Abstract
Integrin α2β1 mediates the binding of several epithelial and mesenchymal cell types to collagen. The composition of the surrounding plasma membrane, especially caveolin-1- and cholesterol-containing membrane structures called caveolae, may be important to integrin signaling. On cell surface α2β1 integrin was located in the raft like membrane domain, rich in GPI-anchored proteins, rather than in caveolae. However, when antibodies were used to generate clusters of α2β1 integrin, they started to move laterally on cell surface along actin filaments. During the lateral movement small clusters fused together. Finally α2β1 integrin was found inside caveolae and subsequently internalized into caveosome-like perinuclear structures. The internalization process, unlike cluster formation or lateral redistribution, was dependent on protein kinase Cα activity. Caveolae are known to be highly immobile structures and α2β1 integrin clusters represent a previously unknown mechanism to activate endocytic trafficking via caveolae. The process was specific to α2β1 integrin, because the antibody-mediated formation of αV integrin clusters activated their internalization in coated vesicles and early endosomes. In addition to natural ligands human echovirus-1 (EV1) gains entry into the cell by binding to α2β1 and taking advantage of α2β1 internalization via caveolae.
INTRODUCTION
Caveolae are cave-like invaginations of the cell surface (for reviews see Kurzchalia and Parton, 1999; Pelkmans and Helenius, 2002; Parton 2003). They have been described as highly immobile and not involved in constitutive endocytic trafficking (Thomsen et al., 2002). Their internalization can be activated, for example, by the phosphatase inhibitor okadaic acid (Parton et al., 1994). In endothelial cells the interaction of albumin docking protein gp60 and caveolin-1 initiates the endocytosis of caveolae (Minshall et al., 2000). Caveolins are membrane proteins that are molecular markers of caveolae and shown to be crucial for the formation of these structures (Fra et al., 1995). Caveolins can be associated with cell surface growth factor receptors (Couet et al., 1997) and cell adhesion receptors (Wary et al., 1996, 1998; Wei et al., 1999) and caveolae might therefore play a role in cellular signaling. In addition, caveolae have been suggested to participate in the uptake of folate by pinocytosis, but this is still under discussion (Parton, 2003). Caveolin-deficient mice have given novel insight into the function of caveolae. Caveolin-1 gene knockout leads to pulmonary defects, vasoconstriction, or dilatation abnormalities and resistance to diet-induced obesity (Drab et al., 2001; Razani et al., 2001; Schubert et al., 2001; Sotgia et al., 2002; Razani et al., 2002). Simian virus 40 (SV40) has been shown to be internalized through caveolae and detailed studies focused on the virus entry mechanism have produced a lot of new information about the biology of caveolae (Pelkmans et al., 2001, 2002). We have recently shown that human echovirus 1 (EV1) is another virus using caveolae in its entry (Marjomäki et al., 2002). Subsequent to internalization via caveolae, EV1 was shown to be localized in perinuclear structures, which contained caveolin-1 (Marjomäki et al., 2002). Such structures include caveosomes, recently identified endocytic organelles, which participate in the entry of SV40. However, the entry routes of SV40 and EV1 seem not to be identical (Marjomäki et al., 2002) and EV1 was found to be a unique tool to study the cell biology of caveolae. Importantly, EV1 uses α2β1 integrin, a collagen receptor, to bind to cell surface (Bergelson et al., 1992) and therefore, the entry of EV1 can be used to study the role of integrins in caveolae function. Previous studies have shown that integrins can be coprecipitated with caveolin-1 (Wary et al., 1996, 1998), suggesting that caveolae form a platform for integrin-mediated signaling.
Here, we show that α2β1 integrin is located in raft like membrane domains rather than in caveolae. However, the formation of α2β1 integrin clusters triggers their lateral redistribution along cell surface to caveolae and consequently activates the internalization of caveolae in a protein kinase Cα (PKCα)-dependent manner. Integrin α2β1 represents a novel mechanism to activate caveolae-mediated endocytosis. The molecular mechanisms of endocytosis and recycling of β1, β2, and αV integrins have been studied in detail (Bretscher 1992; Fabbri et al., 1999; Ng et al., 1999a; Laukaitis et al., 2001), but integrin trafficking has been described to take place in endosomes and in an endocytic recycling pathway. Thus α2β1 seems to have unique activities compared with other integrins. In agreement with this our results show that the clusters of αV integrin are internalized by a different mechanism, namely in clathrin-coated entry vesicles and early endosomes.
MATERIALS AND METHODS
Cells, Viruses, and Antibodies
SAOS cells (ATCC, Manassas, VA) were transfected with an expression construct encoding α2 integrin (SAOS-α2β1 cells; Ivaska et al., 1999). Cells transfected with an empty expression vector (SAOS-pAW) were used as a control. EV1 (Farouk strain, ATCC) was propagated in GMK cells and purified in sucrose gradients as described previously (Marjomäki et al., 2002). The following antibodies were used: rabbit antisera against purified EV1 (Marjomäki et al., 2002), EEA1 (Mu et al., 1995), transferrin (Zymed, South San Francisco, CA), ERK (Santa Cruz Biotechnology, Santa Cruz, CA), caveolin-1 (Transduction Laboratories, Lexington, KY; N20 antibody, Santa Cruz), and phosphorylated protein kinase Cα (Upstate Biotechnology, Lake Placid, NY) as well as monoclonal antibodies (mAb) against total protein kinase Cα (Upstate), caveolin-1 (Transduction Laboratories and Zymed), tubulin (Sigma, St. Louis, MO), myc (9E10, ATCC), integrin α2 subunit (MCA2025, Serotec, Raleigh, NC), integrin αV subunit (L230, ATCC), and phosphorylated ERK (Transduction Laboratories). Conjugation of anti-α2 and anti-αV antibodies to Alexa 488 was done using a mAb labeling kit (Molecular Probes, Inc., Eugene, OR).
Transfections
SAOS-α2β1 cells were transfected with FuGENE reagent (Boehringer Mannheim, Indianapolis, IN) and the cells were used for experiments after an expression time of 25-40 h. The GFP constructs of actin (CLONTECH, Palo Alto, CA), caveolin-1 (caveolin-GFP; from Dr. Ari Helenius, Institute of Biochemistry, ETH-Hoenggerberg, Switzerland; Pelkmans et al., 2001) wild-type Eps15 (DIIId2; from Dr. Alice Dautry-Varsat, Pasteur Institute, Paris; Benmerah et al., 1998), dominant negative (DN) Eps15 (d95/925; from Dr. Alice Dautry-Varsat, Pasteur Institute, Paris; Benmerah et al., 1999), wild-type PKCα (Dr. Peter J. Parker, Cancer Research, UK; Ng et al., 1999b), DN PKCα (T497A kinase-dead, substrate-binding mutant; Dr. Peter J. Parker, Cancer Research, UK; Mostafavi-Pour et al., 2003), and DN PKCε (kinase dead form; Ivaska et al., 2002b) were used. DN MEK (1E8; from Dr. Natalie Ahn, University of Colorado; Holmström et al., 1999), constitutively active MEK (1R4F; from Dr. Natalie Ahn, University of Colorado; Holmström et al., 1999), and DN Ras (asn-17-ras; from Dr. Larry Feig, Tufts University; Feig and Cooper, 1988) were cotransfected to the cells with pEGFP-C2 (CLONTECH). A myc tagged AP180C (from Dr. Dieter Blaas, University of Vienna; Ford et al., 2001) was revealed from transfected cells by myc labeling.
