Abstract
Decreased expression of interleukin (IL)-7 receptor α (CD127) on CD8+ T cells in progressive HIV disease suggests a role for CD127 regulation in HIV immunopathogenesis. The direct effect of HIV on CD127 expression has not been explored to explain these in vivo findings. Peripheral blood mononuclear cells (PBMCs) or isolated CD8+ T cells from healthy individuals were cultured with either X4 (HIV-1IIIB), R5 (HIV-1BaL), dual tropic (HIV-1CS204), or replication-incompetent (HIV8E5) strains of HIV. Both X4 and R5 strains transiently decreased CD127 expression on CD8+ T cells in PBMC cultures but had no effect on isolated CD8+ T cell cultures. Isolated CD8+ T cells exposed to either (1) PBMCs incubated with HIV and cultured in a transwell or (2) supernatants from PBMCs incubated with HIV resulted in decreased CD127 expression. Under no conditions did the replication-incompetent HIV strain affect CD127 expression. As observed in vivo, infection of PBMCs with HIV in vitro results in the downregulation of CD127 surface expression on CD8+ T cells. Collectively, these data indicate that soluble factor(s) released as a result of HIV infection regulate CD127 expression. Further elucidation of the mechanism(s) of CD127 downregulation will provide important insights into the immunopathogenesis of HIV disease.
Altered expression and activity of CD127 have been observed in several human diseases, including latent Epstein–Barr virus (EBV) and cytomegalovirus (CMV), and chronic HIV and hepatitis C virus (HCV) infection, the implications of which have yet to be fully understood.1–3 In HIV infection, we and others have demonstrated that CD127 is expressed on a lower proportion of CD4+ and CD8+ T cells compared to that observed in HIV-uninfected individuals.2,4 Interleukin (IL)-7 activity is important for T cell development, homeostasis, memory development, and T cell effector functions. Despite the increase in IL-7 in HIV disease,5 cytolytic T cell activity (CTL) becomes impaired with disease progression. We and others have shown that a decrease in IL-7 responsiveness in T cells is also a feature of progressive disease.6,7 The loss of CD127 expression has also been correlated with the expansion of effector CD8+ T cells with cytolytic activity during chronic HIV infection.1 Impaired CD4+ and CD8+ T cell function and decreased CD127 expression have recently been linked to abnormal activation of the immune system in HIV+ individuals and resulting immunodeficiency.2
Expression of CD127 on both naive and memory CD8+ and CD4+ T cells has recently been shown to be inversely correlated with CD8+ T cell exhaustion in the context of persistent exposure to antigen2 and the expansion of effector CD8+ T cells during chronic HIV infection.8 The cellular mechanism linking antigen persistence to decreased CD127 expression is not known. We and others have shown that IL-7 decreases CD127 expression on CD8+ T cells.9,10 This indicates a biological process to explain correlations of increased plasma IL-7 with decreased CD127 in HIV patients.11 The recent observation that HIV tat, to the exclusion of other HIV and non-HIV proteins, decreases CD127 on CD8+ T cells12 suggests a degree of pathogen specificity and that CD127 downregulation is not simply a nonspecific response to immune activation. Although in vitro infection has been used to study mechanisms of HIV-induced alterations of cytokine and cytokine receptor expression, its effect on CD127 expression has not been investigated. Here we begin to examine the mechanisms by which HIV decreases CD127 expression on CD8+ T cells and thereby understand the downregulation that has been observed in in vivo HIV infection.
To first determine if in vitro HIV-1 infection results in a CD127 downregulation on CD8+ T cells, peripheral blood mononuclear cells (PBMCs) (106 cells/ml) from HIV− individuals were incubated with various strains of HIV-1: dual tropic (HIV-1CS204), X4 tropic (HIV-1IIIB), R5 tropic (HIV-1Ba-L), and replication-incompetent (HIV-18e5) (supernatant from 8e5 cells). Briefly, PBMCs were pretreated with 2 μg/ml of polybrene (Sigma-Aldrich) for 1 h at 37°C, 5% CO2 and washed in phosphate-buffered saline (PBS). Cells were then incubated with HIV (MOI of 0.01), supernatants from 8e5 cells containing HIV-1 virions defective in reverse-transcriptase activity (10 ng HIV-1 p24/ml), or mock infected with equivalent volumes of PBMC culture supernatant for 3–4 hours at 37°C, 5% CO2 and washed. Cells were then cultured (106 cells/ml) for 96 h and CD127 expression on CD8+ T cells was measured every 24 h by flow cytometry using a Beckman Coulter ALTRA flow cytometer and the EXPO version 2.0 software package. The impact of a given stimulus on the expression of CD127 on CD8+ T cells was calculated as follows: [(% CD8+CD127+ T cells in the presence of stimulus)/(% CD8+CD127+ T cells in medium alone)]×100. One-way ANOVA analysis was used to compare virus-induced changes over time (relative to uninfected controls at each time point) followed by the Dunnett's posttest, with p<0.05 considered statistically significant (SigmaStat 3.0, Ashburn, VA).
