Abstract
Objectives
Exposure to glucocorticoid levels inappropriately high for current maturation alters fetal hypothalamo-pituitary adrenal axis (HPAA) development. In an established fetal sheep model we determined if clinical betamethasone doses used to accelerate fetal lung maturation have persistent effects on fetal HPAA hypotensive-stress responses.
Study design
Pregnant ewes received saline (n=6) or betamethasone (n=6); two 110 µg/kg body weight doses injected 24h apart (106/107 and 112/113 days gestational age - term 150 days). Basal ACTH and cortisol and responses to fetal hypotension were measured before and five days after the first and 14 days after the second course.
Results
Basal ACTH and cortisol were similar with treatment. HPAA responses to hypotension increased after the second but not first course and ACTH:cortisol ratio increased indicating central HPAA effects
Conclusions
Results demonstrate latency in emergence of fetal HPAA hyper-responsiveness following betamethasone exposure that may explain hyper-responsiveness in full-term but not preterm neonates.
Keywords: prenatal glucocorticoids, fetus, prematurity, hypothalamo pituitary adrenal axis, hypotension, lung maturation
INTRODUCTION
The incidence of preterm delivery is rising in the developed world. Currently about 10% of pregnancies are delivered preterm1 and most mothers in North America, Europe and Australia who threaten premature labor receive synthetic glucocorticoids in accordance with NIH consensus conference recommendations2 to decrease neonatal mortality and morbidity by accelerating fetal lung maturation 3. The window of 24 to 34 weeks of gestation when prenatal glucocorticoid administration to pregnant women is recommended2 parallels a critical phase of development of many fetal systems including the hypothalamo-pituitary adrenal axis (HPAA)4,5. To avoid potential side effects NIH guidelines recommend only a single course of prenatal glucocorticoid administration2. Nevertheless, in the last two decades many pregnant women have received multiple courses6,7 as the original findings of Liggins and Howie8 indicated that benefits of maternal glucocorticoid administration may only persist for 7 to 10 days.
The therapeutic benefits of accelerated lung maturation are accompanied by exposure of the fetus to an inappropriate level of fetal glucocorticoids for the current stage of fetal maturation. Animal studies show that fetal exposure to levels of glucocorticoids higher than those appropriate for the current stage of maturation can re-set the hypothalamo-pituitary-adrenal axis’ (HPAA) feedback set point with sustained consequences for the stress response in offspring9–14 that potentially mediate later life neuropsychological and cardiovascular disorders9,10,12,15–22.
Many pregnancies in which this treatment is administered eventually go to term and these fetuses will have experienced a very different intrauterine developmental history. One report shows that 76% of pregnancies in which the antenatal glucocorticoid therapy is given do not deliver before 32 weeks and 37% deliver after 37 weeks23. Thus it is important to determine any treatment effects on fetuses remaining in utero to permit the effects of glucocorticoids themselves without any effects of prematurity. Human studies have shown several outcomes: a transient suppression24,25, no effects of prenatal betamethasone treatment on baseline ACTH and cortisol levels26,27 or a blunted response to stress26,28,29 in preterm newborns, i.e. when the time between treatment and delivery was short. Similarly, fetal exposure to betamethasone or dexamethasone resulted in a dose-dependent (transient) reduction in basal plasma cortisol concentrations in guinea pig and non-human primate fetuses30,31. On the other hand, cortisol response to stress was greater in full-term newborns when the time between treatment and delivery was longer32. In agreement with, Sloboda et al.33 have shown in sheep that three single weekly intramuscular maternal injections of 500 µg.kg−1 betamethasone starting at 104 days gestation (dGA, term 150 days) lead to increased basal cord levels of ACTH at term. However, this dose was higher than the clinically 2×8–12mg betamethasone 24h apart (equivalent to 2×110–170 µg/kg weight adjusted to a 70 kg woman). Generally, the potential of prenatal synthetic glucocorticoid exposure to alter the HPAA responsiveness permanently appears to vary as a function of dose and timing as has been shown in rats, guinea pigs and sheep10,34,35. Better appreciation of these relations is necessary to understand programming effects of prenatal glucocorticoids on HPAA function.
Although modulation of fetal HPAA function by various glucocorticoid interventions has been examined in response to hypoxemia and CRH stimulation before 36–39 we know of no studies in which fetal HPAA response to hypotension modulated by a clinically relevant glucocorticoid administration has been examined. We hypothesized that prior exposure to single course of betamethasone at the time, dose and route of clinical administration would increase fetal HPAA sensitivity to a hypotensive challenge40 and repetition of betamethasone exposure enhances the effects. Fetal exposure to hypotension is a clinically relevant challenge to fetuses of high-risk pregnancies41 likely to affect fetuses who experienced prior exposure to betamethasone. We studied fetal sheep since they have a neuroendocrine developmental profile similar to humans42. Importantly, maternal administration of betamethasone to accelerate fetal lung maturation was developed in this animal model 43.