Flotation Gradient Centrifugation
SAOS-α2β1 cells were lysed with 1% Triton X-100 in PBS supplemented with protease inhibitors for 30 min on ice. The homogenate was adjusted to a sucrose density of 40.6%, overlaid with 35% sucrose and then filled with 5% sucrose. The gradient was centrifuged for 35,000 rpm at 4°C in a Beckman Sw 41Ti rotor for 18 h.
Integrin Clusters
Antibody against α2 integrin was added in DMEM (supplemented with 0.1% serum, low-DMEM), incubated for 1 h on ice, and washed. Cells were subsequently incubated with Alexa-conjugated goat anti-mouse IgG antibody on ice and washed. Formation of integrin clusters was allowed to occur at 37°C for 30-90 min. Clusters of αV integrins were allowed to form using an anti-αV subunit mAb and Alexa-conjugated anti-mouse IgG antibody. As a control, anti-α2 and anti-αV mAbs, directly labeled with Alexa 488, were used alone without the clustering secondary antibodies.
Measurement of Internalization
The internalization of EV1 or integrin clusters was estimated by confocal microscopy using the three-dimensional (3D) LSM program, version 1.4.2 (Carl Zeiss, Jena, Germany). Cells were incubated with EV1 for 1 h and fixed with 3% paraformaldehyde for 20 min. After fixation, EV1 was labeled without permeabilization using a primary antibody and then the anti-rabbit Alexa 546 conjugate. After a 5-min permeabilization with 0.2% Triton X-100 EV1 was again labeled but now using anti-rabbit Alexa 488 conjugate. Thus, plasma membrane associated EV1 was stained with both Alexa 546 and 488 conjugates and was seen as yellow when the red and green channels were merged. Green signal representing internalized EV1 was measured. First the double-labeled cells were imaged as z-stacks with the confocal microscope. Then, in the 3D for LSM program the volume of internalized vesicles and the total amount of fluorescence was measured. Alltogether 20 cells were measured in the experiment. In parallel experiments the clusters of α2 or αV integrins were allowed to form in the presence of Alexa-conjugated secondary antibody and to be internalized. Samples were fixed as above. The internalized integrins had green color, and the plasma membrane associated integrins were labeled with Alexa 546 conjugate. Again yellow color in merge images represented integrin clusters on the plasma membrane and green signal represented internalized clusters.
Protein Kinase C Activation
SAOS-α2β1 cells were starved overnight in low-DMEM. Cells were then treated in different ways: 1) stimulated with 1 μM PMA 30 min at 37°C; 2) incubated with EV1 for 1 h on ice, washed, and incubated for 30 min at 37°C; 3) pretreated with 10 μM safingol at 37°C for 30 min, followed by EV1 treatment together with 10 μM safingol as described above; 4) incubated with anti-α2 mAb Fab fragment (2 μg) for 1 h on ice, washed, and incubated for 30 min at 37°C; 5) α2β1 integrin clustering as explained above and incubated for 30 min at 37°C; 6) control SAOS-α2β1 cells were treated with low-DMEM for 1 h on ice and then incubated for 30 min at 37°C. The treated cells were lysed with 30 mM octylglucopyranoside (Sigma) with protease inhibitors for 30 min on ice.
SDS-PAGE and Immunoblotting
Samples were separated in 12% SDS-polyacrylamide gels and electroblotted onto PVDF membrane (Millipore, Bedford, MA). Aerolysin overlay assay was performed as described (Fivaz et al., 2002). Primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Richmond, CA) were used. Bands were detected by chemiluminescence (Pierce, Rockford, IL).
Immunofluorescence and Confocal Microscopy
Subconfluent SAOS-α2β1 cultures were incubated with EV1 for various time periods and then fixed with methanol at -20°C for 6 min or with 4% paraformaldehyde for 20 min at room temperature. Cross-absorbed goat secondary antibodies against rabbit (Alexa 488, 546, or 633 nm; Molecular Probes, Inc.) and mouse (Alexa 488, 546, or 633 nm; Molecular Probes, Inc.) antibodies were used for labeling. The cells were examined with an Axiovert 100 M SP epifluorescence microscope (Carl Zeiss) equipped with a confocal setup (Zeiss LSM510). Images were acquired using a Plan Neofluar objective (63×, 1.25 oil) and a digital resolution of 512 × 512. False colocalization signals were avoided by scanning fluorescence from different excitation wavelengths separately.
Live Cell Microscopy
SAOS-α2β1 cells were stained with Alexa 546-conjugated mutant aerolysin (ASSP; Fivaz et al., 2002) together with an anti-α2 mAb on ice for 1 h. Alternatively, cells transfected with cDNA encoding caveolin-1-GFP (green fluorescent protein) or actin-GFP were treated with anti-α2 mAb. After washes the cells were transferred to a preheated sample stage (27/37°C) at a confocal microscope (Carl Zeiss Axiovert 100M with LSM510). Anti-mouse Alexa 488 (aerolysin-containing cells) or anti-mouse Alexa 546 (GFP-containing cells) was added and z-stacks through the cells were imaged at 5- or 10-min intervals. CO2 independent medium (Sigma) was used in all steps. 3D projections of the selected images were created for each time point using LSM 510 3.0 and AxioVision Inside4D 3.0 (Carl Zeiss). These image series were processed and edited using Corel Photo-Paint 8, Ulead Media Studio ProVideo Editor 5.02a VE, and Quick Time Pro.0.2.
Electron Microscopy
SAOS-α2β1 cells were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 1 h, and then postfixed in 1% osmium tetroxide, dehydrated, stained with uranyl acetate, and embedded in LX-112. For visualizing clusters of α2 or αV integrin, cells were treated with anti-α2 or αV mAb for 1 h on ice, then with rabbit antibodies against mouse IgG (Sigma), and finally with protein A gold (10-nm particles, G. Posthuma and J. Slot, Utrecht, The Netherlands), both for 1 h on ice. Cells were then either fixed immediately or incubated for 1 min to 2 h at 37°C in complete culture medium. Further preparation for electron microscopy was performed as described above.
Statistical Analysis
For analyzing the transfected samples in Figures 5C and 6B the binomial t test (comparison of two experimental percentual figures) was used. For analyzing the differences in binding of EV1 to cells (Figure 7B) multivariate analysis of variance (MANOVA) was used with the least significant difference (LSD) as a post hoc test.
Figure 5.
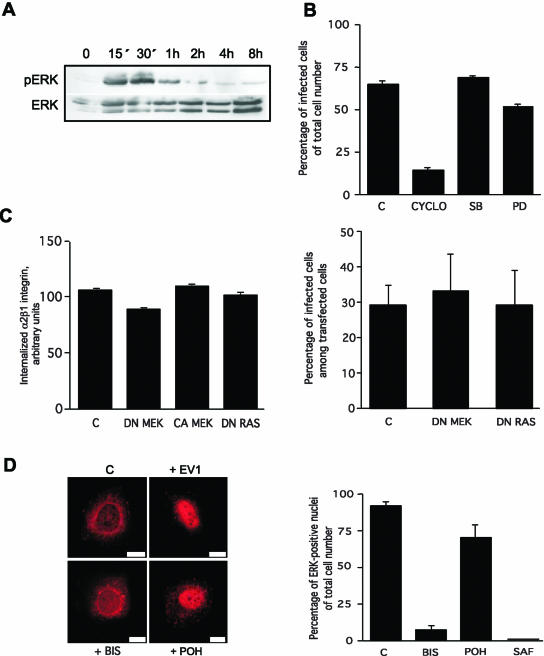
Binding of α2β1 integrin to EV1 induces the phosphorylation of ERK MAP kinase in a PKCα activity-dependent manner. (A) ERK is transiently phosphorylated <15 min after α2β1 integrin binding to EV1 and the phosphorylation lasts ∼1 h (Western blot). (B) Inhibitors of ERK activation (PD) and p38 kinase (SB) do not inhibit infection in contrast to methyl β-cyclodextrin (CYCLO), an inhibitor of cholesterol metabolism and caveolae. (C) Transient transfections with cDNAs encoding the dominant negative MEK (DN MEK) or dominant negative Ras (DN Ras) do not inhibit α2β1 integrin internalization or infection. Constitutively active MEK (CA MEK) does not increase the internalization of α2β1 integrin either. (D) ERK is translocated to the nucleus 4 h p.i. The general PKC inhibitor bisindolylmaleimide (5 μM, BIS) inhibits the nuclear translocation of ERK, unlike a Ras inhibitor, perillyl alcohol (500 μM, POH). Quantification of ERK positive nuclei after treatments with bisindolylmaleimide (BIS), another PKC inhibitor safingol (10 μM, SAF) or Ras inhibitor (POH). Error bars show SE values (B and C: the graph on the left, D) or 95% confidence limits (C: the graph on the right).