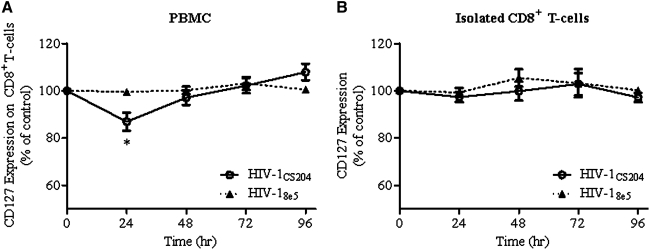
We found that infection of PBMCs with all HIV strains resulted in a decrease in CD127 expression on CD8+ T cells. As illustrated with HIV-1CS204 (Fig. 1A), CD127 expression was significantly lower after 24 h of culture (p=0.003 by ANOVA and p<0.05 by Dunnett's pairwise test versus baseline). This decrease was transient, as CD127 levels returned to baseline after 48 h. Receptor expression returned to levels observed in mock-infected cells by 96 h. Infection of PBMCs with the replication-incompetent HIV-18e5 did not alter CD127 expression on CD8+ T cells suggesting that active viral replication is required for the downregulation of CD127 (Fig. 1A). The regulation of CD127 expression by host cytokines altered in HIV infection (e.g. IL-7 and IL-4) has been investigated in vitro, and both IL-7 and IL-4 have been shown to decrease CD127 expression on CD8+ T cells.8,9,13 In the present study, the addition of anti-IL-7 or anti-IL-4 antibodies did not reverse the downregulation of CD127 observed on CD8+ T cells with HIV-infected PBMC cultures, nor was the concentrations of IL-7 or IL-4 in culture supernatants found to be elevated (data not shown).
FIG. 1.
In vitro HIV infection decreases CD127 expression in human peripheral blood mononuclear cells (PBMCs) but not in isolated CD8+ T cell cultures. PBMCs or isolated CD8+ T cells were exposed to HIV in vitro for 3–4 h, washed, and cultured for a further 72 h. The expression of CD127 on CD8+ T cells was measured by flow cytometry every 24 h. The expression of CD127 on CD8+ T cells over time was decreased in (A) PBMC cultures after 24 h (p=0.003 by one-way ANOVA and *p<0.05 by Dunnett's test versus mock-infected cells) but not in (B) isolated CD8+ T cells cultured with HIV-1CS204 (dual-tropic) strain. Data are shown as % expression compared to mock-infected cells (i.e., parallel, uninfected PBMC cultures) and depict the results of four (PBMC) or six (isolated CD8+ T cells) experiments. Cells infected with the replication-incompetent strain HIV-18e5 were also analyzed as a negative control. Statistical significance was determined by the one-way ANOVA and Dunnett's posttests (*p<0.05), comparing CD127 expression to mock-infected cells.
To determine if HIV directly decreases CD127 expression on CD8+ T cells isolated CD8+ T cells were cultured with each HIV-1 strain in parallel to the PBMC experiments described above. Isolation of CD8+ T cells was performed using MACS CD8 Microbeads (Miltenyi Biotec) following the manufacturer's instructions. In contrast to the results observed with PBMCs, no significant changes in surface CD127 expression were detected on isolated CD8+ T cells following incubation with HIV-1CS204 (Fig. 1B), HIV-1IIIB, or HIV-1Ba-L (data not shown). As above, replication-incompetent HIV-18e5 did not affect CD127 expression (Fig. 1B).