MATERIAL AND METHODS
Animal care and surgical instrumentation
All procedures were approved by the Cornell University Animal Use and Care Committee. Rambouillet-Colombia ewes of known gestational age were acclimated to the animal facilities for at least five days before surgery and kept in rooms with controlled light/dark cycles (14 h light/10 h dark: lights off at 2100 and lights on at 0700) and fasted 24 h before surgery. At 101 ± 1 (mean ± SEM) days gestation (dGA), ewes were sedated with intramuscular ketamine (Ketaflo, Abbott, Abbott Park, IL). General anesthesia was provided with 1 to 2.5% isoflurane (Isoflo, Abbott). Ewes were instrumented with a polyvinyl carotid arterial catheter to record fetal blood pressure (FBP) and jugular vein catheter for administration of drugs. Following laparotomy and hysterotomy fetuses were instrumented with catheters in the left carotid artery and jugular vein, with their tips in the ascending aorta and superior vena cava, respectively. An amniotic cavity catheter was placed to correct FBP. The abdomen was closed and catheters maintained patent via infusion of heparinized saline (12.5 UI/ml - 0.5 ml/h). Ewes received 0.5 g ampicillin (AMP-Equine; Pfizer Animal Health, New York, NY) i.v. and 0.5g ampicillin into the amniotic cavity 12 hourly for three days and phenylbutazone orally (Phenylzone paste, Schering-Plough, Kenilworth, NJ) 0.5 g twice daily for three days for post-operative analgesia.
Experimental protocol
Pregnant sheep were randomized to saline (n=6) and betamethasone treated (n=6) groups following at least three days recovery. They received two betamethasone courses or equivalent volume of saline intramuscularly at 106 and 107 dGA and 112 and 113 dGA. Each course of betamethasone consisted of two doses of 110 µg/kg betamethasone phosphate (Celestan solubile, Essex, Munich, Germany) 24 h apart or an equal volume saline. Average maternal weights in the control group were 52.3±2.23 kg and in the betamethasone group 54.5±2.0 kg. Average betamethasone dose was 6.0±0.22g. Fetal HPAA activity was examined in response to three hypotensive challenges: 105 dGA, i.e. before the first course of treatment, at 111 dGA, i.e. four days after the first and before the second course of treatment and at 127 dGA, i.e. 14 days after the second course of treatment. FBP and fetal amniotic pressure were recorded continuously. Pressures were monitored using calibrated pressure transducers (Cobe, Lakewood, CO). FBP was corrected for the amniotic pressure and mean FBP calculated.
Hypotensive challenge
The hypotensive challenge was induced by fetal jugular vein infusion of sodium nitroprusside (SNP - 10 µg/ml, Sigma-Aldrich, Deisenhofen, Germany) beginning at 0.1 ml/min. The SNP infusion rate was increased stepwise from 0.2 ml/min to 3.2 ml/min by doubling the infusion rate every 2 min. Fetal and maternal arterial blood samples were taken prior to the stress responses for measurement of blood gases, hemoglobin concentration and oxygen saturation using a blood gas analyzer (ABL600, Radiometer, Copenhagen, Denmark; measurements corrected to 39 °C) and a hemoximeter (OSM2, Radiometer). Plasma ACTH and cortisol were determined 30 min before, at the end (0 min), and 15 min, 60 min and 120 min after the end of the SNP infusion.1 ml blood was collected in chilled EDTA tubes, plasma separated by centrifugation at 4 °C for 10 min at 3.000 g, flash frozen and stored at −80 °C.
Hormone analyses
Fetal plasma cortisol was measured the Coat-A-Count radioimmunoassay kit (Diagnostic Products, Los Angeles, CA) with a sensitivity of 5.5 nmol/L. For plasma pools measuring 27.60 nmol/L and 138 nmol/L, intra-assay coefficients of variation (CV) were 4.14 % and 3.3 % respectively. Fetal ACTH was measured by chemiluminescence enzyme immunometric assay (DPC Immulite assay, Diagnostic Products). Assay sensitivity was 1.98 pmol/L. All samples were measured in one assay. For samples measuring 4.38 pmol/L and 46.48 pmol/L, intra-assay CVs were 4.75 % and 3.74 % respectively. ACTH:cortisol ratio was calculated to examine relative pituitary and adrenal responses to hypotension.
Data acquisition and statistical analysis
FBP and amniotic pressure were amplified and digitized using a 16-bit-analog-digital interface card and a data acquisition system (Windaq, DATAQ Instr., Akron, OH) at a sample rate of 256 Hz and stored on a hard disc of a PC. Baseline FBP was averaged over 10 min before the hypotensive challenge.
The integrated FBP decrease (FBPint) was used as the index of the challenge to the fetal HPAA and estimated using the area under the curve with the formula:
Changes of hormone values from baseline and treatment differences within the respective hypotensive challenges were tested with two-way ANOVA for repeated measures and Student-Newman-Keuls post-hoc test. Differences in blood gases, FBP and hormone values between the gestational ages were also tested using two-way ANOVA for repeated measures and the Student-Newman-Keuls post-hoc test. Results are given as mean ± S.E.M and only p values < 0.05 considered significant.