Figure 6.
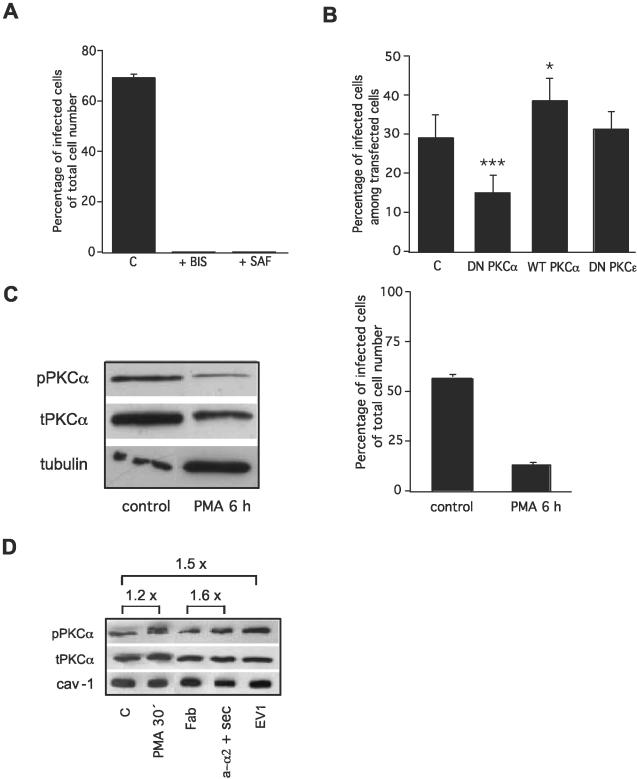
PKCα activity is crucial for EV1 replication and the clustering of α2β1 increases PKCα phosphorylation. (A) EV1 infection is prevented after inhibition of PKC activity with either bisindolylmaleimide (BIS) or safingol (SAF). (B) Dominant negative PKCα inhibits infection (p < 0.001, binomial t test), whereas over-expression of wild-type PKCα increases infection to some extent (p < 0.05, binomial t test). Dominant negative PKCε does not have any effect on infection. (C) PKCα is downregulated after a chronic treatment (6 h) with phorbol ester (PMA), which is shown by Western blotting. This treatment also inhibits EV1 infection. (D) Phosphorylation of PKCα detected by Western blotting during EV1 entry (EV1), a short treatment (30 min) with PMA and clustering of α2β1 integrin by anti-α2 mAb (a-α2 + Sec) and Fab of anti-α2 mAb as its control. Negative control is marked as C. In C and D antibodies against phosphorylated PKCα, total PKCα, tubulin and caveolin-1 were used. Error bars show SE values (A and C) or 95% confidence limits (B).
Figure 7.
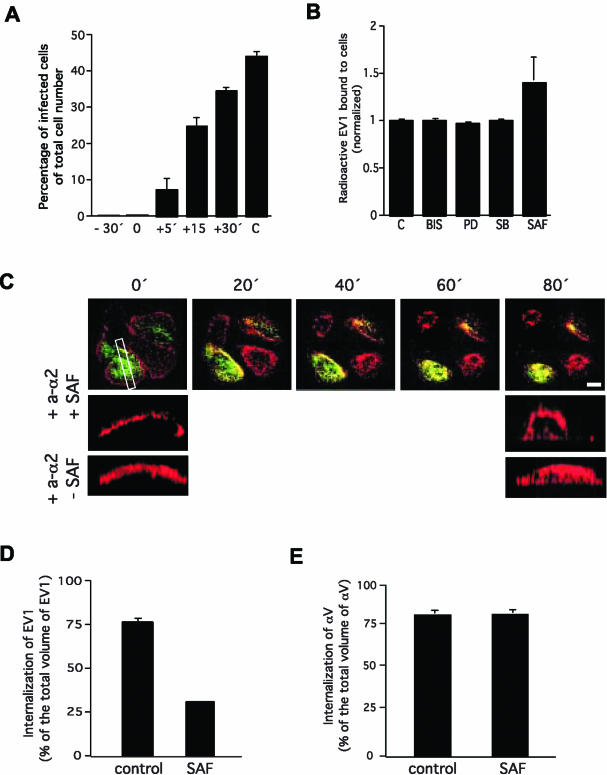
Integrin α2β1-mediated internalization of EV1 via caveolae is dependent on PKCα activity. (A) Safingol can be added to cells 30 min before EV1 (-30′), at the same time as EV1 (0), or 5 min after EV1 (+5′) to prevent the infection. (B) Safingol does not affect the ability of α2β1 integrin to bind radioactive EV1. (C) Live cell imaging showing that safingol does not prevent the clustering-related redistribution leading to colocalization of α2β1 integrin (red) and caveolin-1 (green) on the plasma membrane. To show that safingol inhibits integrin (red) internalization a rectangular slice was taken from the center area of one safingol treated and one nontreated cell (both in the presence of clustering antibodies) and projections were viewed from the side. Caveolin-1 has been excluded from these images. Note that safingol is causing some rounding of cells. (D) Safingol prevents the internalization of EV1 (100 MOI). Internalization was quantified by labeling EV1 before and after permeabilization with different colors and then taking Z-scans through the cells with a confocal microscope and measuring the internalized vesicles using 3D for LSM software. (E) Safingol has no effect on the internalization of antibody induced αV clusters. Error bars show SE values.
