Summary
Targeting a specific arm of signaling pathways is of great interest. In this issue, Bosco et al. (2012) exploit the interactive interface between Rac GTPase and its effector p67phox to specifically inhibit ROS production, without perturbing other Rac-mediated cellular processes.
The coordinated assembly of multiprotein complexes is essential for the transmission of multiple downstream signaling pathways. Chemical tools that interfere with the assembly of such complexes are highly desirable to tease out the intracellular signaling networks. Upon stimulation, NADPH oxidase, a key cellular enzyme responsible for generating the reactive oxygen species (ROS), is assembled into an active complex at the membrane. ROS is the central weapon of phagocytes to combat the invading microorganisms. It also plays important regulatory functions during neutrophil chemotaxis (Hattori et al., 2010). Inefficient generation of ROS due to genetic mutations of the components of NADPH oxidase leads to chronic granulomatous disease (CGD), which is typically characterized by inability to fight infection and aberrant inflammation. Beyond their well-characterized traditionally roles in innate immunity and inflammation, more recently ROS and NADPH oxidase are actively being scrutinized as the key mediators of multitude of pathological conditions caused by oxidative stresses, including neurological diseases, cardiovascular pathologies, and cancer (Bedard and Krause, 2007; Kleinschnitz et al., 2010; Williams and Griendling, 2007). Accordingly, specific pharmacological inhibitors of ROS production by NADPH oxidase are being sought after for the therapeutic benefits of various human pathologies contributed by the oxidative stresses.
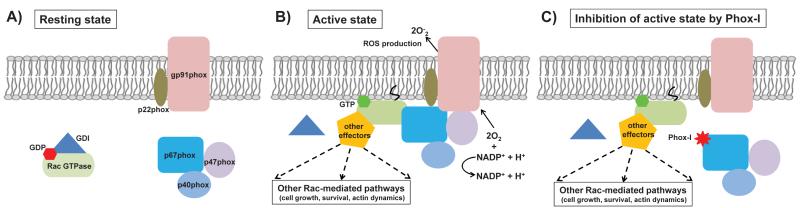
Targeting the assembly of active NADPH oxidase complex is an efficient way to inhibit ROS production. The central components of active NADPH oxidase consist of two membrane-bound subunits, gp91phox (or Nox2) and p22phox, and four cytosolic proteins, p47phox, p67phox, p40phox, and small GTPase Rac. In response to external stimuli, the cytosolic components are translocated to the membrane and interact with the membrane-bound components to assemble an active NADPH complex, which then transfer electrons from NADPH to oxygen to generate the reactive superoxide anion (Figure 1). A series of protein-protein interactions are critical for an efficient assembly of fully active NADPH oxidase complex. For example, the C-terminal part of cytosolic subunit p67phox interacts with p47phox, while the N-terminal half of p67phox is capable of binding to Rac. The GTP-bound activated Rac recruits p67phox to the membrane, hence facilitating the assembly of NADPH oxidase complex and its activation. As demonstrated by a small molecule inhibitor (Gao et al., 2004), targeting Rac activity is a legitimate strategy to interfere with the assembly of NADPH complex and ROS production. However, given the pleiotropic regulatory functions of Rac in a wide range of cellular functions, the “global” inhibition of Rac activity inevitably comes with the risk of unintended side effects, concomitant with inhibition of ROS generation. On the other hand, the “pathway-selective” inhibition of downstream of Rac signaling may specifically abrogate ROS production, without affecting other Rac-dependent cellular processes. Since the specific interactions between Rac and its effectors dictate consequent downstream cellular responses, chemically exploiting such interactive interfaces may pave the way for the “pathway-selective” inhibition.
Figure 1.
Assembly of NADPH oxidase complex and its inhibition by Phox-I. A) In resting state, the membrane-bound and cytosolic components of NADPH oxidase are segregated. The inactive GDP-bound Rac GTPase also resides in the cytoplasm, in association with Rho-GDP dissociation inhibitor (GDI). B) Upon stimulation, the cytosolic components are translocated to the membrane and interact with the membrane-bound components to assemble an active NADPH complex, which then transfer electrons from NADPH to oxygen to generate the reactive superoxide anion. The GTP-bound activated Rac recruits p67phox to the membrane, hence facilitating the assembly of NADPH oxidase complex and its activation. The active Rac also interact with other effectors to mediate downstream cellular responses such as the organization of cytoskeleton. C) A small molecule Phox-I disrupts the interaction between Rac and p67phox effector and specifically inhibits ROS production, without affecting other Rac-mediated pathways.
