
Features of ligand binding in the β2AR structure and a conceptual model of signaling pathways to G-proteins and arrestins in β2AR activation. (A) Side view of the structure of active-state β2AR in the complex with the agonist BI-167107 (PDB ID 3SN6), with helices V/VI and III/VII color-coded orange and blue, respectively, to indicate that they interact with the correspondingly colored fragments of the ligands in (B). (B) Chemical structures of the ligands used in the current 19F-NMR studies. Orange highlights the head groups, green the common ethanolamine moieties and blue the substituents to the amino group of the ethanolamine tail. Ligand names are shown on the right, with published pharmacological efficacy indicated in parentheses.
The changes observed by 19F-NMR (Fig. 3) provide additional insight into correlations between the chemical structure of the ligands and their interactions with β2AR. Binding of full agonists (such as isoproterenol and formoterol) imparts a large shift in the conformational equilibrium of both helices VI (Cys2656.27) and VII (Cys3277.54). Binding of partial agonists, which either completely lack (as in tulobuterol) or have much weaker (as in clenbuterol) polar features on their aromatic heads, has a smaller influence on the conformational equilibrium of helix VI than full agonists, but has almost as much impact on helix VII. In addition, the β-arrestin-biased ligands carvedilol and isoetharine, which differ from their corresponding “unbiased” prototypes (inverse agonist carazolol and full agonist isoproterenol, respectively) only in the tail moieties, strongly impact the helix VII equilibrium. A key observation is that the 1D 19F-NMR spectrum of β2AR(C265A, C327S, TETC341) bound to isoetharine, which is one of the most potent biased agonists, is similar to those of β2AR(C265A, C327S, TETC341) bound to carazolol and isoproterenol (Fig. 2B). This shows that the C-terminus of the non-transmembrane helix VIII, where Cys341 is located, is not directly involved in the β-arrestin signaling pathway. In the absence of structural data on a complex of β2AR with β-arrestin, it is tempting to further hypothesize from this data that the position of Cys341 on helix VIII is located outside of the interface with bound arrestin.
These observations on structure–activity relationships for β-arrestin biased β2AR ligands, and unbiased full and partial agonists, provide insights into β2AR signaling pathways that suggest the existence of parallel pathways for G-protein and β-arrestin signaling (Fig. S3). Although these pathways appear interconnected on several levels and may involve other activation-related rearrangements in the cytoplasmic and periplasmic loops (45), the present 19F-NMR results point to a significant level of decoupling of the two pathways. The implicated links between ligand structure and function may serve as a platform for future rational design of specific ligands.
Given the above observations on the correlation between ligand structure and receptor response, a connection can now be made between the chemical structures of pharmacological ligands and their signaling pathways acting either through G-proteins or β-arrestins. The general principle that links the chemical space of the ligands to the specific changes in conformational plasticity at the signaling cytoplasmic receptor surface may be preserved in other GPCRs. The use of 19F-NMR may thus prove to be a widely applicable tool for structure–activity relationship studies of GPCR ligands.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Roadmap Initiative grant P50 GM073197 for technology development and purchase of the 19F-NMR probe, and by PSI:Biology grant U54 GM094618 for GPCR biology studies. The authors thank J. Velasquez for help on molecular biology; C. Cornillez-Ty, T. Trinh, and K. Allin for help on baculovirus expression; J. Gatchalian for ligand binding assays; P. Stanczak for assistance with the paramagnetic titration experiment; V. Cherezov for helpful discussions; I. Wilson for careful review and scientific feedback on the manuscript; K. Kadyshevskaya for assistance with figure preparation; and A. Walker for assistance with manuscript preparation. R.C.S. is a founder and member of the board of directors of Receptos, a GPCR structure-based drug discovery company.
Footnotes
Materials and Methods
References (46-54)
References and Notes
- 1.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115:2971. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shenoy SK, Lefkowitz RJ. beta-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake MT, et al. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 5.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 6.Cherezov V, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson MA, et al. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16:897. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum DM, et al. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wacker D, et al. Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J Am Chem Soc. 2010;132:11443. doi: 10.1021/ja105108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen SG, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westfield GH, et al. Structural flexibility of the G{alpha}s {alpha}-helical domain in the {beta}2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A. 2011;108:16086. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 14.Jaakola VP, et al. Identification and characterization of amino acid residues essential for human A2A adenosine receptor : ZM241385 binding and subtype selectivity. J Biol Chem. 2010;285:13032. doi: 10.1074/jbc.M109.096974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu F, et al. Structure of an Agonist-Bound Human A2A Adenosine Receptor. Science. 2011;332:322. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr Opin Struct Biol. 2011;21:541. doi: 10.1016/j.sbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Nygaard R, Frimurer TM, Holst B, Rosenkilde MM, Schwartz TW. Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol Sci. 2009;30:249. doi: 10.1016/j.tips.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Gether U, et al. Structural instability of a constitutively active G protein-coupled receptor. Agonist-independent activation due to conformational flexibility. J Biol Chem. 1997;272:2587. doi: 10.1074/jbc.272.5.2587. [DOI] [PubMed] [Google Scholar]
- 19.Ghanouni P, et al. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor. J Biol Chem. 2001;276:24433. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 20.Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor. Proc Natl Acad Sci U S A. 2001;98:5997. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granier S, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 22.Swaminath G, et al. Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- 23.Swaminath G, et al. Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem. 2004;279:686. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- 24.Yao X, et al. Coupling ligand structure to specific conformational switches in the beta2-adrenoceptor. Nat Chem Biol. 2006;2:417. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- 25.Yao XJ, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc Natl Acad Sci U S A. 2009;106:9501. doi: 10.1073/pnas.0811437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bokoch MP, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463:108. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danielson MA, Falke JJ. Use of 19F NMR to probe protein structure and conformational changes. Annu Rev Biophys Biomol Struct. 1996;25:163. doi: 10.1146/annurev.bb.25.060196.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.See supporting material and methods on Science Online.
