Abstract
Adenovirus relies on numerous interactions between viral and host cell proteins to efficiently enter cells. Undoubtedly, post-translational modifications of host and cellular proteins can impact the efficiency of this cell entry process. Ubiquitylation, once simply thought of as a modification targeting proteins for proteasomal degradation, is now known to regulate protein trafficking within cells, protein-protein interactions, and cell signaling pathways. Accumulating evidence suggests that protein ubiquitylation can influence all stages of the life cycle of other viruses such as cell entry, replication, and egress. Until recently the influence of ubiquitylation has only been documented during adenovirus replication. This review highlights the most recent evidence demonstrating direct engagement of host ubiquitylation and SUMOylation machinery by adenovirus during cell entry. Additionally, potential roles for host protein ubiquitylation and the potential for adenovirus regulation of host ubiquitylation machinery during cell entry are discussed.
INTRODUCTION
From cell attachment to evading the host immune system, adenoviruses must efficiently utilize their ~40-encoded proteins to productively infect cells. Twelve of these virally encoded proteins are present in adenovirus virions (Figure 1), each potentially playing an important role in mediating virus entry into host cells (Nemerow et al., 2009; Russell, 2009) or establishing a niche in the nucleus for viral gene transcription and replication (Chen et al., 2007; Hindley et al., 2007; Karen and Hearing, 2011). Adenovirus, like all viruses, has evolved strategies to usurp host cell molecules for their own benefit. While several well characterized interactions between virion structural proteins and host cell factors have been documented as the virus enters cells (Nemerow et al., 2009), recent reports suggest that there are virus-host interactions during adenovirus cell entry which are still poorly characterized. Furthermore, both virus and host may have evolved strategies to manipulate these interactions between host and viral proteins though a variety of post-translational modifications.
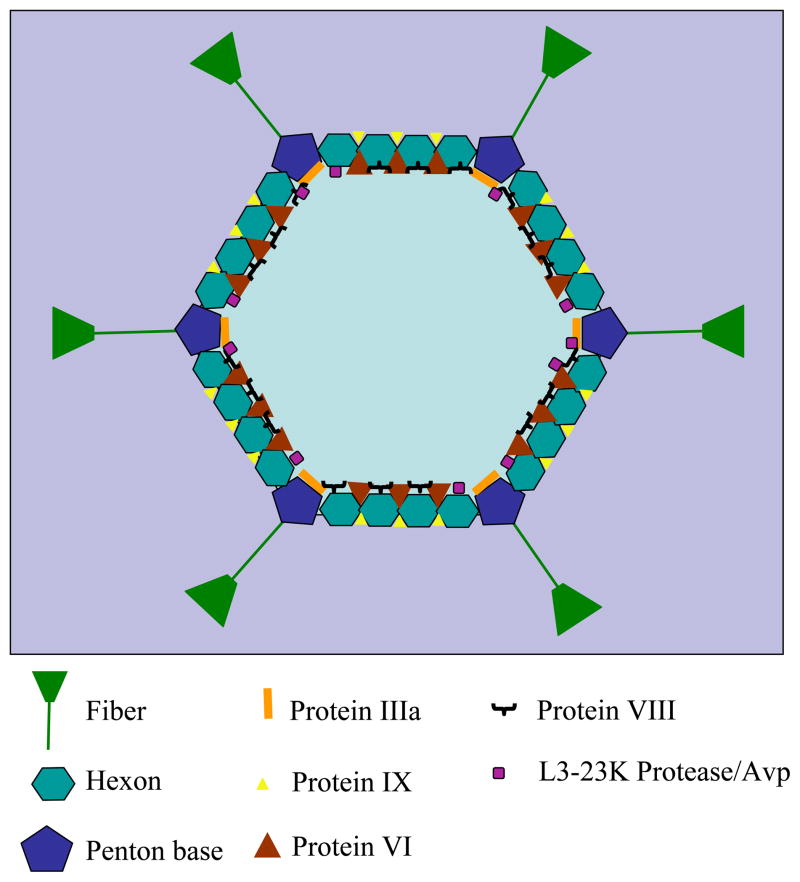
Figure 1. Adenovirus capsid structure.
Diagram showing the 7 capsid proteins plus the L3-23K protease. Protein IIIa, protein VI and protein VIII are located in the interior of the capsid. Also contained in the capsid is the L3-23K protease/AVP. Not shown are the Terminal protein, Protein Mu, Protein VII, and Protein V, where are all associated with the viral DNA (also not shown). Adapted from (Nemerow et al., 2009)
Ubiquitylation of viral and host proteins can influence a variety of processes in virus entry, replication and egress. While numerous reports have highlighted the interaction of adenovirus proteins with the ubiquitin/proteasome system (UPS) during viral replication (Querido et al., 2001; Harada et al., 2002; Blanchette et al., 2004; Woo and Berk, 2007; Schwartz et al., 2008; Isobe et al., 2009; Blackford et al., 2010), recent evidence suggests that ubiquitylation of adenovirus structural proteins and host cell proteins can influence the cell entry of adenoviruses (Gout et al., 2010; Wodrich et al., 2010). A greater understanding of the influence of the UPS in adenovirus cell entry is likely to identify novel mechanisms which viruses use to enter cells and may also identify new targets for antiviral therapeutics. This review will highlight recent observations implicating ubiquitin as an important posttranslational modification of host and cellular proteins during adenovirus cell entry. Interactions of adenovirus structural proteins with host ubiquitylation machinery and potential regulation of adenovirus receptors by the ubiquitin modifications will be discussed.
Adenovirus Cell Entry
Adenoviruses (Ads) are non-enveloped, double-stranded DNA viruses that cause upper respiratory tract, gastrointestinal tract and ocular infections. There are >50 human serotypes, which are classified into 7 subgroups (A–G)(Aoki et al., 2011; Seto et al., 2011). Human Ads bind to cells via an interaction between the adenovirus fiber protein and a primary attachment receptor. Subgroups A, E and F use the Coxsackievirus B and Adenovirus Receptor (CAR) as their primary attachment receptor (Freimuth et al., 1999). Subgroups C and D also use CAR in addition to other receptors (subgroup C can also use heparan sulfate proteoglycans (HSPG), MHC-I and VCAM-I and subgroup D viruses can also use sialic acid and CD46) (Bergelson et al., 1997; Hong et al., 1997; Freimuth et al., 1999; Huang et al., 1999; Arnberg et al., 2000; Chu et al., 2001; Dechecchi et al., 2001). Subgroup B viruses use CD46, HSPG, CD80/86, and Demsoglein 2 (Gaggar et al., 2003; Sharma et al., 2009; Wang et al., 2011). Additionally, more recent studies suggest the cell attachment during systemic infection may rely on the association of soluble serum cofactors with the capsid (Shayakhmetov et al., 2005; Parker et al., 2006; Alba et al., 2009).