RESULTS
Maternal and fetal arterial blood gases, pH and Hb were in the physiological range at the beginning of the repeated hypotensive challenges and did not differ between the groups throughout the study (Table 1). Basal FBP was not different between control and betamethasone treated fetuses at all gestational ages (Table 1). As a measure of cardiovascular development44, basal FBP increased with gestational age in both groups (Table 1). All fetuses were alive and well at the end of the study.
| baseline 105 dGA |
5 days after the 1st treatment 111 dGA |
14 days after the 2nd treatment 127 dGA |
||||
|---|---|---|---|---|---|---|
| vehicle | betamethasone | vehicle | betamethasone | vehicle | betamethasone | |
| Fetal | ||||||
| FBP (mmHg) | 38 ± 1.2 | 37 ± 1.3 | 40 ± 1.0 | 37 ± 0.8 | 44 ± 1.4 * | 43 ± 1.8 * |
| pH | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.35 ± 0.01 | 7.36 ± 0.03 | 7.36 ± 0.01 | 7.37 ± 0.01 |
| pCO2 (mmHg) | 46.3 ± 1.4 | 48.4 ± 0.9 | 49.0 ± 1.6 | 47.8 ± 1.7 | 48.8 ± 1.5 | 47.9 ± 1.3 |
| pO2 (mmHg) | 27.8 ± 1.0 | 26.6 ± 1.1 | 24.7 ± 1.3 | 24.9 ± 0.6 | 24.0 ± 1.2 | 27.7 ± 1.8 |
| Hb (mg dl−1) | 8.0 ± 0.3 | 8.8 ± 0.2 | 8.0 ± 0.3 | 7.7 ± 0.3 | 9.6 ± 0.4 | 8.7 ± 0.2 |
| O2 sat (%) | 75.6 ± 1.3 | 78.0 ± 2.8 | 76.4 ± 6.6 | 80.5 ± 1.9 | 74.5 ± 2.4 | 80.5 ± 2.3 |
| Maternal | ||||||
| pH | 7.46 ± 0.02 | 7.48 ± 0.01 | 7.44 ± 0.01 | 7.45 ± 0.01 | 7.46 ± 0.02 # | 7.50 ± 0.01 |
| pCO2 (mmHg) | 32.0 ± 1.9 | 34.1 ± 0.9 | 31.6 ± 3.4 | 33.2 ± 1.4 | 31.5 ± 2.1 | 34.6 ± 1.0 |
| pO2 (mmHg) | 123.0 ± 6.0 | 111.1 ± 5.5 | 117.5 ± 7.2 | 111.8 ± 2.7 | 119.0 ± 4.5 | 112.9 ± 2.3 |
| Hb (mg dl−1) | 8.5 ± 0.7 | 9.7 ± 0.5 | 8.9 ± 0.4 | 8.7 ± 0.2 | 9.4 ± 0.4 | 9.0 ± 0.3 |
| O2 sat (%) | 96.2 ± 0.6 | 97.9 ± 0.6 | 97.9 ± 0.7 | 97.9 ± 0.4 | 98.3 ± 0.7 | 98.3 ± 0.3 |
Exposure to SNP produced a prompt decrease in FBP that returned to baseline levels within 15 min of cessation of infusion, i.e. before the next ACTH and cortisol measurement). FBPint was similar in the control and betamethasone treated groups at baseline (105 dGA; 56102 ± 2425 vs. 57286, ± 3148 mmHg*min) and 14 days after the second treatment course (127 dGA, 61378 ± 1762 vs. 55126 ± 3186 mmHg*min). However, at 111 dGA, i.e. four days after the first betamethasone course, FBPint was lower in betamethasone treated fetuses compared to controls (59406 ± 2557vs. 45068 ± 3613 mmHg*min; p<0.05).