RESULTS
On Cell Surface α2β1 Integrin Is in Membrane Rafts
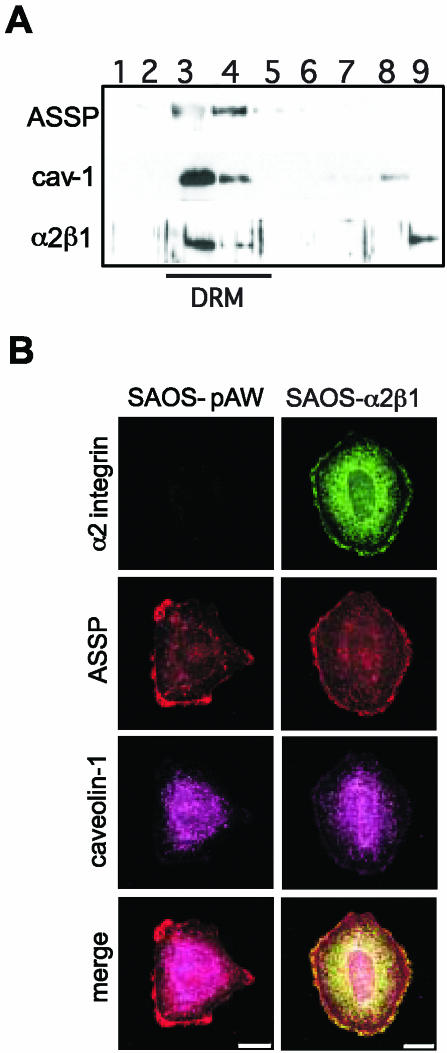
Flotation gradient centrifugation indicated that α2β1 integrin in SAOS-α2β1 cells (human osteosarcoma cells transfected with α2 integrin cDNA) is found in fractions containing detergent resistant membranes (DRM; Figure 1A). The same fractions contained glycosyl phosphatidyl inositol anchored proteins (GPI-APs), well-known components of raft type cholesterol-sphingolipid-rich microdomains and caveolin-1, a hallmark protein for caveolae and caveosomes (Figure 1A). GPI-APs were recognized using Alexa 546-conjugated mutant aerolysin (ASSP; Fivaz et al., 2002). Because flotation gradient centrifugation cannot be used to separate different DRM domains, confocal microscopy was used. When cells were plated on plastic in the presence of serum, patches of ASSP were found at the cell peripheries, suggesting the presence of raft domains. In addition, some intracellular staining was seen (Figure 1B). In SAOS-α2β1 cells α2β1 integrin colocalized with ASSP positive membrane domains (Figure 1B), indicating that ligand free α2β1 is present in raft domains. Caveolin-1 was mainly seen inside the cell, most clearly around the nucleus. Some caveolin-1 was also seen on the cell surface in accordance with the fact that the SAOS cell surface is rich in caveolae (Marjomäki et al., 2002). No clear colocalization of caveolin-1 and α2β1 integrin was detected (Figure 1B).
Figure 1.
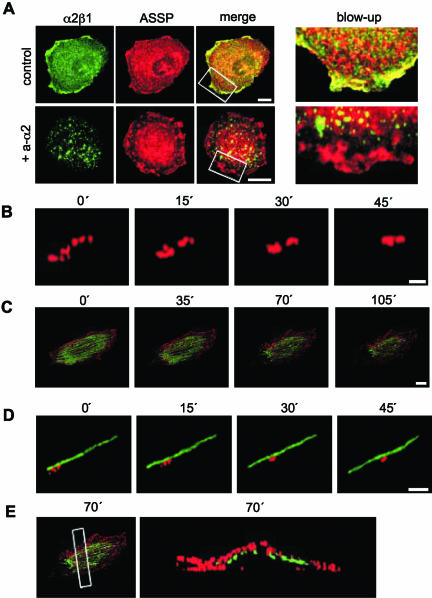
Integrin α2β1 is located in lipid rafts. (A) In flotation gradient centrifugation of 1% Triton X-100 lysed cell homogenate α2β1 integrin, caveolin-1, and GPI-APs (ASSP) localize at the 5-35% sucrose interphase. The localization of detergent-resistant membranes (DRM) have been indicated. Western blot of gradient fractions 1-9 (from top to bottom). (B) Lipid rafts containing GPI-APs (ASSP) are enriched in α2β1 integrin in SAOS-α2β1 cells. SAOS-pAW cells are α2β1 negative control cells. Indirect immunofluorescence labeling was used to reveal α2β1 integrin and caveolin-1, whereas GPI-APs were labeled directly with an ASSP-Alexa 546 conjugate. Confocal images are 3D projections of scanned cells. Scale bar, 10 μm. In merge images yellow color indicates colocalization of GPI-APs and α2β1 integrin.
Clustering of α2β1 Integrin Leads to Lateral Redistribution following Actin Filaments and Internalization in Caveolae-like Structures
Cluster formation was first induced using primary anti-α2 integrin antibodies together with secondary antibodies. The fate of the clustered integrins was followed by live cell confocal microscopy, and the data were processed to obtain projections of the 3D images of the cells at different time points. Concomitant staining of cells with Alexa 546-conjugated ASSP was used to mark the GPI-APs containing domains. During the follow-up period integrins formed clusters (Figure 2A) that started to move along the cell surface. Concomitantly, small clusters fused together (Figure 2B, Video 1). Moving integrin clusters followed cortical actin microfilaments, i.e., filaments close to the cell surface (Figure 2, C-E, Video 2). Destruction of microfilaments with cytochalasin D (5 μg/ml) inhibited the movement of α2β1 integrins out of lipid rafts (our unpublished results). The presence of α2β1 integrins on the cell surface was also confirmed by measuring the relative distribution of internalized vs. cell surface integrins using differential staining before and after permeabilization (our unpublished results).
Figure 2.
Formation of α2β1 clusters initiates redistribution of α2β1 out of lipid rafts. (A) In SAOS-α2β1 cells α2β1 integrin (green) is colocalized with GPI-APs (ASSP, red; control), but after incubation with anti-α2 mAb and secondary antibody (+a-α2) α2β1 integrin is located outside the lipid rafts. Scale bar, 10 μm. Blow-ups show lipid raft areas on cell edges (merge images). (B) A close-up about the formation of α2β1 integrin clusters (red) and integrin redistribution (see Video 1). Scale bar, 0.5 μM. (C) A whole-cell view shows that integrin clusters (red) move along actin filaments (GFP-actin; see Video 2). Scale bar, 10 μM. (D) A close-up of integrin clusters fusing together and moving along actin. Scale bar, 2 μM. B, C, and D are all live images of the same cell, B and D being digitally isolated close ups of C (actin is not shown in B). The live imaging (B-D) was performed at low temperature (27°C) in order to increase temporal resolution. (E) A rectangular slice of the cell from the 70-min time point was taken to verify that the actin filaments were indeed cortical, i.e., close to the plasma membrane. The white rectangle shows the location of this slice.
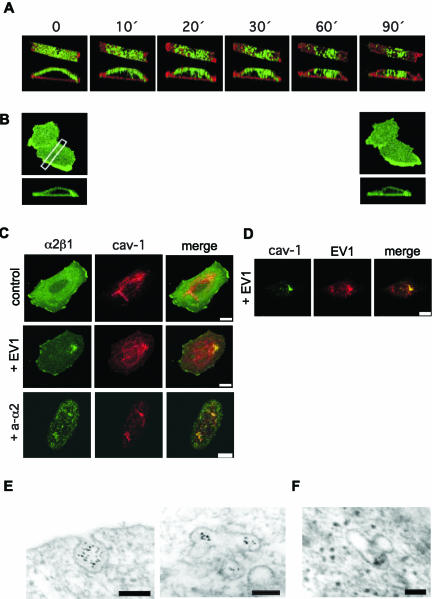
Integrin clusters were subsequently internalized (Figure 3A, Movie 3). Importantly, under the same conditions no integrin clustering or internalization was detected if cells were treated with fluorophore-labeled primary antibody without the secondary antibody (Figure 3B). Furthermore, the conditions used in live cell microscopy had no effects on cell viability or morphology (our unpublished results). Integrin clusters were internalized in caveolin-1-positive vesicles (Figure 3C, Video 4). Integrin α2β1 was immunostained before and after permeabilization of the cells to distinguish between internalized and cell surface integrin. Two hours after antibody-mediated clustering 71 ± 3% of α2β1 integrin was intracellular. Destruction of microfilaments with cytochalasin D (5 μg/ml), which inhibited the movement of α2β1 integrins out of lipid rafts, did not completely prevent the α2β1-mediated EV1 entry and infection (our unpublished results). GPI-APs were also internalized to some extent. However, this is not evident in the images because of the “shadow” rendering method used. In accordance with our previous observations (Marjomäki et al., 2002), EV1 was rapidly internalized into similar vesicles that contained caveolin-1 and α2β1 integrin, and the vesicles had the tendency to accumulate close to the nucleus (Figure 3, C and D). The internalization of clustered integrins was further studied by EM using secondary antibodies and protein A gold. The cell surface invaginations containing clustered integrins appeared to be similar to caveolae and, accordingly, the integrin containing intracellular vesicles resembled caveosomes (Pelkmans et al., 2001; Figure 3E). EV1 labeled with gold particles was shown to accumulate into identical vesicles (Figure 3F). Our data indicate that the formation of α2β1 clusters alone may trigger accumulation of the receptors into caveosome like organelles and that EV1 may behave as a multivalent ligand that activates the same internalization process as the antibodies.