After attachment, an interaction between the adenovirus penton base and αv integrins triggers clathrin-mediated endocytosis (Nemerow and Stewart, 1999; Smith et al., 2010)and likely initiates stepwise disassembly of the adenovirus capsid (Greber et al., 1993), a process necessary for adenovirus escape from endosomal compartments (Greber et al., 1993; Wiethoff et al., 2005). Adenovirus trafficking within endosomal compartments appears to depend upon the nature of Ad-receptor interactions, with subgroup C adenovirus serotypes trafficking to early endosomal compartments prior to endosomal escape (Gastaldelli et al., 2008) while subgroup B viruses traffic to late endosomes/lysosomes prior to endosomal escape (Miyazawa et al., 2001; Shayakhmetov et al., 2003). Within endosomes, the adenovirus capsid partially uncoats, a process necessary for adenovirus rupture of endosomal membranes to access the cytoplasm. Partial capsid disassembly is required to release an internal capsid protein, protein VI (pVI), which was recently shown to rupture endosomal membranes, allowing the partially disassembled virion to enter the cytoplasm (Wiethoff et al., 2005; Maier et al., 2010; Moyer et al., 2011). After endosome escape, adenovirus traffics to the nucleus via microtubules, docking at the nuclear pore and delivers the adenovirus genome to the nucleus. Several groups have suggested that determinants in the major capsid protein, hexon (Smith et al., 2008; Bremner et al., 2009; Strunze et al., 2011), and pVI (Wodrich et al., 2010) influence microtubule-dependent trafficking of adenovirus virions in the cytoplasm. Docking of partially disassembled adenovirus virions at the nuclear envelope occurs at nuclear pore complexes. Roles for nuclear filament protein Nup214, as well as soluble factors, hsc70 and histone H1 have been implicated in nuclear import of the viral genomic DNA (Saphire et al., 2000; Trotman et al., 2001; Strunze et al., 2011).
The Ubiquitin Proteasome System (UPS)
Ubiquitin plays essential roles in multiple cellular processes. Ubiquitin is conjugated to proteins via amide formation between the ubiquitin C-terminus and ε-amino groups on lysine side chains of proteins (Kerscher et al., 2006). Once a ubiquitin is appended to a given protein, subsequent ubiquitin molecules can be either conjugated to another lysine residue of the same protein (multiubiquitylation) or appended to one of seven lysine residues on ubiquitin itself (polyubiquitylation) (Kim and Rao, 2006). Conjugation of ubiquitin to proteins relies on the activity of three classes of enzymes termed E1, E2, and E3-ubiquitin ligases. It is the E3-ubiquitin ligase which provides the substrate specificity and ultimately conjugates ubiquitin to the target protein (Nagy and Dikic, 2010). While protein ubiquitylation was initially studied for its role targeting proteins for proteasomal degradation via appendage of K48 linked polyubiquitin chains to target proteins, more recent work has highlighted a role for mono- or multi-ubiquitylation in protein trafficking and activity and the assembly of signaling complexes (Kim and Rao, 2006). Protein ubiquitylation is highly dynamic, with possibly hundreds of E3 ubiquitin ligases and approximately 100 deubiquitinases (Nijman et al., 2005) tightly regulating ubiquitin modifications of proteins. Numerous examples have highlighted the influence of the UPS on viral replication and egress such as in DNA virus replication in the nucleus (Weitzman et al., 2011) or the budding of enveloped viruses (Bieniasz, 2006). Much less is currently known about the contributions of the UPS during viral entry.
ADENOVIRUS CAPSID PROTEINS AND UBIQUITYLATION
Protein VI
Recently, evidence for direct engagement of the Ad5 capsid of the UPS during cell entry has been suggested (Wodrich et al., 2010). Wodrich et. al. reported the identification of a highly conserved PPxY motif in capsid protein VI (Wodrich et al., 2010). This motif is present within all sequenced human adenoviruses (Wodrich et al., 2010). Present as ~342 copies in the adenovirus virion, protein VI is released from the capsid interior during partial disassembly of the virus within endosomes. Protein VI was recently found to possess all of the in vitro membrane disrupting activity of the adenovirus capsid and antibody neutralization (Maier et al., 2010) or genetic impairment of pVI membrane lytic activity (Moyer et al., 2011) were recently shown to prevent adenovirus membrane rupture. Wodrich et. al. demonstrated that although there was no defect in endosomal membrane rupture by recombinant Ad5 in which the pVI PPSY motif was mutated to PGAA (M1), there was a substantial defect in cytosolic trafficking of the M1 mutant virus to microtubule organizing centers (MTOC) and subsequent nuclear accumulation (Wodrich et al., 2010). PPxY motifs are recognized by WW-domains, commonly found in HECT-domain E3-ubiquitin ligases of the Nedd4 family (Ingham et al., 2004). Recruitment of these Nedd4 family members frequently leads to the ubiquitylation of the PPxY-containing substrate or associated proteins (Sudol, 1996; Harty et al., 2000). In vitro studies demonstrated that protein VI is oligo-ubiquitylated (2–3 ubiquitins) by a cytosolic factor and that this ubiquitylation is abrogated upon mutation of the PPSY motif in Ad5 protein VI to PGAA (Wodrich et al., 2010). When overexpressed in U2OS cells, Ad5 protein VI containing the M1 mutation failed to associate with members of the Nedd4 family of E3 ubiquitin ligases, Nedd4.1, Nedd4.2, AIP4, WWP1 and WWP2 as assessed by co-precipitation or fluorescence colocalization (Wodrich et al., 2010). Knockdown of Nedd4.2, but not Nedd4.1, AIP4 or other Nedd4 family members by siRNA lead to a reduction in Ad5 transduction efficiency as well as reduced colocalization of Ad5 with MTOCs (Wodrich et al., 2010). Further, live cell imaging of the intracellular trafficking of protein VI demonstrated that it was highly mobile, apparently moving in association with vesicular structures in a microtubule dependent manner (Wodrich et al., 2010). Together, these data lead Wodrich et al. to propose that protein VI facilitates the microtubule dependent trafficking of adenovirus capsids toward the MTOC, ultimately leading to nuclear delivery of the viral genome. The exact role for the protein PPxY in adenovirus cell entry remains to be elucidated. Given that the PPxY in protein VI is so highly conserved amongst adenovirus serotypes, it is interesting to consider whether the fixation of this motif in the adenovirus genome resulted from a strict requirement for microtubule dependent movement of the virus within the cytoplasm, or whether it may have evolved to evade host restriction factors which prevented “PPxY-less” adenoviruses from efficiently delivering their genomes to the nucleus for viral replication. Given several reports which indicate that certain cell types support microtubule independent infection by adenoviruses, the latter possibility warrants further exploration.