In this study, Bosco et al (2012) present a novel strategy to rationally design small molecules that target the interactive interface between Rac and its effector p67phox, which allows for the specific inhibition of ROS production by NADPH oxidase complex. To embark on this task, the authors first took a full advantage of previous structural and mutagenesis studies that identified a binding pocket within p67phox critical for interaction with the active Rac (Lapouge et al., 2000). This structural information enabled the structure-based virtual screening of small molecules that might target this binding interface and disrupt the formation of p67phox complex with Rac. From the initial screening of over 700,000 compounds followed by several rounds of molecular docking and data refinement, top 9 candidates predicted to be specific to p67phox were selected. A representative compound Phox-I1 was experimentally tested for its binding to p67phox and ability to disrupt the p67phox interaction with Rac. To this end, they employed the microscale thermophoresis that measures the changes in fluorescent intensity upon protein-protein or protein-small molecule interactions in near-native conditions. Using the purified wild-type and mutant proteins of both p67phox and Rac, they demonstrated the specificity of Phox-I1 in disrupting the interaction between p67phox and GTP-bound active Rac.
Next, to determine if Phox-I1 could inhibit ROS production, they employed cell-based functional assays using the fMLP-induced NADPH oxidase activation in different cell types, including primary murine and human neutrophils. In these assays, Phox-I1 manifested a comparable or better potency in comparison to other known compounds such as ROS scavenger, pan-inhibitor of NADPH oxidase, and Rac-GTP inhibitor. Phox-I1 had no inhibitory effect toward either the PMA-induced ROS production in cells or the glucose oxidase-mediated ROS generation in vitro. Having validated the utility of Phox-I1 in specifically abrogating fMLP-induced ROS production, they went on to carry out the structure-activity relationship (SAR) studies to indentify the necessary chemical moiety and to improve the potency. A substructure search of database identified a series of compounds, which they grouped into two categories; ‘Phox-I1-like’ and ‘Phox-I2-like’. Like Phox-I1, a second generation lead compound Phox-I2 displayed a specific inhibitory activity toward ROS production. More importantly, unlike the general inhibitors of Rac activity (Ferri et al., 2009; Gao et al., 2004), in the presence of Phox-I1 and Phox-I2, the active Rac-mediated F-actin assembly upon fMLP stimulation was unaffected, highlighting their “pathway-selective” inhibition of p67phox effector arm of Rac signaling. Further SAR studies of chemical scaffold of Phox-I1 and Phox-I2 will lead to the development of promising ROS inhibitors with a higher specificity for the clinical utility.
In conclusion, the current body of work by Bosco et al (2012) clearly represents a proof of principle for the novel concept of exploiting chemical biology approaches to tease out cellular functions of pleiotropic regulators, such as Rac GTPase. Unlike the activity-based high throughput screening, the execution of virtual screening requires the preexisting detailed understanding of specific molecular interactions. However, with exponentially growing number of small molecules and computational capacity combined with the protein-protein interaction networks, the rational design of small molecules with exquisite properties to tell apart the complicated cellular circuits may become attainable in the future. Finally, as implicated by this study, harnessing the power of virtual screening may become a standard practice that enables the activity-based high throughput screening more efficient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedard K, Krause KH. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bosco et al. Chem. Bio. 2012;19:xxx–xxx. [Google Scholar]
- Ferri N, Corsini A, Bottino P, Clerici F, Contini A. J Med Chem. 2009;52:4087–4090. doi: 10.1021/jm8015987. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, et al. Proc Natl Acad Sci U S A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, et al. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge K, Smith SJ, Walker PA, Gamblin SJ, Smerdon SJ, Rittinger K. Mol Cell. 2000;6:899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- Williams HC, Griendling KK. J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]