Figure 3.
Clusters of α2β1 integrin are internalized in caveolae. (A) Live cell imaging of cellular internalization of clustered α2β1 integrin. Integrin α2β1 is green and GPI-APs are red (ASSP). A rectangular slice was taken from the center area of one cell, and projections were viewed from above and the side (see Video 3). (B) Live cell imaging with fluorophore (green)-labeled anti-α2 integrin mAb without secondary antibody. A rectangular slice was taken from the center area of one cell, and the projection was viewed from the side. (C) In SAOS-α2β1 cells incubated with anti-α2 mAb and secondary antibody or EV1, α2β1 integrin (green) is colocalized with caveolin-1 (red; see Video 4). Scale bar, 10 μM. (D) Caveolin-1 colocalizes with EV1 2 h p.i. Scale bar, 10 μM. (E) Electron microscopy of gold particles linked to secondary antibodies against anti-α2 mAb indicates that clustered α2β1 integrin is internalized in caveolae (left) and later is found in caveosomes (right). Protein A gold particles (5 nm) label the secondary antibodies that were used to cluster the primary anti-α2 antibodies. Scale bar, 100 nm. (F) A caveosome containing internalized EV1 that is labeled with gold particles (5 nm) is shown for comparison. Scale bar, 100 nm. For EV1 infections MOI 100 was used.
Importantly, in EM we could not see EV1 or α2β1 clusters inside coated vesicles, suggesting that clathrin-dependent processes are not involved in their entry. This was further studied by transient transfection with cDNA coding for dominant negative (DN) Eps15, known to inhibit the internalization of transferrin (Benmerah et al., 1999). As shown in Table 1, DN Eps15 had no effect on α2β1 internalization, although it could inhibit the entry of transferrin. In SAOS-α2β1 cells, transfected AP180C (Ford et al., 2001) proved to be an even more effective inhibitor of transferrin internalization, but without an effect on α2β1 entry (Table 1).
Table 1.
Inhibition of transferrin internalization has no effect on the internalization of α2β1 integrin clusters
| cDNA | No. of cells containing internalized transferrin (% of all transfected cells) | No. of cells containing internalized α2β1 integrin (% of all transfected cells) |
|---|---|---|
| Control | 78 (39/50) | 82 (41/50) |
| EPS15 WT | 84 (42/50) | 92 (46/50) |
| EPS15 DN | 40 (20/50) | 92 (46/50) |
| AP180C | 24 (12/50) | 90 (45/50) |
The number of cells containing internalized transferrin or α2β1 integrin was counted 2 h after α2β1 integrin clustering was induced by antibodies. Transferrin was added 15 min before fixation. Only the cells that had successfully been transfected were analyzed. The figures in parentheses show the number of positive cells/number of cells analyzed.
The Clusters of αV Integrins Are Internalized in Coated Vesicles and Endosomes
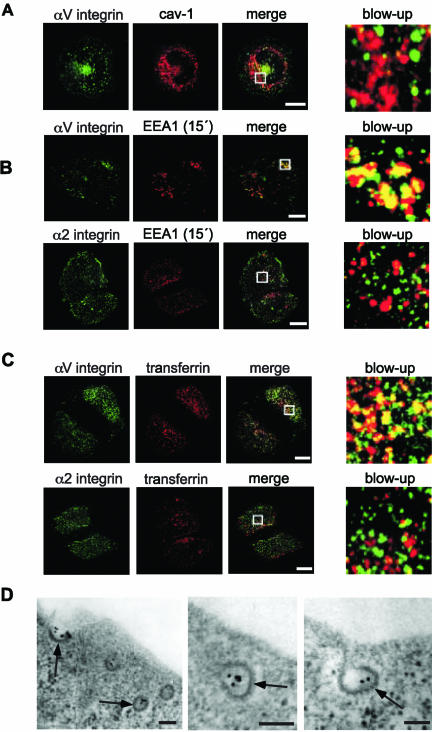
We repeated the same experiment with antibodies against αV integrin subunit and secondary antibodies. In 2 h 93 ± 1% of αV clusters had been internalized. If primary anti-αV antibodies were used alone, no clustering or internalization of αV integrins was detected (our unpublished results). The clusters of αV integrins were internalized in caveolin-1-negative vesicles (Figure 4A). However, some vesicles containing αV clusters were positive for early endosome associated protein (EEA1; Figure 4B) and for endocytosed transferrin (Figure 4C). Importantly, internalized α2β1 clusters were in EEA1 and transferrin negative vesicles (Figure 4, B and C). Finally, electron microscope images revealed that αV clusters labeled with gold particles were found in structures that were morphologically identical to clathrin-coated vesicles (Figure 4D). Picornaviruses that bind to αV integrins, such as parechovirus-1, are known to enter their host cells via the clathrin-dependant pathway (Joki-Korpela et al., 2001).
Figure 4.
Clusters of αV and α2β1 integrins are internalized through distinct pathways. (A) Clusters of αV integrins (green) are not targeted to caveolin-1-containing vesicles (red). Scale bar, 10 μM. (B) Clusters of αV integrins, unlike clusters of α2β1 integrins, are found in vesicles positive for EEA1, a molecular marker for early endosomes. Scale bar, 10 μM. (C) Clusters of αV integrins, unlike clusters of α2β1 integrins, are internalized via the same pathway as transferrin. Scale bar, 10 μM. In A-C, white rectangles show the locations of the blow-ups. (D) αV integrin clusters labeled with gold particles (10 nm) 1 min after internalization can be seen inside coated vesicles by electron microscopy. Clathrin coat is indicated by arrows. Scale bar, 100 nm.
Protein Kinase C Activity Is Essential for the Internalization of α2β1 Integrin via Caveolae
The fact that integrins can mediate cellular signals (Schwartz and Ginsberg, 2002) may influence their internalization. We have previously shown that mitogen activated protein kinases (MAPKs) are regulated by the collagen receptor integrins (Ravanti et al., 1999). Here, extracellular signal regulated kinase (ERK) was activated at very early time points after α2β1 binding to EV1 (Figure 5A). Because a selective inhibitor of ERK activation (20 μM PD98059, Calbiochem, La Jolla, CA; Figure 5B) or transient transfections with cDNAs coding for dominant negative or constitutively active MEK (DN MEK and CA MEK, respectively) had no effect on EV1 infection (Figure 5C), and DN MEK inhibited the internalization of α2β1 integrin only 10% (Figure 5C), we tried to specify other components of the same pathway, upstream of ERK and MEK, using selective inhibitors of Ras and protein kinase C (PKC). A Ras inhibitor (500 μM perillyl alcohol, POH, Aldrich, Milwaukee, WI) had little effect on ERK activation and its consequent transport into nucleus (Figure 5D). In addition, transfections with DN Ras had no effect on α2β1 internalization or EV1 infection (Figure 5C). In contrast, a PKC inhibitor (5 μM bisindolylmaleimide, Calbiochem) almost completely prevented accumulation of ERK in nucleus (Figure 5D). The experiment was repeated with another PKC inhibitor (10-25 μM safingol, Calbiochem) with the same results (Figure 5D).