Penton Base
The adenovirus penton base is a homopentamer present at the vertices of the adenovirus capsid. In addition to anchoring the receptor binding fiber protein to the surface of the adenovirus capsid, binding of the penton base to αv integrins through a conserved RGD motif initiates viral endocytosis (Nemerow and Stewart, 1999; Smith et al., 2010). The adenovirus penton base contains two PPxY motifs in its N-terminal domain, also conserved across all known human serotypes (Galinier et al., 2002). In vitro and in vivo studies by Galinier et al. demonstrate that the Ad2 and Ad3 penton bases also binds to WW-domain containing proteins, with the co-chaperone protein Bcl-2-associated athanogene 3, BAG3, identified as a penton base interacting protein in a yeast two hybrid screen (Galinier et al., 2002; Gout et al., 2010). Galinier et al. further demonstrated that members of the Nedd4 family could also bind penton base via the N-terminal PPxY motif (Galinier et al., 2002). Although Galinier et al. determined that Ad2 penton binding to the Nedd4 family member WWP1 formed a stable complex, they did not determine if the penton base itself was ubiquitylated, nor if deletion of the first PPxY motif affected adenovirus cell entry.
More recently, Gout et al., demonstrated that BAG3 interactions with penton base occur within infected cells as determined by co-immunoprecipitation (Gout et al., 2010). Further, when penton base is overexpressed in cells in culture, it is able to recruit BAG3 to the nucleus in a PPxY-dependent manner (Gout et al., 2010). These experimental results suggest that BAG3 may be important for adenovirus infection. To examine this possibility, Gout et al., demonstrated that siRNA knockdown of BAG3 attenuated transgene delivery by replication-incompetent Ad5 vectors based on a reduction in luciferase expression (Gout et al., 2010). They also showed by single round infectivity assays that less progeny virions were produced in BAG3-knockdown cells (Gout et al., 2010). This study did not demonstrate the effect of mutating the penton base PPxY motifs on viral cell entry or replication. Nonetheless, these data suggest that BAG3 plays a role in adenovirus cell entry. As with the PPxY motifs in protein VI, further study of the penton base PPxY motifs is warranted to elucidate their role in adenovirus cell entry.
L3-23K Protease
Adenovirus virions contain approximately 11 copies of the L3-23K cysteine protease (or Avp) (Greber et al., 1996). Avp has a well defined role in adenovirus virion assembly by proteolytically processing adenovirus capsid preproteins during viral maturation, presumably to lock the capsid into a metastable state necessary for subsequent entry of these newly formed virions into neighboring, uninfected cells (Weber, 1976). It has also been demonstrated that Avp’s protease activity is required for efficient adenovirus cell entry (Cotten and Weber, 1995; Greber et al., 1996). Inhibition of the protease activity via reduction and alkylation of the active site cysteine or oxidation of the active site cysteine by copper chloride treatment reduced adenovirus transgene expression (Cotten and Weber, 1995; Greber et al., 1996). In one report, the defect in cell entry of protease-inactivated virions was found to occur after the virus had escaped from endosomes into the cytoplasm but prior to docking of the virus at the nuclear envelope (Greber et al., 1996). The function of Avp during the cell entry process has previously been ascribed to further proteolytic processing of the capsid, although this phenomenon has not been completely characterized. Interestingly, Avp possesses deubiquitylating and deISGylating activity, which has been observed in vitro and in vivo (Balakirev et al., 2002). While deubiquitylating activity was observed after 12 hrs during infection based on global reduction in the number of ubiquitylated proteins observed in western blots, the deubiquitylating activity of Avp was not examined at earlier times during cell entry (Balakirev et al., 2002). Given the involvement of Avp in adenovirus cell entry, it is interesting to consider whether Avp deubiquitylation of one or several host or viral proteins during adenovirus entry is required for efficient intracellular trafficking to the nucleus.
UBIQUITYLATION AND ADENOVIRUS RECEPTORS
Adenovirus relies on cell surface receptors for cell attachment to initiate infection. Secondary engagement of αv integrins after attachment initiates viral endocytosis. The receptors used by adenovirus are known to influence not only cell tropism (Sharma et al., 2009), but also the intracellular fate of the virus (Wickham et al., 1994; Miyazawa et al., 1999; Carey et al., 2007). A better understanding of how adenovirus receptor cell surface expression and intracellular fate are regulated could improve our understanding of adenovirus pathogenesis. Additionally, since mechanisms controlling adenovirus receptor cell surface expression and intracellular fate could be dysregulated in certain disease states, a better understanding of this process could improve our ability to select appropriate adenovirus vectors for gene therapy.
One potential mechanism influencing adenovirus receptor biology is the UPS. Previous reports demonstrate receptor interactions with UPS machinery during pathogen entry (Khor et al., 2003; Abrami et al., 2006; Coller et al., 2009). Tumor endothelial marker 8 (TEM8), a receptor for Bacillus anthracis lethal toxin, is ubiquitylated within 10 minutes of toxin treatment (Abrami et al., 2006). Mutating one of the 16 lysine residues greatly diminished TEM8 ubiquitylation, which lead to a delay in TEM8 endocytosis in response to toxin treatment, indicating that ubiquitylation of TEM8 is necessary for efficient endocytosis (Abrami et al., 2006). TEM8 ubiquitylation (and subsequent internalization) depended on the Cbl E3 ubiquitin ligase, as knockdown of Cbl abolished toxin-induced TEM8 ubiquitylation and internalization (Abrami et al., 2006). HCV colocalizes with Cbl prior to internalization, though whether Cbl is required for HCV internalization was not determined (Coller et al., 2009). Thus pathogen receptor ubiquitylation may influence internalization.