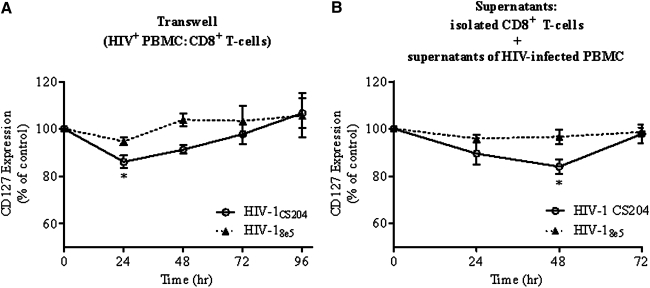
To determine if a soluble factor(s) resulting from in vitro HIV infection of PBMCs mediated the effect on CD127 expression, isolated CD8+ T cells were incubated in a transwell with autologous PBMCs. Incubation of PBMCs previously infected for 24 h with HIV-1CS204 in transwells with isolated CD8+ T cells resulted in the decrease of CD127 expression in the latter cells after 24 h (Fig. 2A, p=0.005 by ANOVA and p<0.05 by Dunnett's pairwise test versus baseline). Similar results were observed in cultures infected with HIV-1IIIB or HIV-1Ba-L strains (data not shown). After 96 h of transwell culture, CD127 expression on isolated CD8+ T cells returned to levels similar to what was observed when PBMCs were mock infected.
FIG. 2.
Soluble factors from in vitro HIV infection decrease the expression of CD127 on isolated CD8+ T cells. (A) Isolated CD8+ T cells were cultured in a transwell with PBMCs that were incubated with HIV-1CS204 for 24 h and then washed. Culture of PBMCs with isolated CD8+ T cells in a transwell continued for a further 72 h and CD127 expression was measured by flow cytometry every 24 h. Statistically significant decreases in CD127 expression were observed at 24 h (p=0.005 by one-way ANOVA and *p<0.05 by Dunnett's test versus mock-infected cells). (B) After an initial 24 h of culture, isolated CD8+ T cells were then cultured for 72 h in culture supernatant collected from PBMCs previously infected with HIV and cultured for 24 h. The expression of CD127 was measured by flow cytometry every 24 h. Cells were incubated with PBMCs or supernatants from PBMCs infected with the replication-incompetent strain HIV-18e5 as a negative control. Statistically significant changes were observed at 24 h (p=0.021 by ANOVA and *p<0.05 by Dunnett's test versus mock-infected cells). Data depict the results of four experiments.
Alternatively, PBMCs were cultured with HIV for 24 h and then their culture supernatants were collected and added to parallel cultures of isolated CD8+ T cells. Expression of CD127 was assessed every 24 h. Similar to the transwell experiments, CD8+ T cells cultured with supernatants from in vitro PBMCs infected by these same strains also showed a decrease in CD127 expression. There appeared to be a decrease by 24 h of incubation, but this did not reach statistical significance until the 48 h time point (p=0.021 by ANOVA and p<0.05 by Dunnett's pairwise test versus baseline) before returning to baseline levels after 72 h (Fig. 2B).
The downregulation of CD127 on CD8+ T cells by in vitro HIV infection appears to be due to the activity of soluble factor(s) present in HIV-infected PBMC cultures. Although these changes are modest, they are in the range of what has been reported with HIV-tat or low concentrations of IL-7, as shown in several reports.12,14 Similarly, the transient nature of CD127 downregulation has also been observed when CD8+ T cells are incubated with IL-7 or IL-4.8,9,13 The observation that HIV-tat, to the exclusion of other HIV proteins, decreases CD127 on CD8+ T cells suggests a degree of pathogen specificity.12 In the present experiments, the concentration of tat in virus-containing supernatants added to isolated CD8+ T cells may have been too low to have a pronounced effect on CD127 expression, but this may increase with viral replication in PBMCs. Analysis of supernatants collected from HIV-1CS204-infected PBMCs suggests that the decrease in CD127 expression was not due to increased IL-7 or IL-4 production. Host cytokines such as IL-7 or IL-4 may work in concert with one another, therefore inhibiting each individually may not reverse the effect of other mediators of CD127. Furthermore, the concentrations of IL-7 and IL-4 required to decrease CD127 expression in vitro exceed levels found in plasma.9,13 Similarly, the concentration of other cytokines in the IL-2R γ-chain sharing family (IL-2, −9, −15, −21), some of which have been shown to decrease CD127 expression,15 may be too low in vitro to alter CD127 expression.