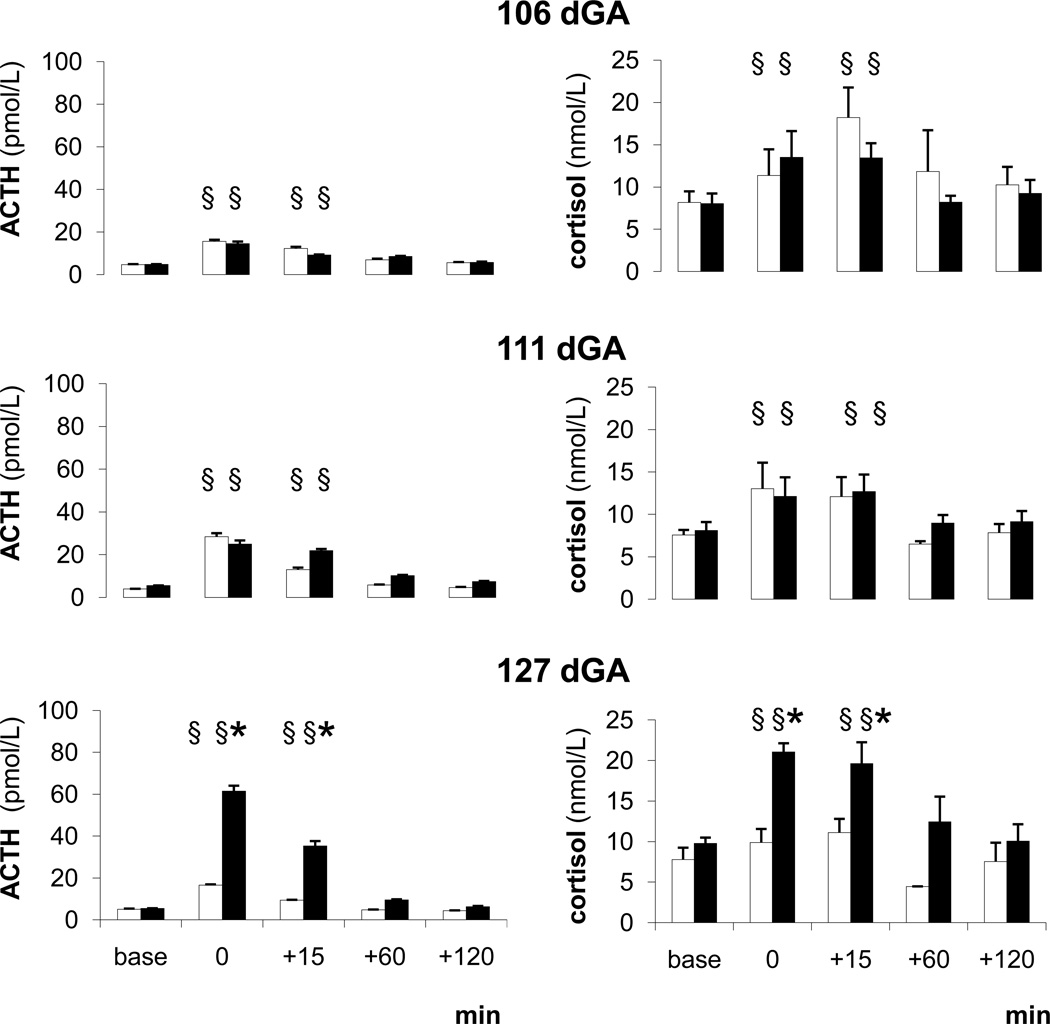
Basal plasma ACTH and cortisol concentrations were similar between the two groups and across all ages studied (Fig. 1). ACTH and cortisol responses to hypotension at 105 dGA, i.e. before betamethasone treatment, and at 111 dGA, i.e. four days after the first betamethasone course, were similar in the two groups (Fig. 1). At 127 dGA, i.e. 14 days after the second treatment course, both ACTH and cortisol responses to hypotension were higher in betamethasone exposed fetuses than controls (Fig. 1). Responses of betamethasone exposed fetuses at 127 dGA were also more pronounced than in the same group at 105 dGA (Fig. 1).
Figure 1. Fetal ACTH and cortisol responses.
ACTH and cortisol responses to the hypotensive challenges in the saline (white) and betamethasone (black) treated fetuses at 105, 111 and 127 dGA
Mean ± SEM, § P < 0.05 compared to baseline, * P < 0.05 compared to controls.
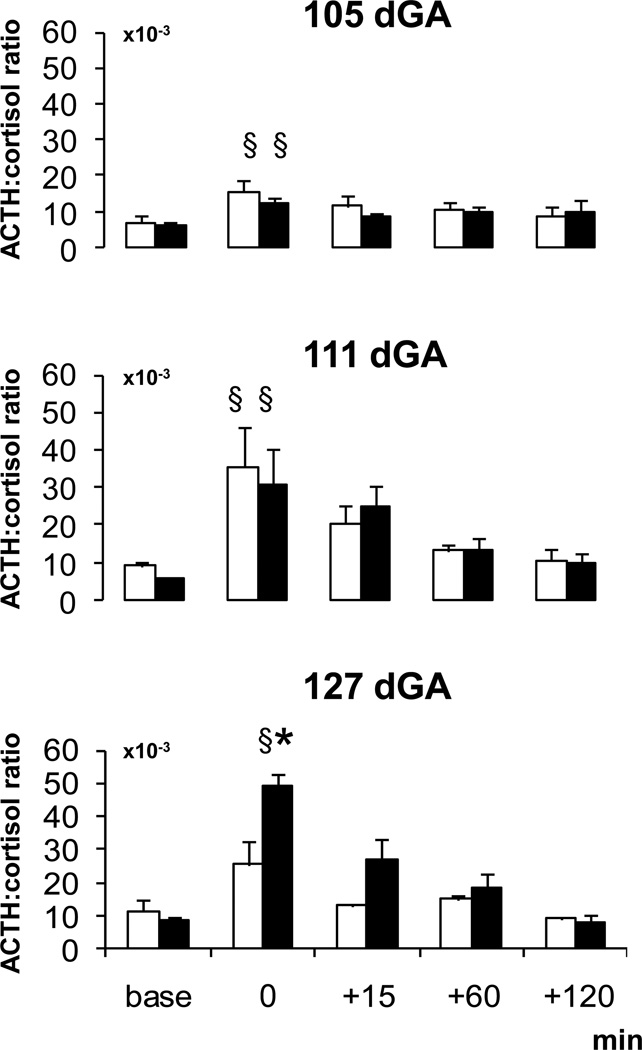
The basal ACTH:cortisol ratio did not differ with age or group (Fig. 2). During the hypotensive challenges, the ACTH:cortisol ratio increased in both groups and at all gestational ages compared to baseline (Fig. 2). At 127 dGA, the increase of the ACTH:cortisol ratio during the hypotensive challenge was greater in betamethasone exposed than control fetuses (Fig. 2) paralleling the increased ACTH and cortisol response in betamethasone exposed fetuses (Fig. 1).