Additionally, influenza virus requires ESCRT machinery for transit of viral ribonucleoproteins (vRNPs) through the endocytic network and into the nucleus (Khor et al., 2003). ESCRT machinery recognizes membrane-bound ubiquitylated cargo and forms intraluminal vesicles and targets these vesicles to lysosome, preventing recycling of the cargo back to the plasma membrane (Raiborg and Stenmark, 2009). Thus, ubiquitylation may not only influence pathogen receptor cell surface expression and internalization, but may also influence intracellular trafficking. Although the influence of ubiquitin-dependent regulation of adenovirus receptors on adenovirus cell entry is currently undefined, inhibition of the UPS has been shown to differentially influence adenovirus cell entry depending on which primary receptor the virus uses (Shayakhmetov et al., 2003). Thus, we have outlined our current understanding of the potential roles of ubiquitin in the biology of several adenovirus receptors below.
Coxsackievirus and adenovirus receptor (CAR)
The Coxsackievirus and adenovirus receptor is a single pass, type I membrane protein residing in apical junction complexes of polarized epithelial cells (Freimuth et al., 2008). Several recent observations suggest that the localization and cell surface levels of CAR may be regulated by ubiquitylation. First, in certain colon cancer cell lines expressing relatively low levels of CAR, treatment of cells with the proteasomal inhibitor, MG132 greatly increased the levels of CAR present on the cell surface as well as the ability of these cells to be transduced with Ad5 vectors (Zhang et al., 2008). Similar observations made for other cell surface receptors have eventually lead to the conclusion that cell surface levels of these receptors are controlled by ubiquitylation. Although Zhang, et al. demonstrated that the elevated cell surface CAR levels in MG132 treated cells were not due to increased mRNA levels, but were likely regulated post-translationally, no evidence for CAR ubiquitylation was reported.
Further support for the potential regulation of CAR cell surface expression by the UPS comes from the observation that greater cell surface expression of CAR, and hence greater transduction by Ad5 vectors, occurs when extracellular domain of CAR is expressed as a GPI-linked protein compared to expression of CAR containing its transmembrane domain (Wang et al., 2007). Interestingly, the cytoplasmic tail of CAR contains 9 lysines. Although mutational analysis of these lysine residues and its effect on CAR cell surface expression was not performed, these lysine residues may serve as ubiquitylation sites. Lack of the cytoplasmic tail could prevent ubiquitin-dependent downregulation of CAR from the cell surface. Alternatively, if CAR is ubiquitylated in its cytoplasmic tail, ubiquitylation may serve as a signal to target CAR for endocytosis and trafficking to lysosomes for degradation. Deleting the cytoplasmic tail, and potentially abolishing ubiquitylation, might allow CAR to be recycled back to the cell surface.
More specific evidence for a role of ubiquitin in the biology of CAR comes from two findings. First, CAR was found to associate with LNX, a protein known to act as an E3-ubiquitin ligase targeting proteins for degradation (Sollerbrant et al., 2003). However, whether CAR associates with LNX during adenovirus infection, and whether the E3-ubiquitin ligase activity of LNX affect adenovirus cell entry has yet to been examined. Second, a role for ubiquitylation in regulating cell surface levels of CAR comes from a recent report that a recently identified splice-variant of CAR, which is thought to mediate infection of polarized airway epithelium, contains an additional intracellular domain encoded by exon 8 (CARex8) and localizes to apical surfaces of polarized lung epithelia rather than apical junctional complexes (Excoffon et al., 2010). This report also found that cell surface levels of CARex8 were regulated by the PDZ- and WW-domain containing protein MAGI-1b. While both the previously identified version of CAR, CARex7, normally localized to apical junctional complexes, and CARex8 appear to be able to bind or colocalize with MAGI-1b, Excoffon et al demonstrated that only CARex8 levels are decreased in the presence of MAGI-1b, suggesting that MAGI-1b regulates CARex8, but not CARex7 degradation. Although not directly addressed by Excoffon et al. it would be interesting to determine whether MAGI-1b dependent degradation of CARex8 involves ubiquitylation of the CAR cytoplasmic tail. Together, these data suggest that CAR cell surface expression, and perhaps cell surface localization is regulated post-translationally by the UPS. Further studies of UPS regulation of CAR cell surface expression might provide important insight into adenovirus tropism, particularly as it relates to the use of adenoviral vectors for gene therapy of cardiovascular disease and cancer.
CD46
Adenovirus Subgroup B viruses can utilize CD46 instead of CAR as their primary receptor. CD46, a membrane complement regulator protein which can bind and inactivate C3b and C4b (Oglesby et al., 1992), is expressed as several alternatively spliced isoforms containing 4 short consensus repeats, 1–3 serine/threonine-rich domains, a transmembrane domain and a cytoplasmic tail (Seya et al., 1999). Only consensus repeats 1 and 2 in the extracellular domain are required for Ad-CD46 interaction (Fleischli et al., 2005; Gaggar et al., 2005; Sakurai et al., 2006). It was hypothesized that the cytoplasmic domain of CD46 could transduce signals. Indeed, CD46 can transduce signals via phosphorylation of the E3-ubiquitin ligase Cbl (Astier et al., 2000). Recent studies indicate Cbl plays a role in ligand-induced ubiquitinylation, down-regulation, and lysosomal targeting of receptors. Interestingly, CD46 is down-regulated after measles infection, but whether or not CD46 downregulation was dependent on Cbl phoshorylation or Cbl E3 ubiquitin ligase activity was not explored (Naniche et al., 1993). Further, CD46 downregulation after adenovirus infection has not been reported.
The ubiquitylation status of CD46, and if Cbl ubiquitylates CD46, have not been explored. However, Shayakhmetov et al. have demonstrated a role for the UPS during infection by subgroup B human adenovirus serotype 35, which uses CD46 as a receptor (Shayakhmetov et al., 2003). Using a recombinant Ad5 in which the fiber was swapped with the adenovirus type 35 fiber protein in the capsid (Ad5/F35), Shayakhmetov et al found that treatment with the proteasome inhibitor MG132 reduced infection of HeLa cells by Ad5/F35 compared to untreated cells, whereas Ad5 infectivity was unchanged with MG132 treatment (Shayakhmetov et al., 2003). Further studies are needed to determine the role of ubiquitylation in the regulation of CD46- endocytosis and trafficking during adenovirus cell entry.