- 29.Evanics F, Kitevski JL, Bezsonova I, Forman-Kay J, Prosser RS. 19F NMR studies of solvent exposure and peptide binding to an SH3 domain. Biochim Biophys Acta. 2007;1770:221. doi: 10.1016/j.bbagen.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Falke JJ, Luck LA, Scherrer J. 19F nuclear magnetic resonance studies of aqueous and transmembrane receptors. Examples from the Escherichia coli chemosensory pathway. Biophys J. 1992;62:82. doi: 10.1016/S0006-3495(92)81787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein-Seetharaman J, Getmanova EV, Loewen MC, Reeves PJ, Khorana HG. NMR spectroscopy in studies of light-induced structural changes in mammalian rhodopsin: applicability of solution (19)F NMR. Proc Natl Acad Sci U S A. 1999;96:13744. doi: 10.1073/pnas.96.24.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballesteros-Weistein numbering is shown as a superscript. The most conserved residue in each transmembrane helix is designated as x.50, where x is the number of the transmembrane helix, and the second number (50) indicates the relative position of the specific residue. The second number decreases towards the N terminus and vice versa. Only residues on transmembrane helices are noted.
- 33.In order to produce β2AR samples that are stabilized enough for NMR measurement, the C-terminus was truncated, the part of intracellular loop 3 that is not involved in G-protein recruitment was removed, and a stabilizing E122W mutation was added. Constructs containing these modifications were also crystallized and characterized as functionally similar to the unmodified β2AR.
- 34.The three 19F-signals in labeled wild type TETβ2AR/carazolol were identified by comparison with signals from β2AR constructs containing single labeled residues, i.e., β2AR(TETC265, C327S, C341A), β2AR(C265A, TETC327, C341A) and β2AR(C265A, C327S,TETC341).
- 35.Ligand binding properties of the cysteine mutants were examined on the membranes from β2AR expressing Sf9 cells. Radioligand binding assay used [3H]Dihydroalprenolol as the reporter ligand, and competition assay used isoproterenol as the competing ligand. The binding affinity of antagonist to the wild type receptor (1.4 nM) is identical to all cysteine mutants (Cys(265) = 1.1 ± 0.1 nM, Cys(327) = 1.4 ± 0.4 nM, Cys(341) = 1.1 ± 0.1 nM) and the affinity of full agonist to the wild type receptor (323 nM) is similar to all cysteine mutants (Cys(265) = 100nM, Cys(327) = 118nM, Cys(341) = 138nM).
- 36.Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3- adrenoceptors. Br J Pharmacol. 2010;160:1048. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisler JW, et al. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nobles KN, et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilty C, Wider G, Fernandez C, Wuthrich K. Membrane protein-lipid interactions in mixed micelles studied by NMR spectroscopy with the use of paramagnetic reagents. Chembiochem. 2004;5:467. doi: 10.1002/cbic.200300815. [DOI] [PubMed] [Google Scholar]
- 40.Liapakis G, et al. The forgotten serine. A critical role for Ser-2035.42 in ligand binding to and activation of the beta 2-adrenergic receptor. J Biol Chem. 2000;275:37779. doi: 10.1074/jbc.M002092200. [DOI] [PubMed] [Google Scholar]
- 41.Strader CD, Candelore MR, Hill WS, Sigal IS, Dixon RA. Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem. 1989;264:13572. [PubMed] [Google Scholar]
- 42.Strader CD, et al. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988;263:10267. [PubMed] [Google Scholar]
- 43.de Graaf C, Rognan D. Selective structure-based virtual screening for full and partial agonists of the beta2 adrenergic receptor. J Med Chem. 2008;51:4978. doi: 10.1021/jm800710x. [DOI] [PubMed] [Google Scholar]
- 44.Katritch V, et al. Analysis of full and partial agonists binding to beta2-adrenergic receptor suggests a role of transmembrane helix V in agonist-specific conformational changes. J Mol Recognit. 2009;22:307. doi: 10.1002/jmr.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahsai AW, et al. Multiple ligand-specific conformations of the beta(2)-adrenergic receptor. Nat Chem Biol. 2011;7:692. doi: 10.1038/nchembio.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.