Figure 2. ACTH:cortisol ratio.
ACTH:cortisol ratio during the hypotensive challenges in the saline (white) and betamethasone (black) treated fetuses at 105, 111 and 127 dGA
Mean ± SEM, § P < 0.05 compared to baseline, * P < 0.05 compared to controls.
COMMENT
Our findings show that betamethasone at doses used clinically to enhance fetal lung maturation in mothers threatening premature labor has persistent effects on fetal HPAA sensitivity and stress response to fetal hypotension. Two courses of betamethasone at the beginning of the last third of gestation augmented the fetal stress response in sheep two weeks later suggesting a glucocorticoid mediated persistent HPAA re-setting towards a higher sensitivity. The increased release of ACTH and cortisol in betamethasone exposed fetuses in response to the hypotensive challenge was accompanied by an increased ACTH:cortisol ratio indicative of a primary betamethasone effect on central HPAA mechanisms. Since the cortisol increase induced by our hypotensive challenge was only about 20% the maximum cortisol output expected at this gestational age45, i.e. far below the adrenal secretory maximum, the ACTH:cortisol ratio indicates that betamethasone treatment primarily enhances pituitary rather adrenal activity.
The developmental increase in ACTH:cortisol ratio independently of treatment group suggests primary HPAA maturation at the hippocampal, hypothalamic and/or pituitary level. Indeed, the increase in ACTH precedes that of cortisol during prepartum maturation of the HPA axis that starts in sheep at approximately 120 dGA42,46. The relative adrenal hypo-responsiveness is in good agreement with the well-known decreased fetal adrenal sensitivity to ACTH at the gestational stage examined47. The ovine fetal adrenals undergo a growth period at 40–90 dGA when they are capable of secreting cortisol47,48, followed by relative quiescence between 90–120 dGA after which adrenal sensitivity starts to increase to a maximum at term47,49.
Our results show that basal ACTH and cortisol levels do not reflect the level of HPAA responsiveness and feedback. Thus, basal ACTH and cortisol levels did not differ between the controls and the betamethasone treated fetuses in spite of the altered responsiveness of the HPAA. Similarly, Sloboda et al.33 did not find elevated basal plasma ACTH and cortisol levels at 125 dGA after three weekly betamethasone injections of 500 µg/kg starting at the same age as our study. The increased HPAA responsiveness in the face of unchanged basal ACTH and cortisol levels reflects alteration of the set point of the reactivity of the axis rather than early maturation of the complete HPAA. Sensitization of the HPAA by betamethasone may indicate both an increased activity of neuroendocrine systems mediating the response to hypotensive stress and/or a glucocorticoid mediated re-setting of the HPAA towards lower negative feedback sensitivity.
Pretreatment with a single course of betamethasone four days before the hypotensive challenge had no effect on the fetal hypotensive stress response, while pre-treatment with two courses of betamethasone one week apart enhanced cortisol and ACTH responses to the hypotensive challenge 14 days later. Theoretically, this difference may be the result of the number of preceding betamethasone treatments, effect of the gestational age when betamethasone treatment (106 versus 112 dGA) was performed, effect of the gestational age when the stress response (111 versus 127 dGA) was performed, or effect of the prolonged time period between the betamethasone pretreatment and the stress challenge (four versus 14 days). It is unlikely that the gestational age at treatment played a major role because both gestational ages are in the period of relative quiescence (90–120 days of gestation) of fetal HPAA function47,49. Baseline plasma ACTH and cortisol concentrations and the responsiveness of the HPAA to hypotension did not differ between 105 and 111 dGA. The similar HPAA response in the control and betamethasone treated fetuses at 111 dGA occurred even in the presence of a slightly lower FBPint in the betamethasone treated fetuses further illustrating the relative quiescence of the HPAA at this age. The endogenous surge of cortisol as a measure of pre-partum HPAA maturation42 and developmental increase in adrenal sensitivity starts at approximately 120 dGA and does not reach its maximum before term 46,47,49. In agreement with that, baseline and peak plasma cortisol and ACTH levels did not differ between 111 and 127 dGA in the control group. We have also shown that the number of glucocorticoid receptors does not change between 110 and 130 dGA in the fetal ovine brain 50. However, we cannot exclude receptor maturation completely because there may have been an increase in the binding of the steroid to the receptor and or steroid receptor complex binding to DNA acceptor sites. Taken together, the extent and nature of the alteration of the stress response appear to result from the latency following fetal betamethasone exposure which agrees with the findings of Sloboda et al.33 who found no change in fetal basal ACTH and cortisol levels at 125 dGA but elevated levels at 146 dGA following three weekly betamethasone injections starting at 104 dGA.