αv Integrins
Integrins, present in all nucleated cells, play a role in a number of cellular processes, including cell adhesion, migration, growth, and differentiation. Integrins are heterodimers composed of an alpha and beta subunit. Engagement of αvβ3, αvβ3 or αvβ5 by the penton base of all but subgroup F adenoviruses can trigger clathrin-mediated endocytosis (Nemerow et al., 2009; Smith et al., 2010). Interestingly, several observations suggest that the intracellular trafficking of adenovirus is greatly influenced by the αv integrin used by the virus for endocytosis (Wickham et al., 1994). Specifically, use of αvβ3 targets the virus for lysosomes, resulting in reduced infectivity (Wickham et al., 1994). Interestingly, several observations suggest that ubiquitylation of αvβ3 integrins may occur upon receptor ligation. First, Tsai et al. demonstrated that Streptococcal pyrogenic exotoxin B (SPE-B) intoxication depends, in part, on binding of SPE-B to αvβ3 via an RGD motif (Tsai et al., 2008). Additionally, the cell surface levels of αvβ3 were decreased after SPE-B binding, and surface levels were restored with treatment of the proteasome inhibitor MG132, suggesting that αvβ3 internalization and, perhaps recycling is regulated by the ubiquitin-proteasome system (Tsai et al., 2008).
Second, Thomas et al. demonstrated that αvβ3 integrin is ubiquitylated in human umbilical vein endothelial cells in response to angiopoietin-2, although whether the alpha or beta chain is ubiquitylated was not described (Thomas et al., 2010). They show that αvβ3 integrin is internalized and ubiquitylated after 10 minutes of angiopoietin-2 ligand treatment (Thomas et al., 2010). No ubiquitylation of αvβ3 integrin was detected in the absence of agniopoietin-2 treatment, demonstrating that ubiquitylation of αvβ3 integrin requires ligand binding. However, it was not determined if αvβ3 integrin was ubiquitylated at the cell surface, resulting in receptor internalization or if ubiquitylation occurred post internalization. Additionally, αvβ3 integrin colocalized with the lysosome marker LAMP1 after 1 hour of angiopoietin-2 treatment, but whether αvβ3 integrin trafficking to lysosomes required ubiquitylation was not determined (Thomas et al., 2010).
To date, no studies determining if αvβ5 integrin is ubiquitylated upon ligand binding have been performed. Initial reports demonstrated that αvβ5 can more greatly support Ad5 infection and endosomal escape than αvβ3 integrins (Wickham et al., 1994). Subsequent studies found that reduced expression of β5 integrin leads to greater targeting of Ad5 to lysosomes (Carey et al., 2007). Although, Carey et al. did not determine which integrin β-subunit was utilized in cells with lower β5 expression. Nonetheless, it would be interesting to determine whether differences in the ubiquitylation of αvβ3 and αvβ5 integrin are observed during Ad5 cell entry and whether integrin ubiquitylation correlates with lysosomal trafficking of Ad5.
SUMOylation
The small ubiquitin-like modifier (SUMO) family of proteins are appended to proteins in a manner similar to ubiquitin and other ubiquitin-like proteins (reviewed in (Geiss-Friedlander and Melchior, 2007)). SUMOylation is another post-translational modification that has recently been shown to influence early events during viral infection. Like ubiquitylation, SUMOylation uses E1, E2, and E3 conjugating enzymes to attach a SUMO to ε-amino groups of lysine residues, though these SUMO conjugating enzymes are different cellular enzymes than those used for ubiquitylation. Protein SUMOylation has been reported to alter protein localization, activity, stability, and promote protein-protein interactions (reviewed in (Geiss-Friedlander and Melchior, 2007)). Although the SUMOylation occurs with great frequency in the nucleus, recent literature suggests that SUMOylation can regulate protein function and trafficking in the cytoplasm as well (reviewed in (Geiss-Friedlander and Melchior, 2007). SUMOylation during viral infection has been reported to promote an antiviral defense, or conversely, promote viral infection (reviewed in (Wimmer et al., 2011)). SUMOylation of HIV integrase protein promotes infection at a step following nuclear entry and prior to integration (Zamborlini et al., 2011). SUMOylation of the Moloney murine leukemia virus capsid protein is required for an early step during viral infection after reverse transcription prior to nuclear entry (Yueh et al., 2006). Although the actual role for SUMOylation at these early stages of retroviral infection are incompletely defined, these data are in line with previous observations that SUMOylation regulates nuclear import of number of cellular proteins (Pichler and Melchior, 2002; Lin et al., 2003; Melchior et al., 2003) or the function of nuclear proteins (reviewed in (Geiss-Friedlander and Melchior, 2007)).
While a role for SUMOylation in the organization of Ad replication centers in the nucleus has been documented (Wimmer et al., 2010), no evidence is available to suggest a role for SUMOylation during Ad cell entry. Nonetheless, one recent report has implicated a role for the nuclear pore-associated SUMO E3 ligase, Nup358/RanBP-2 in facilitating nuclear import of Ad2/5 (Strunze et al., 2011). Nup358/RanBP-2 is required for the import of numerous cellular proteins into the nucleus independent of its sumoylating activity (Walde et al., 2011). Nup358/RanBP-2 was also identified as a cell factor that promotes nuclear import of the HIV pre-integration complex (Brass et al., 2008). Strunze et al. demonstrated that knockdown of Nup358/RanBP-2 had no effect on Ad localization to nuclear pore complexes, but did decrease Ad2/5 capsid uncoating and reduced Ad-mediated GFP expression (Strunze et al., 2011). They also determined that Nup358/RanBP-2 anchors the Ad2/5 capsid to microtubules through interactions with Nup214-Ad2/5 complexes and kinesin. (Strunze et al., 2011). From these data they concluded that Nup358/RanBP-2 serves as a scaffold protein to link Ad to kinesin, which is important for Ad uncoating and genome delivery. Whether or not the sumoylating activity of Nup358/RanBP-2 is required for Ad uncoating, or if the Ad capsid is sumoylated remains to be examined.