On the basis of these findings, we deduced that PKC activity is needed for the activation of signaling pathways by α2β1 integrin. Furthermore, as shown in Figure 6A, the presence of PKC inhibitors reduced the number of EV1-infected cells to background levels. Safingol has been considered to be a selective inhibitor of the α isoform of PKC (Masur et al., 2001). Here, we also performed transfections with DN PKCα cDNA to confirm the role of this isoform in the process (Figure 6B). DN PKCα inhibited EV1 entry and infection, whereas overexpression of wild-type PKCα promoted it (Figure 6B). DN PKCε was used as a control and it had no effect (Figure 6B). The critical role of PKC in EV1 entry and infection was also confirmed in a third set of experiments (Figure 6C), in which chronic treatment of cells with a phorbol ester (PMA, 6 h) was used to downregulate PKC expression. Chronic PMA treatment is known to induce the degradation of both classical and novel PKCs (Srivastava et al., 2002), and it is not specific for PKCα. The effectiveness of the treatment was confirmed by Western blotting (Figure 6C). Concomitantly with a decrease in PKCα level, EV1 infection was prevented (Figure 6C). The amount of active (phosphorylated) PKCα in SAOS-α2β1 cells was analyzed by Western blotting with phosphospecific antibodies. Control SAOS-α2β1 cells contained phosphorylated PKCα and the fact that short PMA treatment (30 min) could only slightly increase the phosphorylation (in five experiments on average 1.5-fold) suggested that most of it might be in the active form (Figure 6D). Some increase (maximally 1.5 fold) in the phosphorylation of PKCα was seen after the cells were exposed to EV1 (Figure 6D). A similar small increase in phosphorylation was seen in the presence of primary anti-α2 and secondary antibodies when compared with the effect of Fab fragments of the same anti-α2 antibody (Figure 6D). We suggest that the increase in PKCα phosphorylation may be a part of the signaling events after integrin clustering. In any case, its activity seems to be critical for integrin signaling.
We next tested the mechanism used by PKCα inhibitors to affect the α2β1 integrin-mediated entry of EV1. If safingol was added to cells 5 min after EV1 almost complete inhibition was still obtained, whereas if added 30 min after EV1 it could no longer prevent infection (Figure 7A). This result limited the action of PKCα to the first minutes of the process. Experiments measuring the number of radioactive EV1 bound to the cell surface indicated that safingol does not inhibit ligand binding to α2β1 integrin (Figure 7B). Actually, in repeated experiments safingol seemed to slightly increase the number of cell bound EV1 viruses (Figure 7B), making it possible to speculate that safingol may actually affect receptor recycling. However, the increase was not statistically significant (multivariate analysis of variance), and therefore it was not studied further. In the presence of safingol EV1 binding to α2β1 integrin could still initiate the lateral redistribution of the integrin out of lipid rafts (our unpublished results). Furthermore, in the presence of safingol clustering antibodies could still initiate the process leading to the co-localization of α2β1 integrin and caveolin-1 (Figure 7C). Thus, safingol had no effect on the lateral movement of α2β1 integrin after cluster formation. Finally, we used confocal microscopy to quantify the number of EV1 particles inside cells and safingol could remarkably reduce the entry of EV1 (Figure 7D). Furthermore, safingol had no effect on the entry of clustered αV integrins (Figure 7E). Thus, the results indicate that the crucial role of PKCα in the process is limited to the activation of caveola entry by α2β1 integrins that have moved from lipid rafts to caveolae.
DISCUSSION
Integrins anchor cells to the extracellular matrix. In addition, they signal and participate in the regulation of cellular functions, such as migration, differentiation, survival, and growth. Like most cell surface receptors also integrins can be internalized after ligand binding. Fibrinogen endocytosis is mediated by αMβ2 integrin in monocytoid cells (Simon et al., 1993) and by αIIbβ3 integrin in platelets (Wencel-Drake et al., 1996). Integrins αVβ3 and αVβ5 can mediate the internalization of vitronectin (Pijuan-Thompson and Gladson, 1997). Some integrins can also recycle (Bretscher 1992; Fabbri et al., 1999). This process seems to be important for migrating epithelial cells (Ng et al., 1999a; Laukaitis et al., 2001). During cell migration β1 integrin trafficking has been described to take place in endosomes and the endocytic recycling pathway (Ng et al., 1999a; Laukaitis et al., 2001) in a PKCα-dependent manner (Ng et al., 1999a). We have recently reported that α2β1 integrin is internalized via caveolae in the process of EV1 entry (Marjomäki et al., 2002). This represents a novel internalization mechanism for integrins. Integrin α2β1 is a collagen receptor. It is expressed on platelets, epithelial cells, and also on many mesenchymal cell types, including fibroblasts, chondrocytes, osteoblasts, and endothelial cells. Integrin α2β1 mediates internalization of collagen and therefore it may participate in the regulation of matrix turnover (Segal et al., 2001). In endothelial cells α2β1-mediated collagen endocytosis may play a special role in the formation of capillary tube and lumen (Davis and Camarillo, 1996). It is not known whether integrins that are internalized in caveolae can recycle.
Many members of the integrin family are used by different viruses for attachment to the cell surface and for subsequent internalization into the host cell (Wang, 2002; Bomsel and Alfsen, 2003). It has been suggested that viruses have evolved to recognize cell adhesion receptors because of their relatively low affinity to natural ligands (Wang, 2002). The integrins may, however, play an active role in the entry process. For example, Kaposi's sarcoma associated herpes virus (human herpes virus 8) has been shown to use α3β1 integrin as its receptor and to activate α3β1-dependent signaling pathways, including focal adhesion kinase (Akula et al., 2002). Thus, virus binding may activate integrin associated cellular signaling, which could promote or modulate virus entry. Integrin α2β1 differs from other integrin type virus receptors by being the only integrin known to mediate virus entry through caveolae instead of clathrin-coated vesicles (Marjomäki et al., 2002). Caveolae are also involved in the entry of SV40 (Pelkmans et al., 2001; Pelkmans et al., 2002), mouse polyoma virus (Richterova et al., 2001), filoviruses (Empig and Goldsmith, 2002), and some bacteria (Shin et al., 2000).
Despite the fact that α2β1 integrin is internalized in caveolae our results indicate that after synthesis it is targeted to lipid rafts together with GPI-APs. The binding of both natural ligands and EV1 to α2β1 integrin may cause the formation of a receptor cluster. Here, using antibodies to induce the cluster formation we were able to show that α2β1 clustering alone can initiate the lateral redistribution of α2β1 out of raft domain. The integrins seem to follow actin microfilaments and during the process smaller clusters fuse together forming larger ones. Lateral redistribution of α5β1 integrin on the cell surface has been detected during electric field directed fibroblast locomotion (Brown and Loew, 1994). When integrins moved toward the cathode they also seemed to form large clusters (Brown and Loew, 1994). However, typically α5β1 is redistributed in endocytic vesicles in which the integrin colocalizes with transferrin receptor (Laukaitis et al., 2001). Thus, the lateral movement of α2β1 on cell surface from rafts to caveolae indicates that α2β1 acts in a different way than fibronectin receptors or other integrins studied so far. In migrating cells it is not known, whether α2β1 is among the β1 integrins that are guided to the endocytic recycling pathway (Ng et al., 1999a). However, integrin clustering after the binding of multivalent ligands seems to initiate a very different process.