The gradual FBP increase observed over the experimental period reflects cardiovascular maturation44. Betamethasone treatment did not affect maturation of basal cardiovascular nor did it induce major differences of NO stimulated vasodilatation. The absent effects on fetal cardiovascular maturation are independent of the acute but transient increase of FBP following BM treatment for about 24 hours51. 52 HPAA stimulation by sodium nitroprusside during the hypotensive challenge is possibly a mixed stimulus to fetal ACTH secretion since NO itself can modulate medullary and hypothalamic function52,53. Since this affects both control and the betamethasone treated fetuses, it is unlikely to be responsible for differences between the groups.
While antenatal glucocorticoid therapy has unquestionable benefits such as lung maturation and cardiovascular adaptation on postnatal requirements that are due to acute cardiovascular glucocorticoid effects54, there remain many unanswered questions on the cost-benefit ratio and the precise nature of potential harm to the developing fetus and offspring, especially in relation to the dose and timing of maternal glucocorticoid exposure as well as the time and nature of HPAA stimulation following previous betamethasone exposure. Apart from the fetal HPAA hyper-responsiveness to a hypotensive challenge following betamethasone treatment at the dose used clinically shown here, a single injection of 500µg/kg betamethasone to pregnant sheep at 104 dGA but not repeated injections increased the ACTH and cortisol responses to CRH+AVP at 6 months postnatal age34,. No effect was found at 1 yr of age34. Repeated but not single maternal betamethasone injections elevated ACTH responses to CRH+AVP at 2 years of age and suppressed cortisol responses at 3 years of age55. While the evidence from long-term problems resulting from human fetal exposure is limited, it does exist. Apart from altered HPAA activity, various adverse outcomes 55 have been reported in offspring exposed to synthetic glucocorticoids. These include an increased risk of postnatal aggressive/destructive behavior, increased distractibility and hyperactivity, blunted response to cardiovascular stress, and inhibited neuromotor development and behavioral performance 56–59. However, not all findings support these negative outcomes60–62.
In summary, the present study demonstrates that prenatal betamethasone exposure at the dose used clinically results in altered development of HPAA function and has a persistent effect in augmenting the HPAA response to a hypotensive challenge during fetal life. This enhanced response may be due to increased neuroendocrine activity and/or decreased negative feedback regulation and is not reflected in basal ACTH and cortisol levels. The latency in emergence of fetal HPAA hyper-responsiveness following fetal betamethasone exposure is potentially the most important factor for the development of the increased fetal HPAA sensitivity and may explain hyper-responsiveness in full-term but not preterm neonates. Further studies are needed to examine whether the re-setting of the HPAA depends on a critical time window of exposure or repetition of glucocorticoid treatment and to understand the dynamics of changes in HPAA sensitivity during development and in later life following the clinical dose of betamethasone.
Acknowledgements
We are grateful to Dr. Xiu-Ying Ding for expert surgical assistance, Sue Jenkins for statistical analysis, Stella Vincent for hormone analysis, and Jill McDonald, Daemon Ferguson and Kate Franko for animal care.
This work was supported by the Max Kade Foundation and HL068649.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conducted in Ithaca, NY, USA
DISCLOSURE: The authors report no conflict of interest.