CONCLUDING REMARKS
Here we have summarized recent observations which suggest that the UPS may influence adenovirus cell entry. These preliminary observations raise many important questions regarding the contributions of ubiquitin to the adenovirus cell entry process. What is the role of recruitment of the Nedd4 family members of E3 ubiquitin ligases during adenovirus infection? It has previously been determined that mutating the motif in pVI responsible for Nedd4 family recruitment decreases adenovirus entry. Would mutating the motif in the penton base also decrease adenovirus entry, and if so, since the penton base and pVI recruit the same family of E3-ubiquitin ligases, would mutations of the motifs in both proteins further decrease adenovirus entry? Additionally, how does the viral protease, which contains deubiquitinylating capabilities, contribute to adenovirus entry? Is deubiquitinylating by the viral protease essential for adenovirus cell entry, and if so, what protein(s) is the protease targeting?
Additionally, recent observations suggest that ubiquitylation of adenovirus receptors can regulate their cell surface expression and intracellular trafficking, although the importance of this regulation during adenovirus cell entry is unexplored. Are cellular receptors utilized by adenovirus for entry ubiquitylated after adenovirus attachment, and if so, how does this affect adenovirus transduction? Does ubiquitylation of these cellular receptors result in downregulation and degradation, which might decrease adenovirus transduction? Additionally, does ubiquitylation of these cellular receptors promote signaling to cellular pathways that are essential to adenovirus entry? Is Ad intracellular trafficking influenced by viral or host protein SUMOylation? Answers to these questions should shed considerable light on the biology of adenoviruses and may provide useful insight into novel targets for antiviral therapeutics.
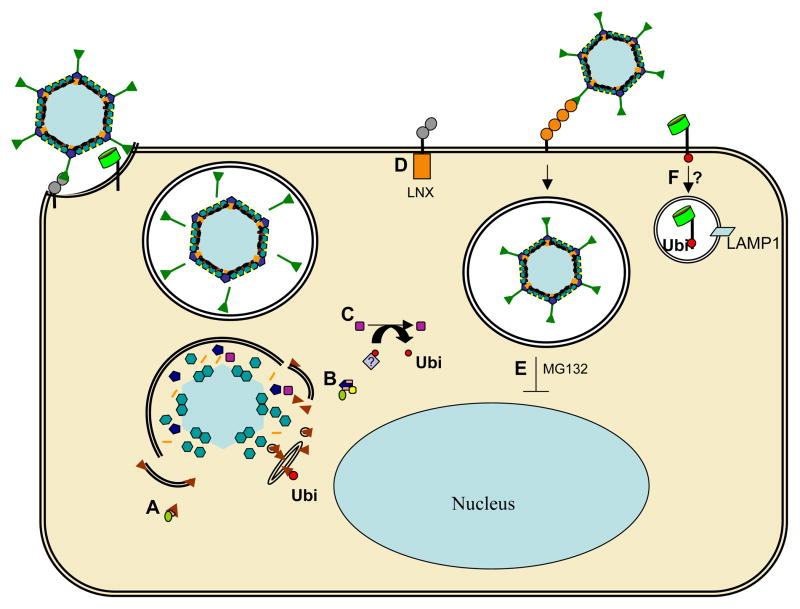
Figure 2. Potential roles for ubiquitlyation during adenovirus cell entry.
A) Protein VI contains a conserved PPxY motif, responsible for colocalization with Nedd4 family members of E3-ubiquitin ligases and in vitro pVI ubiquitylation. Mutation of the PPxY motif decreases specific infectivity at an entry step post endosomal escape. B) The penton base contains 2 conserved PPxY motifs, and Nedd4 E3-ubiquitin ligase family members bind penton base via the PPxY motifs. Additionally, penton base binds BAG3 in a PPxY-dependent manner. BAG3 knockdown decreases adenovirus specific infectivity. C) L3-23K protease/Avp activity is required during adenovirus cell entry. Avp possesses deubiquitylating activity, but it has not been determined if the deubiquitylating activity is required for adenovirus specific infectivity. D) CAR associates with the E3-ubiquitin ligase LNX. Not shown-MG132 treatment increases CAR surface expression and increases adenovirus infectivity. E) Treatment with MG132 decreases adenovirus infectivity for viruses that utilize the CD46 receptor. F) Upon adenovirus binding to αvβ3 integrins, integrin ubiquitlyation and trafficking to lysosomes (LAMP1) may occur.
Acknowledgments
The authors wish to thank Dr. Harald Wodrich for the many helpful discussions of the role of ubiquitin in viral cell entry. C.M.W acknowledges financial support from the NIAID (AI082430), and AHA (2261306). S.A.M. acknowledges financial support from the NIAID (AI007508).