When α2β1 integrin clusters were found in caveolae like invaginations on the cell surface their colocalization with caveolin-1 was observed. Previous studies have shown that integrins can be coprecipitated with caveolin-1 (Wary et al., 1996, 1998; Wei et al., 1999). Our results indicate, that in the case of α2β1 this association is obvious only after integrin clustering and redistribution. Caveolae are immobile and their internalization requires an activating stimulus (Thomsen et al., 2002). For example, the interaction of albumin-docking protein gp60 and caveolin-1 in endothelial cells (Minshall et al., 2000) and the phosphatase inhibitor okadaic acid (Parton et al., 1994) can trigger the internalization of caveolae. Clustering of α2β1 integrin represents a novel mechanism to activate caveolae.
The formation of integrin clusters is known to be important for signaling events (Miyamoto et al., 1995). Furthermore, caveolin-1 may participate in integrin-mediated signaling (Wary et al., 1996, 1998). The interaction of α2β1 with collagen can activate several signaling pathways, including MAPKs (Ivaska et al., 1999; Ravanti et al., 1999), the ζ isoform of PKC (PKCζ; Xu and Clark, 1997), and protein phosphatase 2A (PP2A; Ivaska et al., 2002a). Here, PKCα activity was needed for the internalization of α2β1 integrin in caveolae. Inhibition of PKCα could not prevent the cluster formation or the lateral movement of α2β1 on cell surface. Its inhibitory effect was seen only during a limited time period, suggesting that PKCα activity is obligatory only for the entry of caveolae. Interestingly, PKCα may directly bind to the cytoplasmic domain of integrin β1 subunit (Ng et al., 1999a). Furthermore, PKCα seems to be a resident protein of caveolae, which are major cell surface locations for this enzyme (Smart et al., 1994; Mineo et al., 1998). In caveolae PKCα might be constantly active (Smart et al., 1994; Mineo et al., 1998). Accordingly, control SAOS-α2β1 cells contained phosphorylated PKCα, and PMA could only slightly increase its phosphorylation. A similar small increase in the phosphorylation of PKCα was seen after the cells were exposed to EV1 or antibodies forming α2β1 clusters. Previous studies support the idea that PKCα is needed for caveola-related signaling functions (Smart et al., 1994; Mineo et al., 1998).
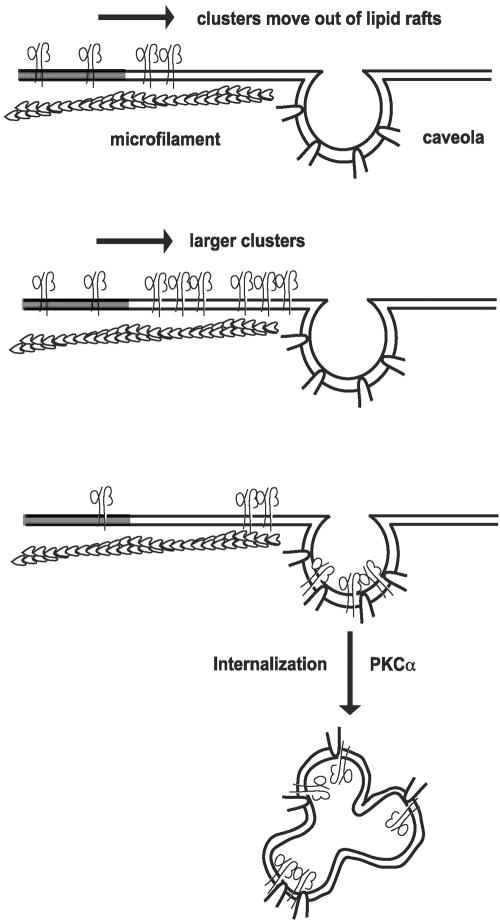
We have identified the formation of α2β1 integrin clusters as a key event leading to the relocation of α2β1 to caveolae. Furthermore, α2β1 clusters trigger the internalization of caveolae (the process has been summarized in Figure 8). Endocytosis via caveolae may be utilized by the natural ligands of α2β1 integrin. In addition, EV1 has evolved to take advantage of this phenomenon by binding to α2β1 on the cell surface. PKCα appears to be an important regulator of the entry process. The data indicate that the function of α2β1 is unique when compared with any other integrin.
Figure 8.
The proposed model of α2β1 integrin-mediated activation of caveolae. Antibody or virus-mediated formation of α2β1 integrin clusters leads to lateral movement on cell surface in which integrin clusters seem to follow microfilaments. Often smaller integrin clusters seem to fuse to each other. Clustering and lateral movement are followed by PKCα dependent internalization in caveolae.
Supplementary Material
Acknowledgments
We thank Arja Mansikkaviita and Raimo Pesonen for technical assistance and Dr. Daniel J. White for critical reading of the manuscript. We thank Dr. Dieter Blaas, Dr. Alice Dautry-Varsat, Dr. Natalie Ahn, Dr. Larry Feig, Dr. Ari Helenius, and Dr. Harald Stenmark for cDNAs and other reagents. The work was supported by grants from the Academy of Finland, the Sigrid Jusélius Foundation, and the Finnish Cancer Association. P.U. has a fellowship from the Finnish Cancer Institute.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0588. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0588.
Abbreviations used: CA, constitutively active; DN, dominant negative; DRM, detergent resistant membranes; EEA1, early endosome associated protein; ERK, extracellular signal regulated kinase; EV1, echovirus-1; GPI-APs, glycosyl phosphatidyl inositol anchored proteins; MAPK, mitogen activated protein kinase; MOI, multiplicity of infection; PKC, protein kinase C; SV40, simian virus 40.