References
- 1.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010 Jan;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antenatal corticosteroids revisited: repeat courses - National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol. 2001 Jul;98(1):144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- 3.Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, Wallace EM. Patterns of antenatal corticosteroid prescribing 1998–2004. Aust N Z J Obstet Gynaecol. 2007 Feb;47(1):42–45. doi: 10.1111/j.1479-828X.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- 4.Kari MA, Raivio KO, Stenman UH, Voutilainen R. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res. 1996 Aug;40(2):319–324. doi: 10.1203/00006450-199608000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003 Nov;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 6.Banks BA, Cnaan A, Morgan MA, et al. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. Am J Obstet Gynecol. 1999 Sep;181(3):709–717. doi: 10.1016/s0002-9378(99)70517-x. [DOI] [PubMed] [Google Scholar]
- 7.Guinn DA. Repeat courses of antenatal corticosteroids: the controversy continues. Am J Obstet Gynecol. 2004 Mar;190(3):585–587. doi: 10.1016/j.ajog.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct;50(4):515–525. [PubMed] [Google Scholar]
- 9.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005 May 28–Jun 3;365(9474):1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 10.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001 Feb;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002 Nov;13(9):373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 12.Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004 Jul 1;558(Pt 1):305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006 Apr 1;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010 Sep;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996 Dec;64(6):412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 16.Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin Exp Pharmacol Physiol. 2001 Nov;28(11):938–941. doi: 10.1046/j.1440-1681.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 17.Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol. 2001 Nov;28(11):957–961. doi: 10.1046/j.1440-1681.2001.03559.x. [DOI] [PubMed] [Google Scholar]
- 18.Bertram CE, Hanson MA. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction. 2002 Oct;124(4):459–467. doi: 10.1530/rep.0.1240459. [DOI] [PubMed] [Google Scholar]
- 19.Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005 Apr;29(2):209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008 Sep;22(5):438–466. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 21.Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens. 2009 Mar;18(2):144–152. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006 Sep;195(3):633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 24.Dorr HG, Versmold HT, Sippell WG, Bidlingmaier F, Knorr D. Antenatal betamethasone therapy: effects on maternal, fetal, and neonatal mineralocorticoids, glucocorticoids, and progestins. J Pediatr. 1986 Jun;108(6):990–993. doi: 10.1016/s0022-3476(86)80946-5. [DOI] [PubMed] [Google Scholar]
- 25.Parker CR, Jr, Atkinson MW, Owen J, Andrews WW. Dynamics of the fetal adrenal, cholesterol, and apolipoprotein B responses to antenatal betamethasone therapy. Am J Obstet Gynecol. 1996 Feb;174(2):562–565. doi: 10.1016/s0002-9378(96)70428-3. [DOI] [PubMed] [Google Scholar]
- 26.Davis EP, Townsend EL, Gunnar MR, et al. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology. 2004 Sep;29(8):1028–1036. doi: 10.1016/j.psyneuen.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Battin MR, Bevan C, Harding JE. Repeat doses of antenatal steroids and hypothalamic-pituitary-adrenal axis (HPA) function. Am J Obstet Gynecol. 2007 Jul;197(1):40 e41–46 e46. doi: 10.1016/j.ajog.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Davis EP, Townsend EL, Gunnar MR, et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J Perinatol. 2006 Mar;26(3):147–153. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 29.Ashwood PJ, Crowther CA, Willson KJ, et al. Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. Am J Obstet Gynecol. 2006 Mar;194(3):861–867. doi: 10.1016/j.ajog.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 30.McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001 May;13(5):425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- 31.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005 Apr;29(2):227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Developmental psychobiology. 2011 Mar;53(2):175–183. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloboda DM, Newnham JP, Challis JR. Effects of repeated maternal betamethasone administration on growth and hypothalamic-pituitary-adrenal function of the ovine fetus at term. J Endocrinol. 2000 Apr;165(1):79–91. doi: 10.1677/joe.0.1650079. [DOI] [PubMed] [Google Scholar]
- 34.Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002 Jan;172(1):71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008 Dec;149(12):6406–6415. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- 36.McDonald TJ, Hoffmann GE, Myers DA, Nathanielsz PW. Hypothalamic glucocorticoid implants prevent fetal ovine adrenocorticotropin secretion in response to stress. Endocrinology. 1990 Dec;127(6):2862–2868. doi: 10.1210/endo-127-6-2862. [DOI] [PubMed] [Google Scholar]
- 37.Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol. 1995 Jun;268(6 Pt 1):E1096–E1107. doi: 10.1152/ajpendo.1995.268.6.E1096. [DOI] [PubMed] [Google Scholar]