BIBLIOGRAPHY
- Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, Nicklin SA, van Rooijen N, Custers J, Goudsmit J, Barouch DH, McVey JH, Baker AH. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Benko M, Davison AJ, Echavarria M, Erdman DD, Harrach B, Kajon AE, Schnurr D, Wadell G. Toward an integrated human adenovirus designation system that utilizes molecular and serological data and serves both clinical and fundamental virology. J Virol. 2011;85:5703–5704. doi: 10.1128/JVI.00491-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- Balakirev MY, Jaquinod M, Haas AL, Chroboczek J. Deubiquitinating function of adenovirus proteinase. J Virol. 2002;76:6323–6331. doi: 10.1128/JVI.76.12.6323-6331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Blackford AN, Patel RN, Forrester NA, Theil K, Groitl P, Stewart GS, Taylor AM, Morgan IM, Dobner T, Grand RJ, Turnell AS. Adenovirus 12 E4orf6 inhibits ATR activation by promoting TOPBP1 degradation. Proc Natl Acad Sci U S A. 2010;107:12251–12256. doi: 10.1073/pnas.0914605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette P, Cheng CY, Yan Q, Ketner G, Ornelles DA, Dobner T, Conaway RC, Conaway JW, Branton PE. Both BC-box motifs of adenovirus protein E4orf6 are required to efficiently assemble an E3 ligase complex that degrades p53. Mol Cell Biol. 2004;24:9619–9629. doi: 10.1128/MCB.24.21.9619-9629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Bremner KH, Scherer J, Yi J, Vershinin M, Gross SP, Vallee RB. Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe. 2009;6:523–535. doi: 10.1016/j.chom.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B, Staudt MK, Bonaminio D, van der Loo JC, Trapnell BC. PU.1 redirects adenovirus to lysosomes in alveolar macrophages, uncoupling internalization from infection. J Immunol. 2007;178:2440–2447. doi: 10.4049/jimmunol.178.4.2440. [DOI] [PubMed] [Google Scholar]
- Chen J, Morral N, Engel DA. Transcription releases protein VII from adenovirus chromatin. Virology. 2007;369:411–422. doi: 10.1016/j.virol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Chu Y, Heistad D, Cybulsky MI, Davidson BL. Vascular cell adhesion molecule-1 augments adenovirus-mediated gene transfer. Arterioscler Thromb Vasc Biol. 2001;21:238–242. doi: 10.1161/01.atv.21.2.238. [DOI] [PubMed] [Google Scholar]
- Coller KE, Berger KL, Heaton NS, Cooper JD, Yoon R, Randall G. RNA interference and single particle tracking analysis of hepatitis C virus endocytosis. PLoS Pathog. 2009;5:e1000702. doi: 10.1371/journal.ppat.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M, Weber JM. The adenovirus protease is required for virus entry into host cells. Virology. 1995;213:494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75:8772–8780. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Gansemer ND, Mobily ME, Karp PH, Parekh KR, Zabner J. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One. 2010;5:e9909. doi: 10.1371/journal.pone.0009909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischli C, Verhaagh S, Havenga M, Sirena D, Schaffner W, Cattaneo R, Greber UF, Hemmi S. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J Virol. 2005;79:10013–10022. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. Curr Top Microbiol Immunol. 2008;323:67–87. doi: 10.1007/978-3-540-75546-3_4. [DOI] [PubMed] [Google Scholar]
- Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Liszewski MK, Atkinson JP, Lieber A. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinier R, Gout E, Lortat-Jacob H, Wood J, Chroboczek J. Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligases. Biochemistry. 2002;41:14299–14305. doi: 10.1021/bi020125b. [DOI] [PubMed] [Google Scholar]
- Gastaldelli M, Imelli N, Boucke K, Amstutz B, Meier O, Greber UF. Infectious adenovirus type 2 transport through early but not late endosomes. Traffic. 2008;9:2265–2278. doi: 10.1111/j.1600-0854.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gout E, Gutkowska M, Takayama S, Reed JC, Chroboczek J. Co-chaperone BAG3 and adenovirus penton base protein partnership. J Cell Biochem. 2010;111:699–708. doi: 10.1002/jcb.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Webster P, Weber J, Helenius A. The role of the adenovirus protease on virus entry into cells. EMBO J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Harada JN, Shevchenko A, Pallas DC, Berk AJ. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J Virol. 2002;76:9194–9206. doi: 10.1128/JVI.76.18.9194-9206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci U S A. 2000;97:13871–13876. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley CE, Lawrence FJ, Matthews DA. A role for transportin in the nuclear import of adenovirus core proteins and DNA. Traffic. 2007;8:1313–1322. doi: 10.1111/j.1600-0854.2007.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Reddy V, Dasgupta N, Nemerow GR. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol. 1999;73:2798–2802. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- Isobe T, Hattori T, Kitagawa K, Uchida C, Kotake Y, Kosugi I, Oda T, Kitagawa M. Adenovirus E1A inhibits SCF(Fbw7) ubiquitin ligase. J Biol Chem. 2009;284:27766–27779. doi: 10.1074/jbc.M109.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen KA, Hearing P. Adenovirus core protein VII protects the viral genome from a DNA damage response at early times after infection. J Virol. 2011;85:4135–4142. doi: 10.1128/JVI.02540-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Khor R, McElroy LJ, Whittaker GR. The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic. 2003;4:857–868. doi: 10.1046/j.1398-9219.2003.0140.x. [DOI] [PubMed] [Google Scholar]
- Kim I, Rao H. What’s Ub chain linkage got to do with it? Sci STKE. 2006;2006:pe18. doi: 10.1126/stke.3302006pe18. [DOI] [PubMed] [Google Scholar]
- Lin X, Sun B, Liang M, Liang YY, Gast A, Hildebrand J, Brunicardi FC, Melchior F, Feng XH. Opposed regulation of corepressor CtBP by SUMOylation and PDZ binding. Mol Cell. 2003;11:1389–1396. doi: 10.1016/s1097-2765(03)00175-8. [DOI] [PubMed] [Google Scholar]
- Maier O, Galan DL, Wodrich H, Wiethoff CM. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology. 2010;402:11–19. doi: 10.1016/j.virol.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Schergaut M, Pichler A. SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem Sci. 2003;28:612–618. doi: 10.1016/j.tibs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Miyazawa N, Crystal RG, Leopold PL. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J Virol. 2001;75:1387–1400. doi: 10.1128/JVI.75.3.1387-1400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa N, Leopold PL, Hackett NR, Ferris B, Worgall S, Falck-Pedersen E, Crystal RG. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J Virol. 1999;73:6056–6065. doi: 10.1128/jvi.73.7.6056-6065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J Virol. 2011;85:2631–2641. doi: 10.1128/JVI.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Dikic I. Ubiquitin ligase complexes: from substrate selectivity to conjugational specificity. Biol Chem. 2010;391:163–169. doi: 10.1515/bc.2010.021. [DOI] [PubMed] [Google Scholar]