As seen in vivo, in vitro HIV infection of PBMCs results in the downregulation of CD127 expression on CD8+ T cells. This effect was transient and appeared to require infection of cells in culture, as culturing of isolated CD8+ T cells with virus did not alter CD127 expression. The requirement for active viral replication is supported by the observation that when PBMCs were cultured with the replication-incompetent viral strain HIV8e5, CD127 expression was unaltered. In addition, this affect is mediated by soluble factor(s) present in the culture supernatants of PBMCs infected with HIV in vitro, though this does not appear to be due to known mediators of CD127 expression (i.e., low concentrations of IL-4, IL-7, or HIV-1 tat). Studies investigating other potential host or viral factors influencing this observed effect are ongoing. Lastly, this study suggests that the mechanisms involved in the downregulation of CD127 in HIV infection may include factors other than, or in addition to, IL-7 or IL-4. Further elucidation of these mechanisms will provide important insights into the immunopathogenesis of HIV disease.
Acknowledgments
A.K. performed the research, analyzed data, and helped with writing the article. A.M.C assisted in the data analysis and drafted and edited the article. J.B.A. assisted in the design of the research, data analysis, and drafted and edited the article. All authors read and approved the final version. This research was supported by grants from the Ontario HIV Treatment Network (OHTN) (Grant ROGB131), the Canadian Institutes of Health Research (Grant HOP84649), and the Canadian Federation of AIDS Research (Grant 019014). A.M.C. is a recipient of an OHTN fellowship and J.B.A. is an OHTN career scientist.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Boutboul F. Puthier D. Appay V, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19(17):1981–1986. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 2.Koesters SA. Alimonti JB. Wachihi C, et al. IL-7Ralpha expression on CD4(+) T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol. 2006;36(2):336–344. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- 3.Golden-Mason L. Burton JR., Jr Castelblanco N, et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44(5):1098–1109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 4.MacPherson PA. Fex C. Sanchez-Dardon J. Hawley-Foss N. Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(5):454–457. doi: 10.1097/00042560-200112150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano LA. Grant RM. Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: Implications for T-cell homeostasis. Nat Med. 2001;7(1):73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 6.Juffroy O. Bugault F. Lambotte O, et al. Dual mechanism of impairment of interleukin-7 (IL-7) responses in human immunodeficiency virus infection: Decreased IL-7 binding and abnormal activation of the JAK/STAT5 pathway. J Virol. 2010;84(1):96–108. doi: 10.1128/JVI.01475-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vranjkovic A. Crawley A. Patey A. Angel JB. IL-7-dependent STAT-5 activation and CD8+ T-cell proliferation are impaired in HIV infection. J Leuk Biol. 2011;89:499–506. doi: 10.1189/jlb.0710430. [DOI] [PubMed] [Google Scholar]
- 8.Paiardini M. Cervasi B. Albrecht H, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174(5):2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 9.Vranjkovic A. Crawley AM. Gee K. Kumar A. Angel JB. IL-7 decreases IL-7 receptor alpha (CD127) expression and induces the shedding of CD127 by human CD8+ T cells. Int Immunol. 2007;19(12):1329–1339. doi: 10.1093/intimm/dxm102. [DOI] [PubMed] [Google Scholar]
- 10.Colle JH. Moreau JL. Fontanet A, et al. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients—reversal by highly active anti-retroviral therapy (HAART) Clin Exp Immunol. 2006;143(3):398–403. doi: 10.1111/j.1365-2249.2006.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasson SC. Zaunders JJ. Zanetti G, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J Infect Dis. 2006;193(4):505–514. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 12.Faller EM. McVey MJ. Kakal JA. MacPherson PA. Interleukin-7 receptor expression on CD8 T-cells is downregulated by the HIV Tat protein. J Acquir Immune Defic Syndr. 2006;43(3):257–269. doi: 10.1097/01.qai.0000230319.78288.f4. [DOI] [PubMed] [Google Scholar]
- 13.Crawley AM. Vranjkovic A. Young C. Angel JB. Interleukin-4 downregulates CD127 expression and activity on human thymocytes and mature CD8(+) T cells. Eur J Immunol. 2011;40(5):1396–1407. doi: 10.1002/eji.200940093. [DOI] [PubMed] [Google Scholar]
- 14.Henriques CM. Rino J. Nibbs RJ. Graham GJ. Barata JT. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood. 2010;115(16):3269–3277. doi: 10.1182/blood-2009-10-246876. [DOI] [PubMed] [Google Scholar]
- 15.Park JH. Yu Q. Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]