- 38.Jellyman JK, Gardner DS, McGarrigle HH, Fowden AL, Giussani DA. Pituitary-adrenal responses to acute hypoxemia during and after maternal dexamethasone treatment in sheep. Pediatr Res. 2004 Dec;56(6):864–872. doi: 10.1203/01.PDR.0000145253.92052.60. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher AJ, Ma XH, Wu WX, et al. Antenatal glucocorticoids reset the level of baseline and hypoxemia-induced pituitary-adrenal activity in the sheep fetus during late gestation. Am J Physiol Endocrinol Metab. 2004 Feb;286(2):E311–E319. doi: 10.1152/ajpendo.00158.2003. [DOI] [PubMed] [Google Scholar]
- 40.Cuthrell WV, Rose JC, Meis PJ. The effect of adrenocorticotropic hormone infusion on subsequent pituitary response in the sheep fetus. American journal of obstetrics and gynecology. 1990 Jul;163(1 Pt 1):170–174. doi: 10.1016/s0002-9378(11)90693-0. [DOI] [PubMed] [Google Scholar]
- 41.Low JA. Determining the contribution of asphyxia to brain damage in the neonate. J Obstet Gynaecol Res. 2004 Aug;30(4):276–286. doi: 10.1111/j.1447-0756.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 42.Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocr Rev. 1989 May;10(2):182–204. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- 43.Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969 Dec;45(4):515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 44.Unno N, Wong CH, Jenkins SL, et al. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am J Physiol. 1999 Jan;276(1 Pt 2):H248–H256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- 45.Lye SJ, Sprague CL, Mitchell BF, Challis JR. Activation of ovine fetal adrenal function by pulsatile or continuous administration of adrenocorticotropin-(1-24). I. Effects on fetal plasma corticosteroids. Endocrinology. 1983 Aug;113(2):770–776. doi: 10.1210/endo-113-2-770. [DOI] [PubMed] [Google Scholar]
- 46.Magyar DM, Fridshal D, Elsner CW, et al. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980 Jul;107(1):155–159. doi: 10.1210/endo-107-1-155. [DOI] [PubMed] [Google Scholar]
- 47.Wintour EM, Crawford R, McFarlane A, Moritz K, Tangalakis K. Regulation and function of the fetal adrenal gland in sheep. Endocrine research. 1995 Feb–May;21(1–2):81–89. doi: 10.3109/07435809509030423. [DOI] [PubMed] [Google Scholar]
- 48.Boshier DP, Holloway H. Morphometric analyses of adrenal gland growth in fetal and neonatal sheep. I. The adrenal cortex. Journal of anatomy. 1989 Dec;167:1–14. [PMC free article] [PubMed] [Google Scholar]
- 49.Rose JC, Morris M, Meis PJ. Developmental aspects of pituitary and adrenal responses to arterial hypotension in neonatal, weanling, and adult sheep. Am J Physiol. 1982 Apr;242(4):E215–E219. doi: 10.1152/ajpendo.1982.242.4.E215. [DOI] [PubMed] [Google Scholar]
- 50.Brodhun M, Coksaygan T, Antonow-Schlorke I, et al. Acute effects of antenatal betamethasone treatment on glucocorticoid receptor density in fetal sheep brain. Acta Neuropathologica. 2003 Oct;106(4):392–392. [Google Scholar]
- 51.Schwab M, Coksaygan T, Nathanielsz PW. Betamethasone effects on ovine uterine and umbilical placental perfusion at the dose used to enhance fetal lung maturation. Am J Obstet Gynecol. 2006 Feb;194(2):572–579. doi: 10.1016/j.ajog.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 52.Rivier C. Role of nitric oxide in regulating the rat hypothalamic-pituitary-adrenal axis response to endotoxemia. Ann N Y Acad Sci. 2003 May;992:72–85. doi: 10.1111/j.1749-6632.2003.tb03139.x. [DOI] [PubMed] [Google Scholar]
- 53.Dias AC, Vitela M, Colombari E, Mifflin SW. Nitric oxide modulation of glutamatergic, baroreflex, and cardiopulmonary transmission in the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H256–H262. doi: 10.1152/ajpheart.01149.2003. [DOI] [PubMed] [Google Scholar]
- 54.Stein HM, Martinez A, Blount L, Oyama K, Padbury JF. The effects of corticosteroids and thyrotropin-releasing hormone on newborn adaptation and sympathoadrenal mechanisms in preterm sheep. Am J Obstet Gynecol. 1994 Jul;171(1):17–24. doi: 10.1016/s0002-9378(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 55.Sloboda DM, Moss TJ, Li S, et al. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. American journal of physiology. Endocrinology and metabolism. 2007 Jan;292(1):E61–E70. doi: 10.1152/ajpendo.00270.2006. [DOI] [PubMed] [Google Scholar]
- 56.French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004 Mar;190(3):588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 57.Karemaker R, Kavelaars A, ter Wolbeek M, et al. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008 Apr;121(4):e870–e878. doi: 10.1542/peds.2007-2454. [DOI] [PubMed] [Google Scholar]
- 58.Karemaker R, Heijnen CJ, Veen S, et al. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr Res. 2006 Dec;60(6):745–750. doi: 10.1203/01.pdr.0000246200.76860.de. [DOI] [PubMed] [Google Scholar]
- 59.Schwab M, Thoms I, Schwab K, et al. Effects of prenatal betamathasone exposure on autonomic function and cortical activity at the age of eight years – a pilot study. J Devel Orig Health Dis. 2009;1(1 Suppl):S 17. [Google Scholar]
- 60.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007 Sep 20;357(12):1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 61.Peltoniemi OM, Kari MA, Lano A, et al. Two-year follow-up of a randomised trial with repeated antenatal betamethasone. Arch Dis Child Fetal Neonatal Ed. 2009 Nov;94(6):F402–F406. doi: 10.1136/adc.2008.150250. [DOI] [PubMed] [Google Scholar]
- 62.Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005 Sep 24;331(7518):665. doi: 10.1136/bmj.38576.494363.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]