- Naniche D, Wild TF, Rabourdin-Combe C, Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993;74(Pt 6):1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- Nemerow GR, Pache L, Reddy V, Stewart PL. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow GR, Stewart PL. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Oglesby TJ, Allen CJ, Liszewski MK, White DJ, Atkinson JP. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992;175:1547–1551. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni S, Lieber A, McVey JH, Nicklin SA, Baker AH. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- Pichler A, Melchior F. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic. 2002;3:381–387. doi: 10.1034/j.1600-0854.2002.30601.x. [DOI] [PubMed] [Google Scholar]
- Querido E, Blanchette P, Yan Q, Kamura T, Morrison M, Boivin D, Kaelin WG, Conaway RC, Conaway JW, Branton PE. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 2001;15:3104–3117. doi: 10.1101/gad.926401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009;90:1–20. doi: 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- Sakurai F, Murakami S, Kawabata K, Okada N, Yamamoto A, Seya T, Hayakawa T, Mizuguchi H. The short consensus repeats 1 and 2, not the cytoplasmic domain, of human CD46 are crucial for infection of subgroup B adenovirus serotype 35. J Control Release. 2006;113:271–278. doi: 10.1016/j.jconrel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Saphire AC, Guan T, Schirmer EC, Nemerow GR, Gerace L. Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J Biol Chem. 2000;275:4298–4304. doi: 10.1074/jbc.275.6.4298. [DOI] [PubMed] [Google Scholar]
- Schwartz RA, Lakdawala SS, Eshleman HD, Russell MR, Carson CT, Weitzman MD. Distinct requirements of adenovirus E1b55K protein for degradation of cellular substrates. J Virol. 2008;82:9043–9055. doi: 10.1128/JVI.00925-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto D, Chodosh J, Brister JR, Jones MS. Using the whole-genome sequence to characterize and name human adenoviruses. J Virol. 2011;85:5701–5702. doi: 10.1128/JVI.00354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T, Hirano A, Matsumoto M, Nomura M, Ueda S. Human membrane cofactor protein (MCP, CD46): multiple isoforms and functions. Int J Biochem Cell Biol. 1999;31:1255–1260. doi: 10.1016/s1357-2725(99)00092-8. [DOI] [PubMed] [Google Scholar]
- Sharma A, Li X, Bangari DS, Mittal SK. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009;143:184–194. doi: 10.1016/j.virusres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ternovoi V, Gaggar A, Gharwan H, Lieber A. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J Virol. 2003;77:3712–3723. doi: 10.1128/JVI.77.6.3712-3723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Cassany A, Gerace L, Ralston R, Nemerow GR. Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport. J Virol. 2008;82:6492–6500. doi: 10.1128/JVI.00557-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Wiethoff CM, Stewart PL, Nemerow GR. Adenovirus. Curr Top Microbiol Immunol. 2010;343:195–224. doi: 10.1007/82_2010_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollerbrant K, Raschperger E, Mirza M, Engstrom U, Philipson L, Ljungdahl PO, Pettersson RF. The Coxsackievirus and adenovirus receptor (CAR) forms a complex with the PDZ domain-containing protein ligand-of-numb protein-X (LNX) J Biol Chem. 2003;278:7439–7444. doi: 10.1074/jbc.M205927200. [DOI] [PubMed] [Google Scholar]
- Strunze S, Engelke MF, Wang IH, Puntener D, Boucke K, Schleich S, Way M, Schoenenberger P, Burckhardt CJ, Greber UF. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe. 2011;10:210–223. doi: 10.1016/j.chom.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Sudol M. Structure and function of the WW domain. Prog Biophys Mol Biol. 1996;65:113–132. doi: 10.1016/s0079-6107(96)00008-9. [DOI] [PubMed] [Google Scholar]
- Thomas M, Felcht M, Kruse K, Kretschmer S, Deppermann C, Biesdorf A, Rohr K, Benest AV, Fiedler U, Augustin HG. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J Biol Chem. 2010;285:23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol. 2001;3:1092–1100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- Tsai WH, Chang CW, Lin YS, Chuang WJ, Wu JJ, Liu CC, Tsai PJ, Lin MT. Streptococcal pyrogenic exotoxin B-induced apoptosis in A549 cells is mediated through alpha(v)beta(3) integrin and Fas. Infect Immun. 2008;76:1349–1357. doi: 10.1128/IAI.01162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U, Wiemann S, Kehlenbach RH. The Nucleoporin Nup358/RanBP2 Promotes Nuclear Import in a Cargo- and Transport Receptor-Specific Manner. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01302.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Chen G, Zhou J, Wu P, Luo D, Huang X, Zhu T, Han Z, Xu G, Wang S, Lu Y, Ma D. Deletion of the intracellular domain of coxsackie and adenovirus receptor (CAR) enhances the expression of itself and boosts the efficiency of current adenovirus-mediated gene therapy in ovarian cancer cell lines in vitro. Cancer Lett. 2007;248:299–307. doi: 10.1016/j.canlet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, Wahl JK, 3rd, Urban N, Drescher C, Hemminki A, Fender P, Lieber A. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976;17:462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman MD, Lilley CE, Chaurushiya MS. Changing the ubiquitin landscape during viral manipulation of the DNA damage response. FEBS Lett. 2011;585:2897–2906. doi: 10.1016/j.febslet.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer P, Schreiner S, Dobner T. Human pathogens and the host cell SUMOylation system. J Virol. 2011 doi: 10.1128/JVI.06227-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer P, Schreiner S, Everett RD, Sirma H, Groitl P, Dobner T. SUMO modification of E1B-55K oncoprotein regulates isoform-specific binding to the tumour suppressor protein PML. Oncogene. 2010;29:5511–5522. doi: 10.1038/onc.2010.284. [DOI] [PubMed] [Google Scholar]
- Wodrich H, Henaff D, Jammart B, Segura-Morales C, Seelmeir S, Coux O, Ruzsics Z, Wiethoff CM, Kremer EJ. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010;6:e1000808. doi: 10.1371/journal.ppat.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JL, Berk AJ. Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export. J Virol. 2007;81:575–587. doi: 10.1128/JVI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh A, Leung J, Bhattacharyya S, Perrone LA, de los Santos K, Pu SY, Goff SP. Interaction of moloney murine leukemia virus capsid with Ubc9 and PIASy mediates SUMO-1 addition required early in infection. J Virol. 2006;80:342–352. doi: 10.1128/JVI.80.1.342-352.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamborlini A, Coiffic A, Beauclair G, Delelis O, Paris J, Koh Y, Magne F, Giron ML, Tobaly-Tapiero J, Deprez E, Emiliani S, Engelman A, de The H, Saib A. Impairment of human immunodeficiency virus type-1 integrase SUMOylation correlates with an early replication defect. J Biol Chem. 2011;286:21013–21022. doi: 10.1074/jbc.M110.189274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NH, Song LB, Wu XJ, Li RP, Zeng MS, Zhu XF, Wan DS, Liu Q, Zeng YX, Zhang XS. Proteasome inhibitor MG-132 modifies coxsackie and adenovirus receptor expression in colon cancer cell line lovo. Cell Cycle. 2008;7:925–933. doi: 10.4161/cc.7.7.5621. [DOI] [PubMed] [Google Scholar]