References
- Akula, S.M., Pramod, N.P., Wang, F.Z., and Chandran, B. (2002). Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108, 407-419. [DOI] [PubMed] [Google Scholar]
- Benmerah, A., Lamaze, C., Begue, B., Schmid, S.L., Dautry-Varsat, A., and Cerf-Bensussan, N. (1998). AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140, 1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303-1311. [DOI] [PubMed] [Google Scholar]
- Bomsel, M., and Alfsen, A. (2003). Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell. Biol. 4, 57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson, J.M., Shepley, M.P., Chan, B.M., Hemler, M.E., and Finberg, R.W. (1992). Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 255, 1718-1720. [DOI] [PubMed] [Google Scholar]
- Bretscher, M.S. (1992). Circulating integrins: α5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO J. 11, 405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M.J., and Loew, L.M. (1994). Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J. Cell Biol. 127, 117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet, J., Sargiacomo, M., and Lisanti, M.P. (1997). Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 272, 30429-30438. [DOI] [PubMed] [Google Scholar]
- Davis, G.E., and Camarillo, C.W. (1996). An α2β1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 224, 39-51. [DOI] [PubMed] [Google Scholar]
- Drab, M. et al. (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449-2452. [DOI] [PubMed] [Google Scholar]
- Empig, C.J., and Goldsmith, M.A. (2002). Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76, 5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri, M., Fumagalli, L., Bossi, G., Bianchi, E., Bender, J.R., and Pardi, R. (1999). A tyrosine-based sorting signal in the β2 integrin cytoplasmic domain mediates its recycling to the plasma membrane and is required for ligand-supported migration. EMBO J. 18, 4915-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig, L.A., and Cooper, G.M. (1988). Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol. Cell. Biol. 8, 3235-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivaz, M., Vilbois, F., Thurnheer, S., Pasquali, C., Abrami, L., Bickel, P.E., Parton, R.G., and van der Goot, F.G. (2002). Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J. 21, 3989-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, M.G., Pearse, B.M., Higgins, M.K., Vallis, Y., Owen, D.J., Gibson, A., Hopkins, C.R., Evans, P.R., and McMahon, H.T. (2001). Simultaneous binding of PtdIns(4, 5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051-1055. [DOI] [PubMed] [Google Scholar]
- Fra, A.M., Williamson, E., Simons, K., and Parton. R.G. (1995). De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. USA 92, 8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström, T.H., Tran, S.E., Johnson, V.L., Ahn, N.G., Chow, S.C., and Eriksson, J.E. (1999). Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells to Fas receptor-mediated apoptosis. Mol. Cell. Biol. 19, 5991-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska, J., Reunanen, H., Westermarck, J., Koivisto, L., Kähäri, V-M., and Heino, J. (1999). Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J. Cell Biol. 147, 401-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska, J., Nissinen, L., Immonen, N., Eriksson, J.E., Kähäri, V-M., and Heino, J. (2002a). Integrin α2β1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3β. Mol. Cell. Biol. 22, 1352-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska, J., Whelan, R.D., Watson, R., and Parker, P.J. (2002b). PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 21, 3608-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joki-Korpela, P., Marjomäki, V., Krogerus, C., Heino, J., and Hyypiä, T. (2001). Entry of human parechovirus 1. J. Virol. 75, 1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia, T., and Parton, R. (1999). Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11, 424-431. [DOI] [PubMed] [Google Scholar]
- Laukaitis, C.M., Webb, D.J., Donais, K., and Horwitz, A.F. (2001). Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 153, 1427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjomäki, V. et al. (2002). Internalization of echovirus 1 in caveolae. J. Virol. 76, 1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur, K., Lang, K., Niggemann, B., Zanker, K.S., and Entschladen, F. (2001). High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol. Biol. Cell 12, 1973-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo, C., Ying, Y., Chapline, C., Jaken, S., and Anderson, R. (1998). Targeting of protein kinase Cα to caveolae. J. Cell Biol. 141, 601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall, R.D., Tiruppathi, C., Vogel, S. M., Niles, W.D., Gilchrist, A., Hamm, H.E., and Malik, A.B. (2000). Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J. Cell Biol. 150, 1057-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, S., Akiyama, S., and Yamada, K. (1995). Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267, 883-885. [DOI] [PubMed] [Google Scholar]
- Mostafavi-Pour, Z., Askari, J.A., Parkinson, S.J., Parker, P.J., Ng, T.T.C., and Humphries, M.J. (2003). Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J. Cell Biol. 161, 155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, F.T. et al. (1995). EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270, 13503-13511. [DOI] [PubMed] [Google Scholar]
- Ng, T., Shima, D., Squire, A., Bastiaens, P.I., Gschmeissner, S., Humphries, M.J., and Parker, P.J. (1999a). PKCα ulregates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 18, 3909-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, T. et al. (1999b). Imaging protein kinase Cα activation in cells. Science 283, 2085-2089. [DOI] [PubMed] [Google Scholar]
- Parton, R.G., Joggerst, B., and Simons, K. (1994). Regulated internalization of caveolae. J. Cell Biol. 127, 1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton, R.G. (2003). Caveolae—from ultrastructure to molecular mechanisms. Nat. Rev. Mol. Cell. Biol. 4, 162-167. [DOI] [PubMed] [Google Scholar]
- Pijuan-Thompson, V., and Gladson, C.L. (1997). Ligation of integrin α5β1 is required for internalization of vitronectin by integrin αvβ3. J. Biol. Chem. 272, 2736-2743. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001). Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473-483. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., and Helenius, A. (2002). Endocytosis via caveolae. Traffic 3, 311-320. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., Puntener, D., and Helenius, A. (2002). Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296, 535-539. [DOI] [PubMed] [Google Scholar]
- Ravanti, L., Heino, J., Lopez-Otin, C., and Kähäri, V.-M. (1999). Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J. Biol. Chem. 274, 2446-2455. [DOI] [PubMed] [Google Scholar]
- Razani, B. et al. (2001). Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 276, 38121-38138. [DOI] [PubMed] [Google Scholar]
- Razani, B. et al. (2002). Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277, 8635-8647. [DOI] [PubMed] [Google Scholar]
- Richterova, Z., Liebl, D., Horak, M., Palkova, Z., Stokrova, J., Hozak, P., Korb, J., and Forstova, J. (2001). Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75, 10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, W., Frank, P.G., Razani, B., Park, D.S., Chow, C.W., and Lisanti, M.P. (2001). Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619-48622. [DOI] [PubMed] [Google Scholar]
- Schwartz, M., and Ginsberg, M. (2002). Networks and crosstalk: integrin signaling spreads. Nat. Cell Biol. 4, E65-E68. [DOI] [PubMed] [Google Scholar]
- Segal, G., Lee, W., Arora, P.D., McKee, M., Downey, G., and McCulloch, C.A. (2001). Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J. Cell Sci. 114, 119-129. [DOI] [PubMed] [Google Scholar]
- Shin, J., Gao, Z., and Abraham, S. (2000). Involvement of cellular caveolae in bacterial entry into mast cells. Science 289, 785-788. [DOI] [PubMed] [Google Scholar]
- Simon, D.I., Ezratty, A.M., Francis, S.A., Rennke, H., and Loscalzo, J. (1993). Fibrin(ogen) is internalized and degraded by activated human monocytoid cells via Mac-1 (CD11b/CD18): a nonplasmin fibrinolytic pathway. Blood 82, 2414-2422. [PubMed] [Google Scholar]
- Smart, E., Foster, D., Ying, Y.-S., Kamen, B., and Anderson., R. (1994). Protein kinase C activators inhibit receptor-mediated potocytosis by preventing internalization of caveolae. J. Cell Biol. 124, 307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia, F. et al. (2002). Intracellular retention of glycosylphosphatidyl inositol-linked proteins in caveolin-deficient cells. Mol. Cell. Biol. 22, 3905-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, J., Procyk, K.J., Iturrioz, X., and Parker, P.J. (2002). Phosphorylation is required for PMA- and cell-cycle-induced degradation of protein kinase Cdelta. Biochem. J. 368, 349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, P., Roepstorff, K., Stahlhut, M., and van Deurs, B. (2002). Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell 13, 238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. (2002). Protein recognition by cell surface receptors: physiological receptors versus virus interactions. Trends Biochem. Sci. 27, 122-126. [DOI] [PubMed] [Google Scholar]
- Wary, K.K., Mainiero, F., Isakoff, S., Marcantonio, E., and Giancotti, F.G. (1996). The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733-743. [DOI] [PubMed] [Google Scholar]
- Wary, K.K., Mariotti, A., Zurzolo, C., and Giancotti, F.G. (1998). A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625-634. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Yang, X., Liu, Q., Wilkins, J.A., and Chapman, H.A. (1999). A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencel-Drake, J.D., Boudignon-Proudhon, C., Dieter, M.G., Criss, A.B., and Parise, L.V. (1996). Internalization of bound fibrinogen modulates platelet aggregation. Blood 87, 602-612. [PubMed] [Google Scholar]
- Xu, J., and Clark, R. (1997). A three-dimensional collagen lattice induces protein kinase C-ζ activity: role in α2 integrin and collagenase mRNA expression. J. Cell Biol. 136, 473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.